

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Aurora A kinase | Bioorg Med Chem Lett 18: 1623-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.068 BindingDB Entry DOI: 10.7270/Q261115V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50004205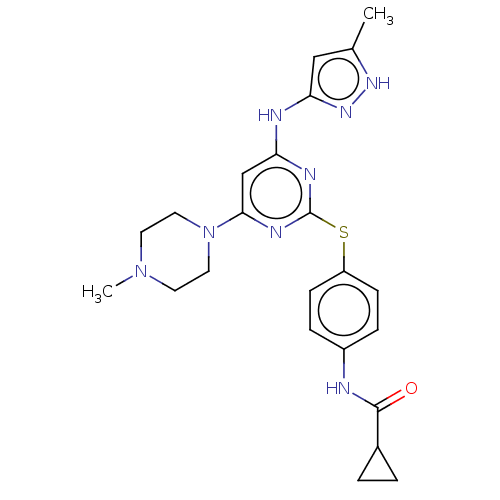 (MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His6-tagged Aurora A (1 to 403 residues) (unknown origin) expressed in baculovirus expression system | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Aurora B kinase | Bioorg Med Chem Lett 18: 1623-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.068 BindingDB Entry DOI: 10.7270/Q261115V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM50004205 (MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His6-tagged Aurora C (1 to 309 residues) (unknown origin) expressed in baculovirus expression system | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Aurora C kinase | Bioorg Med Chem Lett 18: 1623-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.068 BindingDB Entry DOI: 10.7270/Q261115V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM12985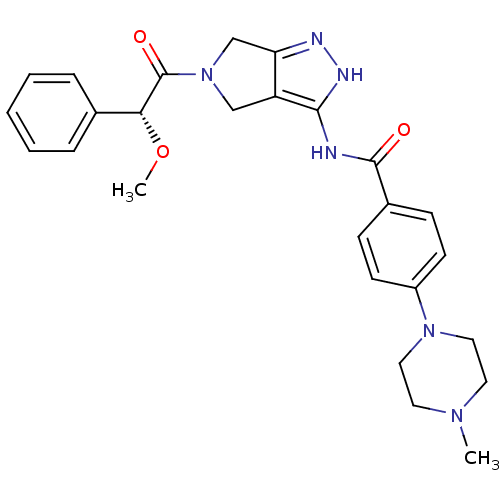 (5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Aurora A kinase | Bioorg Med Chem Lett 18: 1623-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.068 BindingDB Entry DOI: 10.7270/Q261115V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50004205 (MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His6-tagged Aurora B (62 to 344 residues) (unknown origin) expressed in baculovirus expression system | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM12985 (5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Aurora C kinase | Bioorg Med Chem Lett 18: 1623-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.068 BindingDB Entry DOI: 10.7270/Q261115V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM12985 (5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Aurora B kinase | Bioorg Med Chem Lett 18: 1623-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.068 BindingDB Entry DOI: 10.7270/Q261115V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530623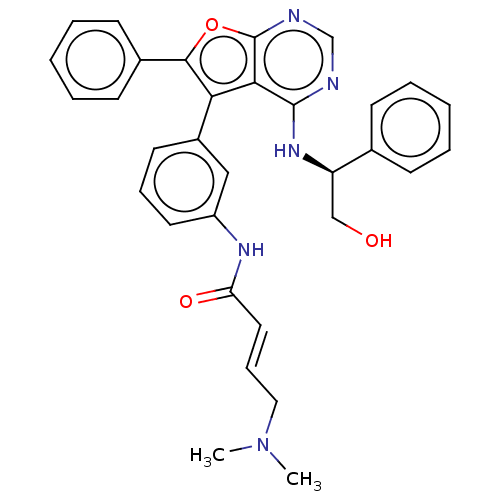 (CHEMBL4521381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530623 (CHEMBL4521381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50175305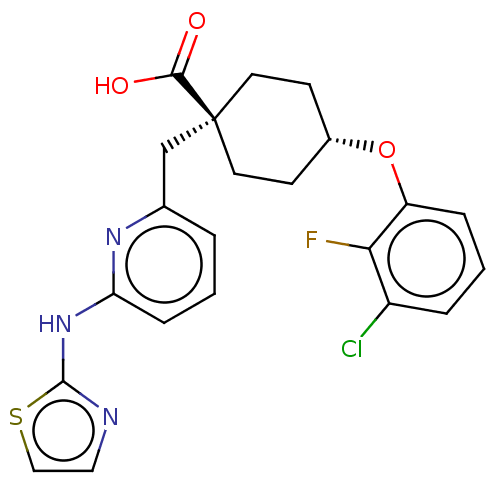 (CHEMBL3600873) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged Aurora A expressed in Escherichia coli using RRR(GLRRASLG)4R-NH2 as substrate after 40 mins in presence of... | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50507492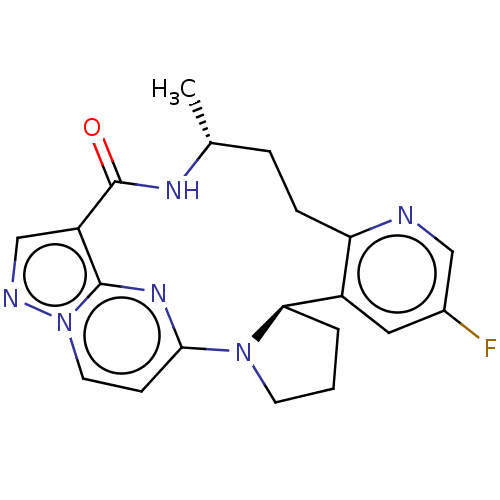 (Loxo-195 | Selitrectinib | US10966985, Compound 33...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wildtype human TRKA using poly (Glu,Tyr) 4:1 as substrate in presence of [gamma-33P]ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468247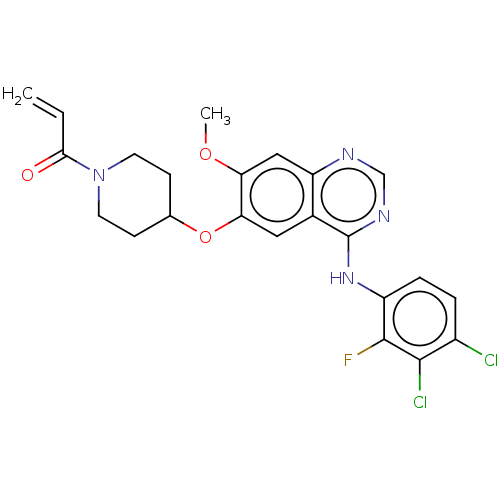 (HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468247 (HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50206389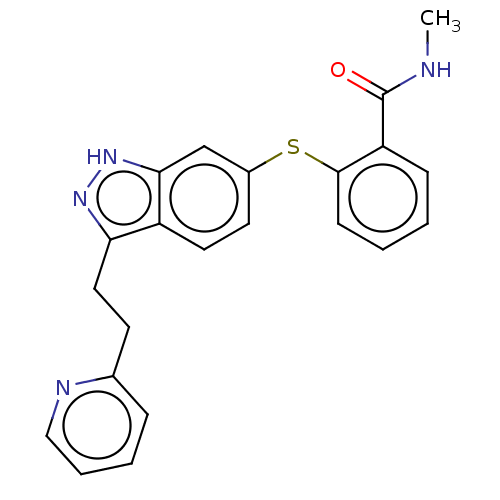 (CHEMBL3939307) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of VEGFR1 (unknown origin) | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 3 (Homo sapiens (Human)) | BDBM50206389 (CHEMBL3939307) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of VEGFR3 (unknown origin) | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM374727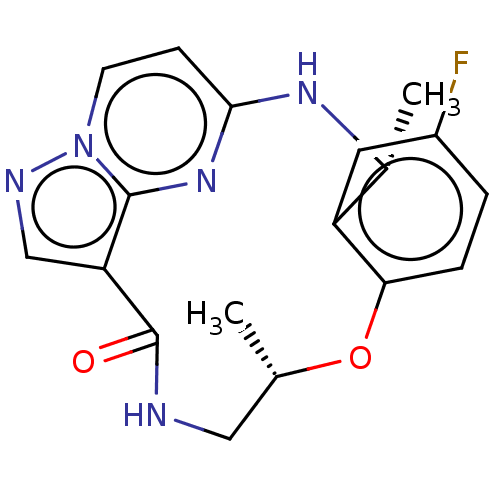 ((7S,13R)-11-fluoro-7,13-dimethyl-6,7,13,14- tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wildtype human TRKA using poly (Glu,Tyr) 4:1 as substrate in presence of [gamma-33P]ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530623 (CHEMBL4521381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530623 (CHEMBL4521381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530618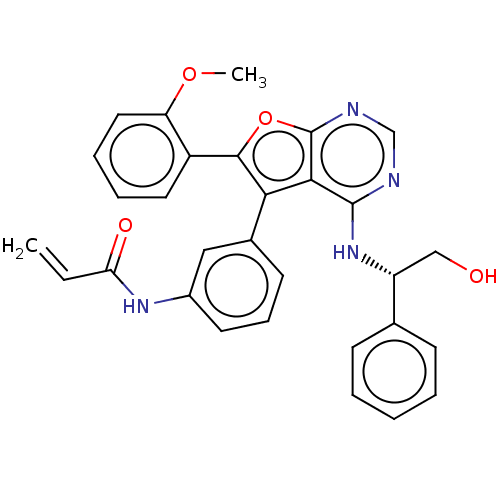 (CHEMBL4559807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530618 (CHEMBL4559807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50206389 (CHEMBL3939307) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) | Eur J Med Chem 124: 186-199 (2016) Article DOI: 10.1016/j.ejmech.2016.08.026 BindingDB Entry DOI: 10.7270/Q2668G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50579500 (CHEMBL4864729) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TRKC (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530622 (CHEMBL4514452) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530622 (CHEMBL4514452) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468247 (HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.218 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468247 (HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.218 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.421 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.421 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50579499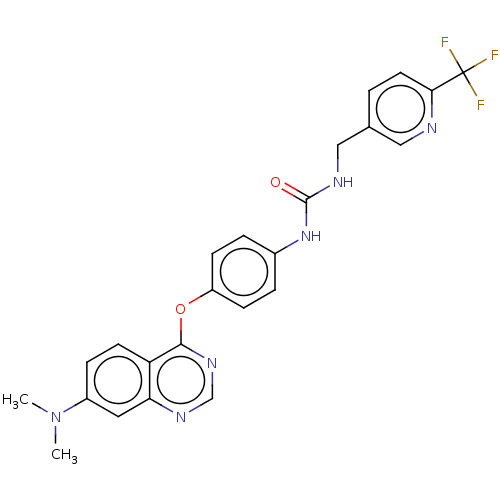 (CHEMBL4847875) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type human partial length CSF1R (I564 to S939 residues) expressed in bacterial expression system by Kinomescan method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01006 BindingDB Entry DOI: 10.7270/Q2W3815D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530618 (CHEMBL4559807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.636 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530618 (CHEMBL4559807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.636 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50579499 (CHEMBL4847875) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human c-Kit by Kinomescan method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01006 BindingDB Entry DOI: 10.7270/Q2W3815D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530615 (CHEMBL4534719) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530615 (CHEMBL4534719) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50579496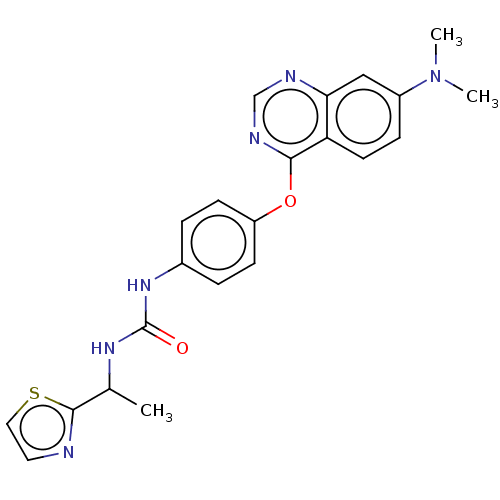 (CHEMBL4851545) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant GST-CSF1R (residues L534 to C972) expressed in Sf9 insect cells using poly(Glu,Tyr) as substrate incubated for 20 min... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01006 BindingDB Entry DOI: 10.7270/Q2W3815D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530622 (CHEMBL4514452) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530622 (CHEMBL4514452) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12178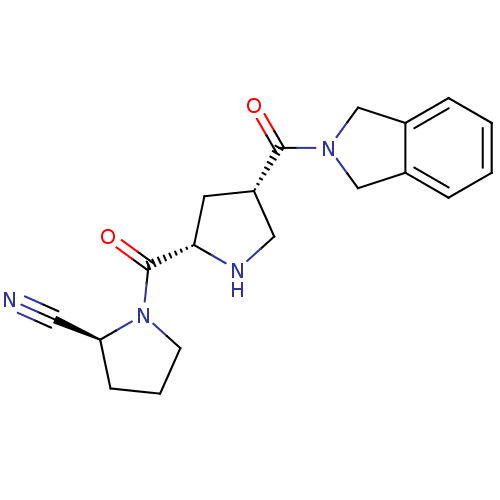 ((2S)-1-{[(2S,4S)-4-(2,3-dihydro-1H-isoindol-2-ylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 16: 3268-72 (2006) Article DOI: 10.1016/j.bmcl.2006.03.037 BindingDB Entry DOI: 10.7270/Q22805V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50579500 (CHEMBL4864729) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TRKB (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320036 (CHEMBL1086235 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320040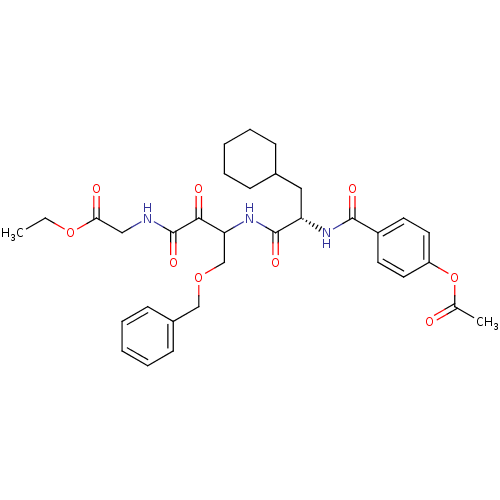 (CHEMBL1085728 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50586463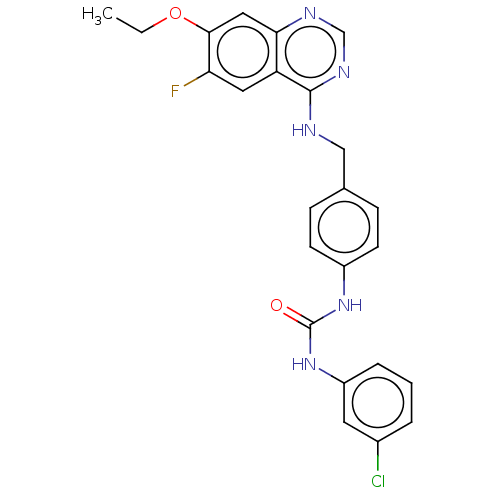 (CHEMBL5083023) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of GST-tagged AURA Ser123 to 401 residues) (unknown origin) expressed in Sf9 insect cells using tetra-LLRASLG peptide as substrate incubat... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320065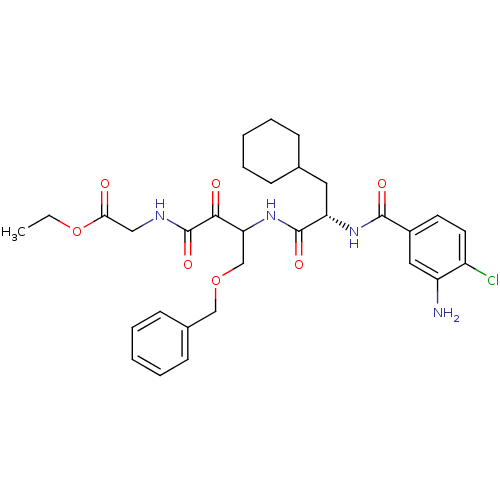 (CHEMBL1085970 | Glycine, N-[(3S)-4-Benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320057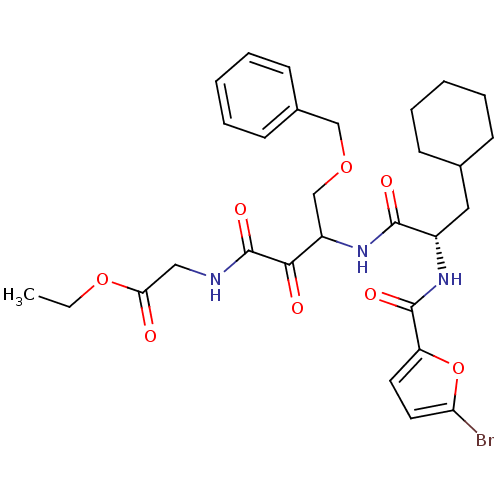 (CHEMBL1083620 | ethyl 2-(4-(benzyloxy)-3-((S)-2-(5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50399540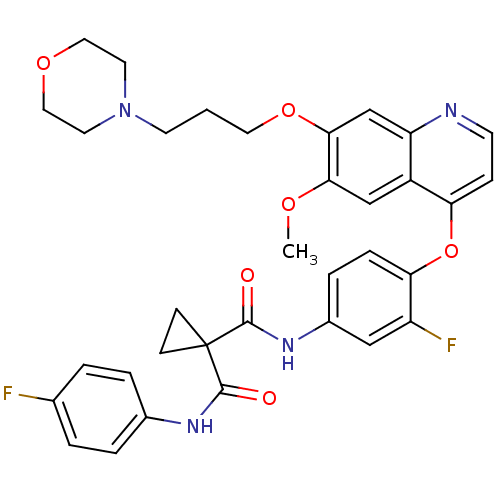 (FORETINIB | US10464902, Foretinib | US10882853, Co...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wildtype human TRKA using poly (Glu,Tyr) 4:1 as substrate in presence of [gamma-33P]ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320039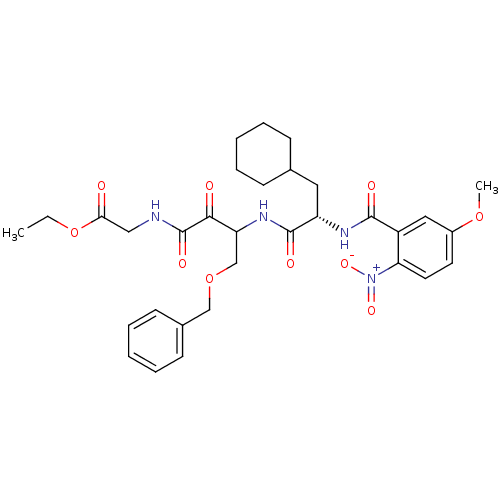 (CHEMBL1085726 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1059 total ) | Next | Last >> |