

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (Rattus norvegicus (rat)) | BDBM364425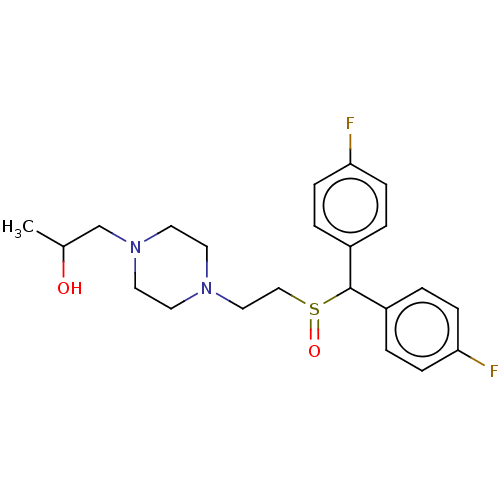 (US10913711, Compound 10d | US11555013, Compound 10...) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | >0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Norepinephrine Transporter Binding Assay. Brains from male Sprague-Dawley rats weighing 200-225 g (Taconic Labs, Germantown, N.Y.) were removed, fron... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7TRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM364427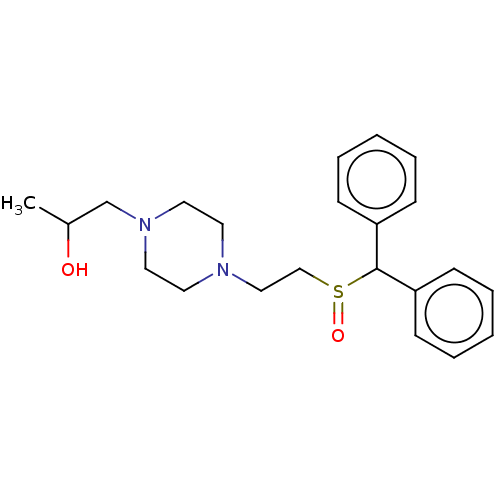 (US10913711, Compound 10a | US11555013, Compound 10...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serotonin Transporter Binding Assay. Brains from male Sprague-Dawley rats weighing 200-225 g (Taconic Labs, Germantown, N.Y.) were removed, midbrain ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7TRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus (rat)) | BDBM364427 (US10913711, Compound 10a | US11555013, Compound 10...) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Norepinephrine Transporter Binding Assay. Brains from male Sprague-Dawley rats weighing 200-225 g (Taconic Labs, Germantown, N.Y.) were removed, fron... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7TRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50221547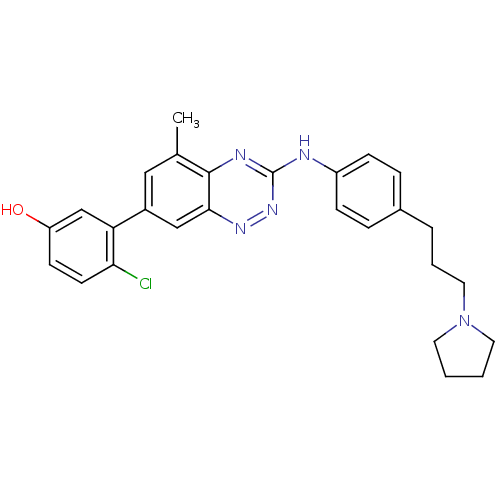 (4-chloro-3-(5-methyl-3-(4-(3-(pyrrolidin-1-yl)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of Abl | Bioorg Med Chem Lett 17: 5812-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.043 BindingDB Entry DOI: 10.7270/Q2M32VGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50007518 ((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01353 BindingDB Entry DOI: 10.7270/Q2BV7MF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50007518 ((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D3R expressed in HEK293 cell membranes incubated for 1 hr by radioligand binding assay | J Med Chem 59: 10676-10691 (2016) Article DOI: 10.1021/acs.jmedchem.6b01373 BindingDB Entry DOI: 10.7270/Q2WQ05RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50007518 ((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human D2L receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation co... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01353 BindingDB Entry DOI: 10.7270/Q2BV7MF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139771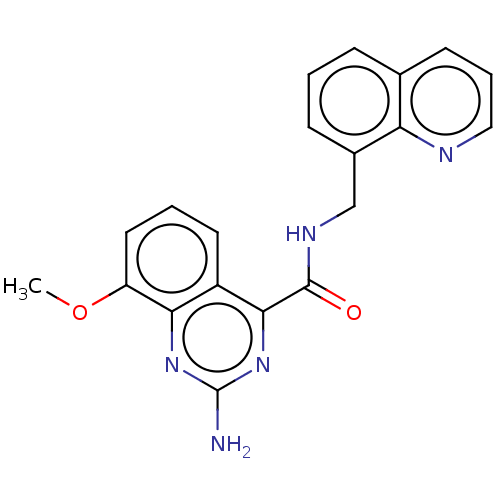 (CHEMBL3765580 | US10138212, Example 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303248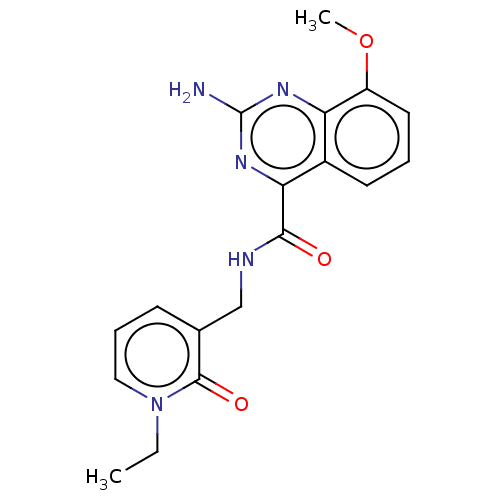 (2-amino-N-[(1-ethyl-2- oxo-3-pyridyl)methyl]-8- me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201006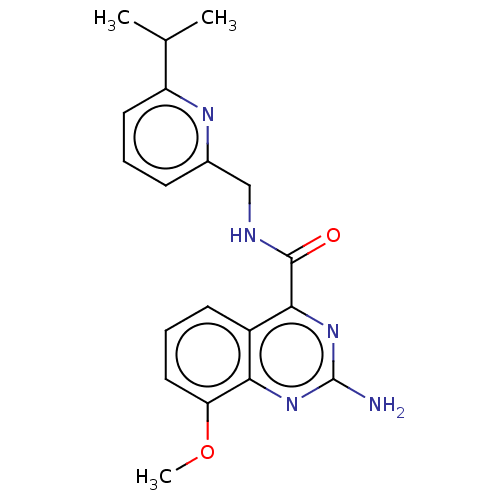 (CHEMBL3923709 | US10138212, Example 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200981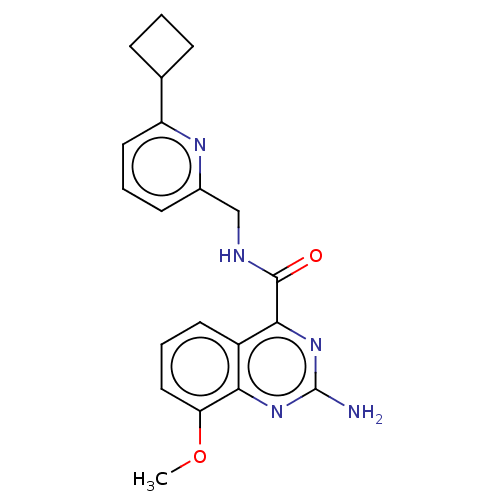 (CHEMBL3960148 | US10138212, Example 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201019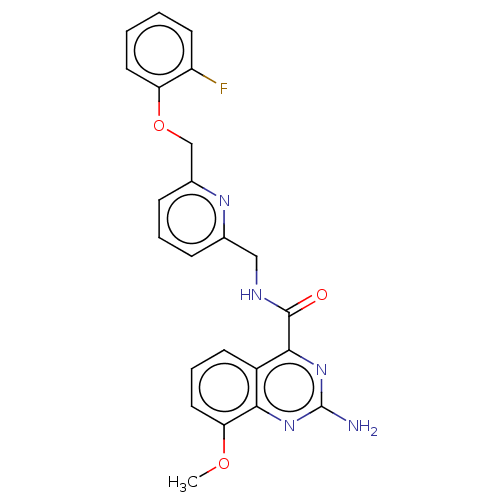 (CHEMBL3973920 | US10138212, Example 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303246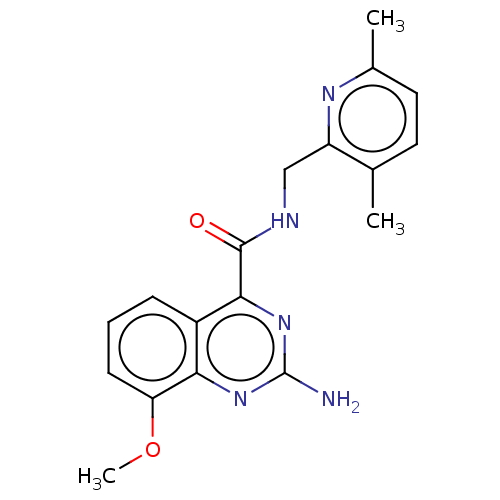 (2-amino-N-[(3,6- dimethyl-2- pyridyl)methyl]-8- me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139773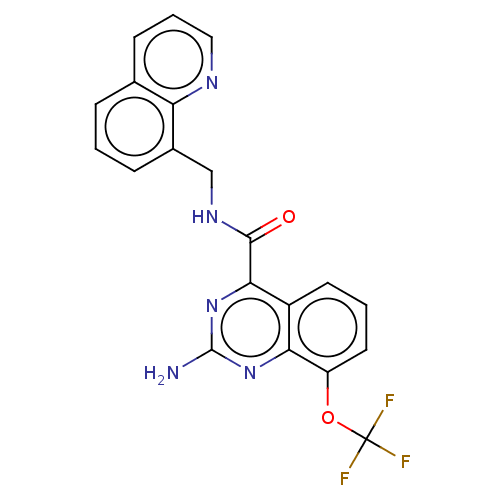 (CHEMBL3765379 | US10138212, Example 101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50007518 ((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D2R expressed in HEK293 cell membranes incubated for 1 hr by radioligand binding assay | J Med Chem 59: 10676-10691 (2016) Article DOI: 10.1021/acs.jmedchem.6b01373 BindingDB Entry DOI: 10.7270/Q2WQ05RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50378001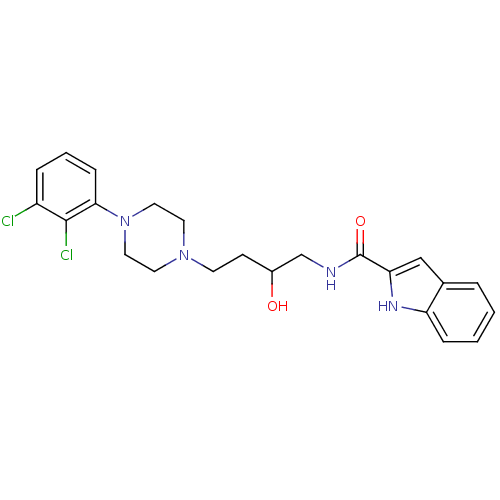 (CHEMBL1627321 | US8748608, 36) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America as Represented by the Secretary of the Department of Health and Human Services; The University of North Texas Health Science Center at Fort Worth US Patent | Assay Description Methods for performing in vitro dopamine receptor binding studies are described in Huang et al. J. Med. Chem. 44:1815-1826 (2001) and Luedtke et al. ... | US Patent US8748608 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50017698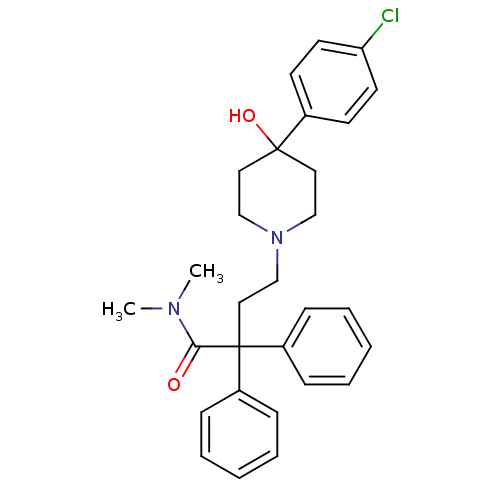 (4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.268 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00611 BindingDB Entry DOI: 10.7270/Q2N301V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50573367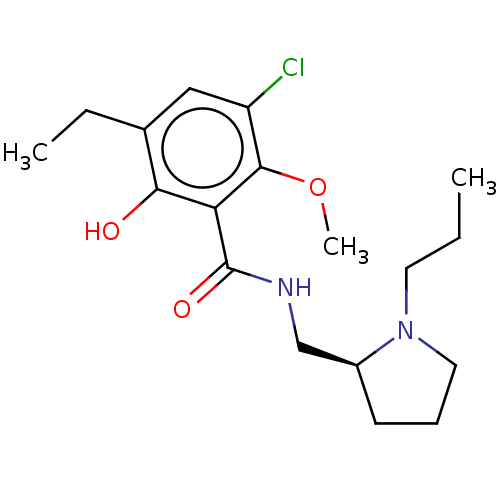 (CHEMBL4865434) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01353 BindingDB Entry DOI: 10.7270/Q2BV7MF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50178623 (CHEMBL110365 | N-(4-(4-(2-methoxyphenyl)piperazin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human dopamine D3 receptor expressed in HEK293 cells | J Med Chem 50: 4135-46 (2007) Article DOI: 10.1021/jm0704200 BindingDB Entry DOI: 10.7270/Q2WW7HDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303251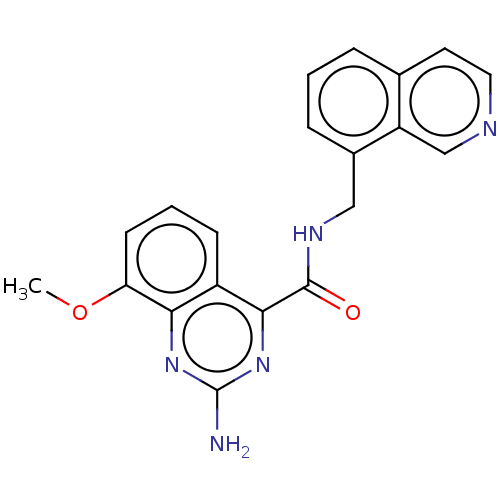 (2-amino-N-(8- isoquinolylmethyl)-8- methoxy-quinaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303181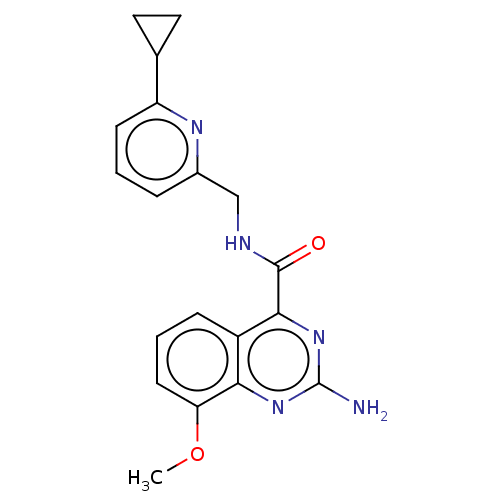 (2-amino-N-[(6- cyclopropyl-2- pyridyl)methyl]-8- m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303280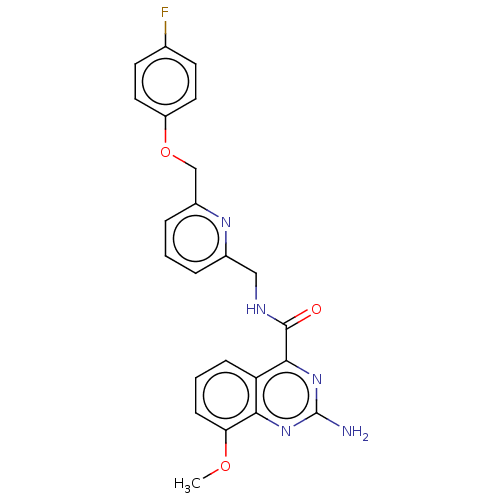 (2-amino-N-[[6-[(4- fluorophenoxy)methyl]- 2-pyridy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161235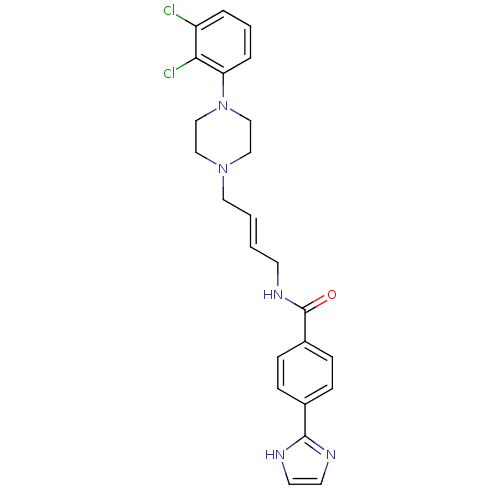 (CHEMBL179351 | N-{4-[4-(2,3-Dichloro-phenyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50221559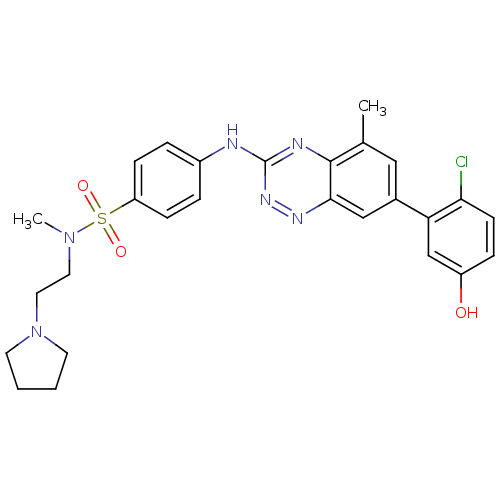 (4-(7-(2-chloro-5-hydroxyphenyl)-5-methylbenzo[e][1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of Abl | Bioorg Med Chem Lett 17: 5812-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.043 BindingDB Entry DOI: 10.7270/Q2M32VGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50131922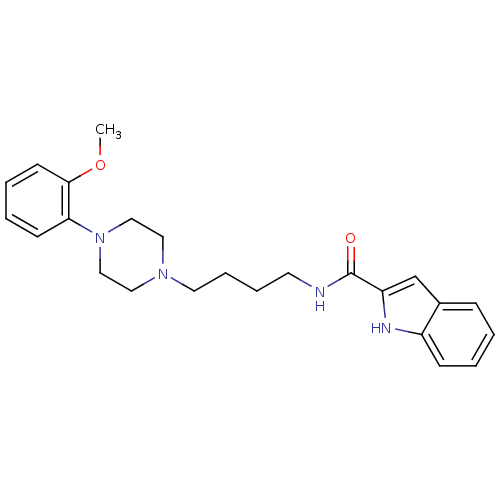 (1H-Indole-2-carboxylic acid {4-[4-(2-methoxy-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3 receptor expressed in HEK293 cells after 60 mins by gamma counting analysis | J Med Chem 55: 6689-99 (2012) Article DOI: 10.1021/jm300482h BindingDB Entry DOI: 10.7270/Q2Q81F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50585129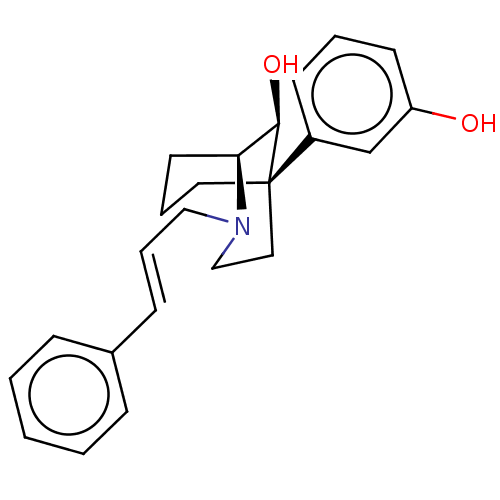 (CHEMBL5077645) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00611 BindingDB Entry DOI: 10.7270/Q2N301V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200989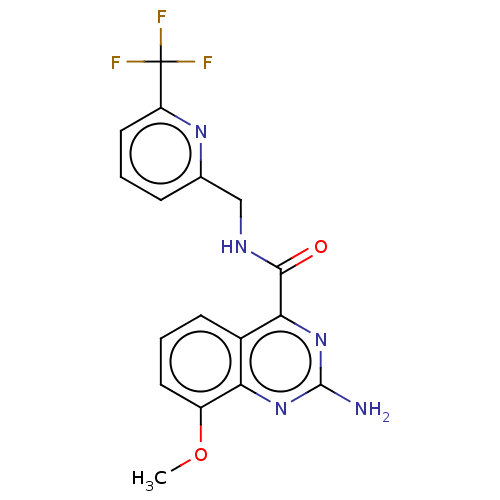 (CHEMBL3906827 | US10138212, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303252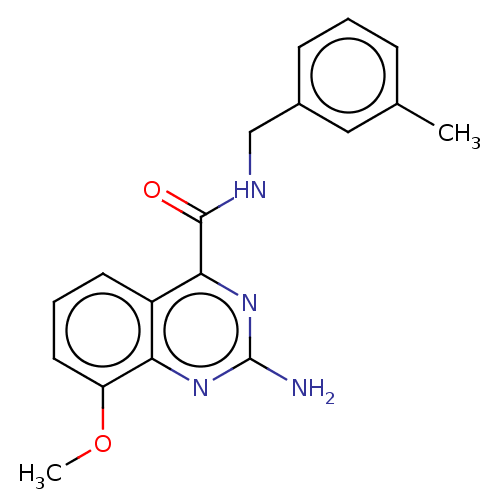 (2-amino-8-methoxy-N- (m- tolylmethyl)quinazoline- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200984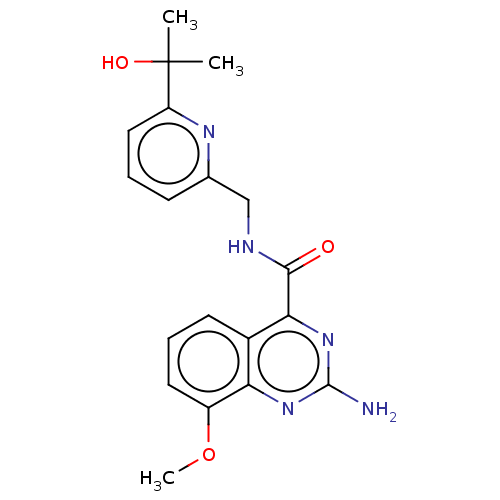 (CHEMBL3932655 | US10138212, Example 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50219103 (CHEMBL241973 | N-(4-(4-(2-methoxyphenyl)piperazin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human dopamine D3 receptor expressed in HEK293 cells | J Med Chem 50: 4135-46 (2007) Article DOI: 10.1021/jm0704200 BindingDB Entry DOI: 10.7270/Q2WW7HDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50129426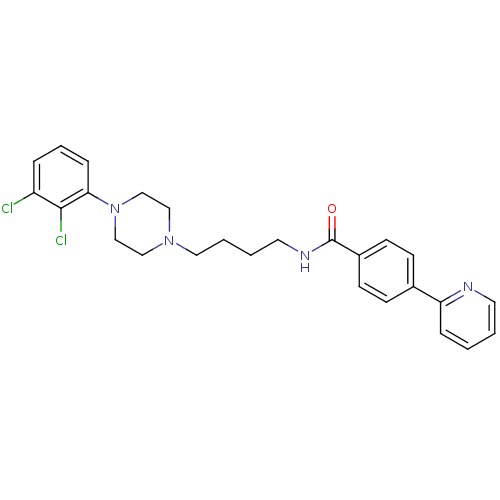 (CHEMBL69451 | N-(4-(4-(2,3-dichlorophenyl)piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human dopamine D3 receptor expressed in HEK293 cells | J Med Chem 50: 4135-46 (2007) Article DOI: 10.1021/jm0704200 BindingDB Entry DOI: 10.7270/Q2WW7HDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161217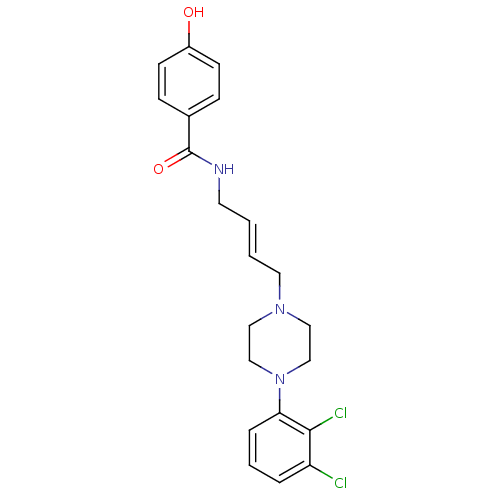 (CHEMBL195057 | N-{4-[4-(2,3-Dichloro-phenyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50221565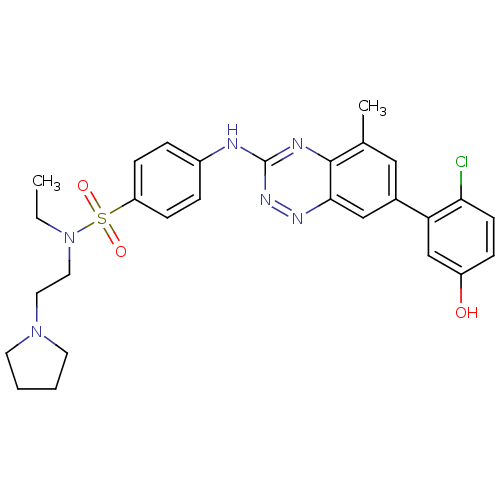 (4-(7-(2-chloro-5-hydroxyphenyl)-5-methylbenzo[e][1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of Abl | Bioorg Med Chem Lett 17: 5812-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.043 BindingDB Entry DOI: 10.7270/Q2M32VGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50156916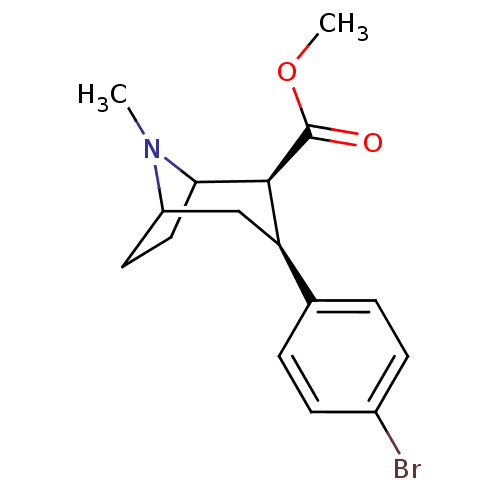 ((1R)-3beta-(4-bromophenyl)tropane-2beta-carboxylic...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN 35428 from DAT in rat striatal membrane | J Med Chem 49: 6621-5 (2006) Article DOI: 10.1021/jm0603973 BindingDB Entry DOI: 10.7270/Q2CZ36SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50573366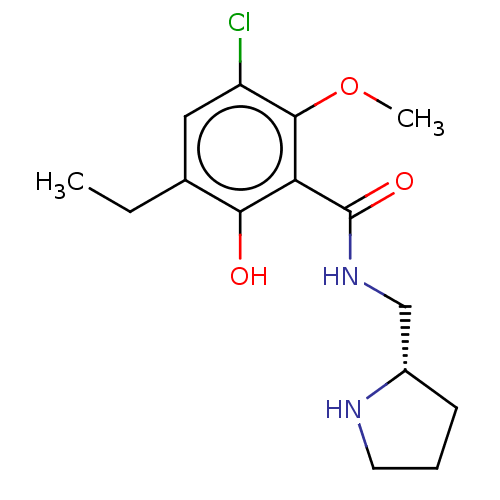 (CHEMBL4861573) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.431 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01353 BindingDB Entry DOI: 10.7270/Q2BV7MF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50573380 (CHEMBL4873313) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.436 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01353 BindingDB Entry DOI: 10.7270/Q2BV7MF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50573381 (CHEMBL4864320) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.444 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01353 BindingDB Entry DOI: 10.7270/Q2BV7MF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50573383 (CHEMBL4850300) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.493 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01353 BindingDB Entry DOI: 10.7270/Q2BV7MF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50573385 (CHEMBL4846561) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.499 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01353 BindingDB Entry DOI: 10.7270/Q2BV7MF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303255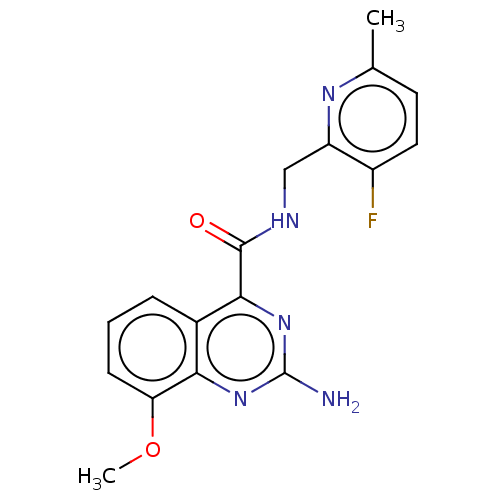 (2-amino-N-[(3-fluoro-6- methyl-2- pyridyl)methyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201017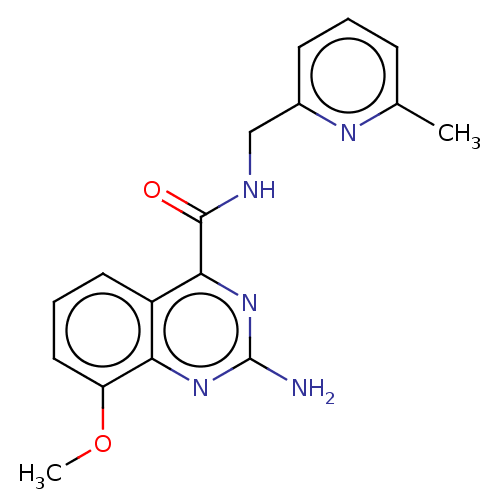 (CHEMBL3941632 | US10138212, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303313 (2-amino-8-fluoro-N-[(3- fluoro-6-methyl-2- pyridyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303298 (2-amino-8-fluoro-N-[(2- pyrazol-1- ylphenyl)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50378018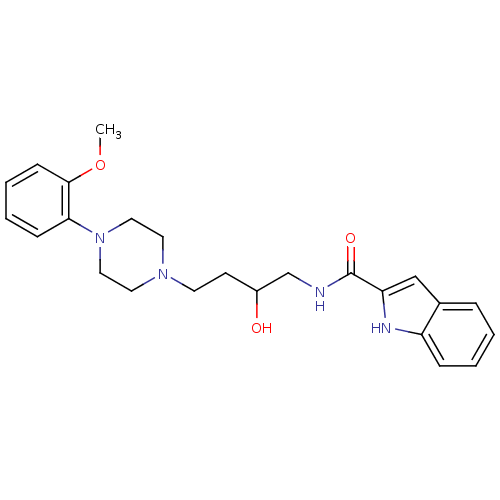 (CHEMBL1627322 | US8748608, 37) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America as Represented by the Secretary of the Department of Health and Human Services; The University of North Texas Health Science Center at Fort Worth US Patent | Assay Description Methods for performing in vitro dopamine receptor binding studies are described in Huang et al. J. Med. Chem. 44:1815-1826 (2001) and Luedtke et al. ... | US Patent US8748608 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM123847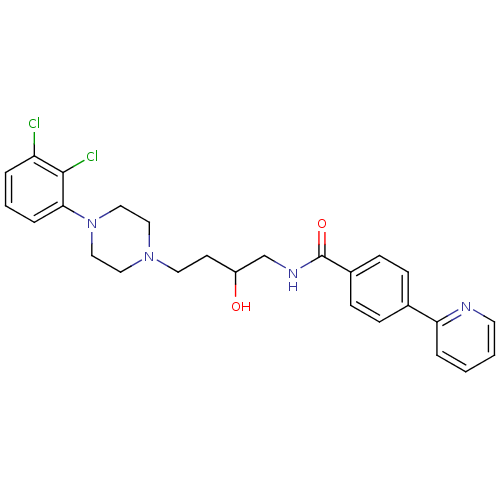 (US8748608, 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America as Represented by the Secretary of the Department of Health and Human Services; The University of North Texas Health Science Center at Fort Worth US Patent | Assay Description Methods for performing in vitro dopamine receptor binding studies are described in Huang et al. J. Med. Chem. 44:1815-1826 (2001) and Luedtke et al. ... | US Patent US8748608 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50129426 (CHEMBL69451 | N-(4-(4-(2,3-dichlorophenyl)piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161238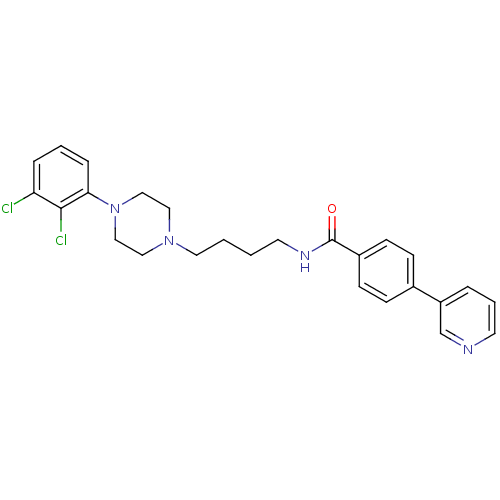 (CHEMBL179960 | N-{4-[4-(2,3-Dichloro-phenyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303145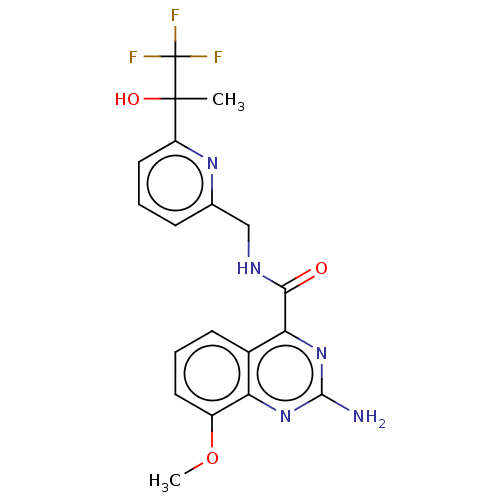 (2-amino-8-methoxy-N- [[6-(2,2,2-trifluoro-1- hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50219111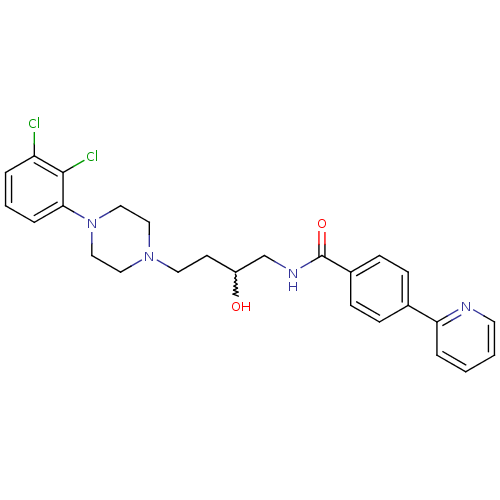 (CHEMBL390253 | N-(4-(4-(2,3-dichlorophenyl)piperaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human dopamine D3 receptor expressed in HEK293 cells | J Med Chem 50: 4135-46 (2007) Article DOI: 10.1021/jm0704200 BindingDB Entry DOI: 10.7270/Q2WW7HDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50221557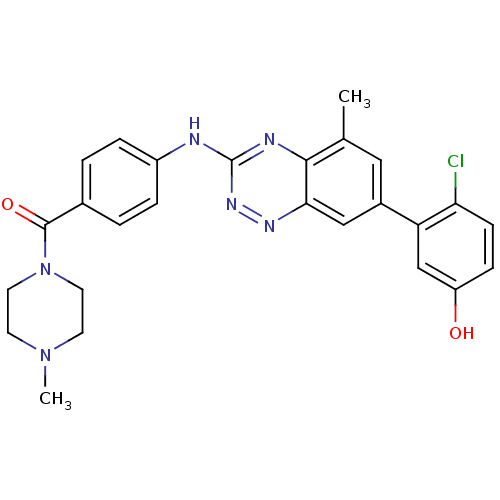 ((4-(7-(2-chloro-5-hydroxyphenyl)-5-methylbenzo[e][...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of Abl | Bioorg Med Chem Lett 17: 5812-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.043 BindingDB Entry DOI: 10.7270/Q2M32VGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 13656 total ) | Next | Last >> |