 Found 1229 hits with Last Name = 'dai' and Initial = 'j'
Found 1229 hits with Last Name = 'dai' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50773

(1-[4-(2-methoxyphenyl)-1-piperazinyl]-3-(1-naphtha...)Show InChI InChI=1S/C24H28N2O3/c1-28-24-11-5-4-10-22(24)26-15-13-25(14-16-26)17-20(27)18-29-23-12-6-8-19-7-2-3-9-21(19)23/h2-12,20,27H,13-18H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Displacement of [125I-HEAT from human alpha1D-adrenoreceptor expressed in CHOK1 cell membranes incubated for 60 mins |
Bioorg Med Chem Lett 28: 547-551 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.068
BindingDB Entry DOI: 10.7270/Q2057JHB |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204245

(CHEMBL3898284)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@]1(C)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r,c:5| Show InChI InChI=1S/C17H15F3N2O2S/c1-16-12-4-2-10(6-12)15(16)9-22(25(16,23)24)13-5-3-11(8-21)14(7-13)17(18,19)20/h2-5,7,10,12,15H,6,9H2,1H3/t10-,12+,15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(RAT) | BDBM50445231
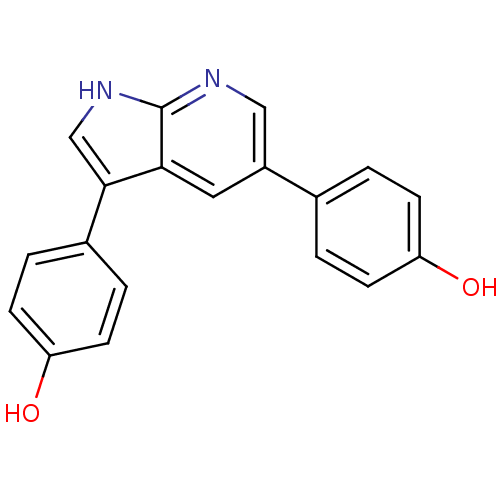
(CHEMBL3102950)Show InChI InChI=1S/C19H14N2O2/c22-15-5-1-12(2-6-15)14-9-17-18(11-21-19(17)20-10-14)13-3-7-16(23)8-4-13/h1-11,22-23H,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 2301, CNRS
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat recombinant 6xHis-tagged DYRK1A catalytic domain (1 to 502) expressed in Escherichia coli BL21 (DE3) using ATP as subst... |
J Med Chem 56: 9569-85 (2014)
Article DOI: 10.1021/jm401049v
BindingDB Entry DOI: 10.7270/Q2BG2QFT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204241

(CHEMBL3917372)Show SMILES [H][C@]12CC[C@]([H])(C1)[C@]1([H])[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r| Show InChI InChI=1S/C16H15F3N2O2S/c17-16(18,19)14-6-12(4-3-11(14)7-20)21-8-13-9-1-2-10(5-9)15(13)24(21,22)23/h3-4,6,9-10,13,15H,1-2,5,8H2/t9-,10+,13-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204251
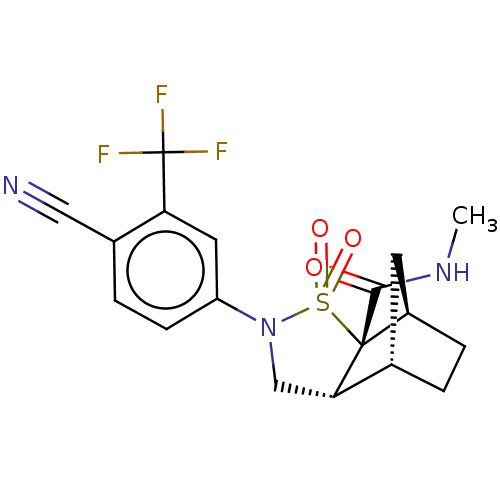
(CHEMBL3921315)Show SMILES [H][C@]12CC[C@]([H])(C1)[C@]1(C(=O)NC)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r| Show InChI InChI=1S/C18H18F3N3O3S/c1-23-16(25)17-12-4-2-10(6-12)15(17)9-24(28(17,26)27)13-5-3-11(8-22)14(7-13)18(19,20)21/h3,5,7,10,12,15H,2,4,6,9H2,1H3,(H,23,25)/t10-,12+,15-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204246

(CHEMBL3893320)Show SMILES [H][C@]12CC[C@]([H])(C1)[C@]1(C(N)=O)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r| Show InChI InChI=1S/C17H16F3N3O3S/c18-17(19,20)13-6-12(4-2-10(13)7-21)23-8-14-9-1-3-11(5-9)16(14,15(22)24)27(23,25)26/h2,4,6,9,11,14H,1,3,5,8H2,(H2,22,24)/t9-,11+,14-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204247
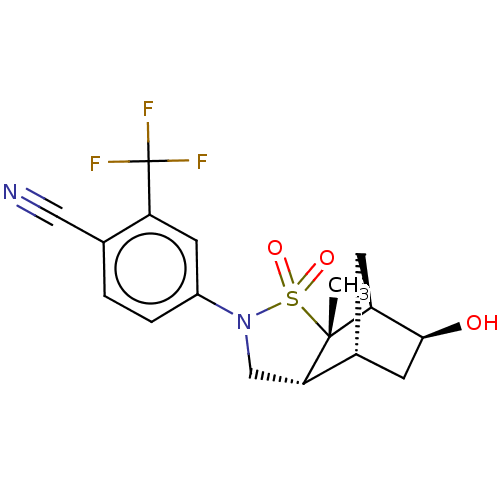
(CHEMBL3920247)Show SMILES [H][C@]12C[C@H](O)[C@]([H])(C1)[C@]1(C)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r| Show InChI InChI=1S/C17H17F3N2O3S/c1-16-13-4-10(5-15(13)23)14(16)8-22(26(16,24)25)11-3-2-9(7-21)12(6-11)17(18,19)20/h2-3,6,10,13-15,23H,4-5,8H2,1H3/t10-,13+,14+,15+,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204243

(CHEMBL3926358)Show SMILES [H][C@]12CC[C@]([H])(C1)[C@]1(C)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r| Show InChI InChI=1S/C17H17F3N2O2S/c1-16-12-4-2-10(6-12)15(16)9-22(25(16,23)24)13-5-3-11(8-21)14(7-13)17(18,19)20/h3,5,7,10,12,15H,2,4,6,9H2,1H3/t10-,12+,15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50773

(1-[4-(2-methoxyphenyl)-1-piperazinyl]-3-(1-naphtha...)Show InChI InChI=1S/C24H28N2O3/c1-28-24-11-5-4-10-22(24)26-15-13-25(14-16-26)17-20(27)18-29-23-12-6-8-19-7-2-3-9-21(19)23/h2-12,20,27H,13-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Displacement of [125I-HEAT from human alpha1A-adrenoreceptor expressed in CHOK1 cell membranes incubated for 60 mins |
Bioorg Med Chem Lett 28: 547-551 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.068
BindingDB Entry DOI: 10.7270/Q2057JHB |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204242

(CHEMBL3902310)Show SMILES [H][C@]12C[C@H](F)[C@]([H])(C1)[C@]1(C(=O)NC)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r| Show InChI InChI=1S/C18H17F4N3O3S/c1-24-16(26)17-13-4-10(5-15(13)19)14(17)8-25(29(17,27)28)11-3-2-9(7-23)12(6-11)18(20,21)22/h2-3,6,10,13-15H,4-5,8H2,1H3,(H,24,26)/t10-,13+,14+,15+,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(RAT) | BDBM50445230
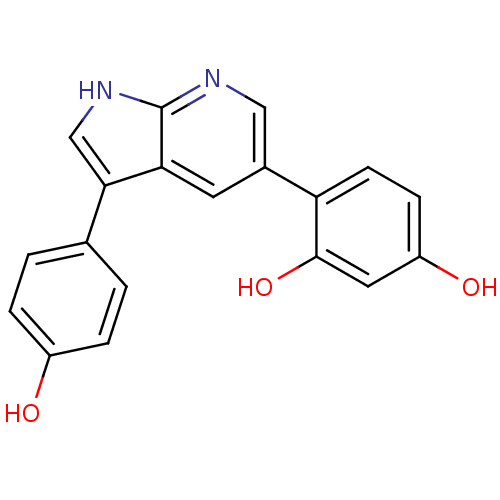
(CHEMBL3102951)Show SMILES Oc1ccc(cc1)-c1c[nH]c2ncc(cc12)-c1ccc(O)cc1O Show InChI InChI=1S/C19H14N2O3/c22-13-3-1-11(2-4-13)17-10-21-19-16(17)7-12(9-20-19)15-6-5-14(23)8-18(15)24/h1-10,22-24H,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 2301, CNRS
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat recombinant 6xHis-tagged DYRK1A catalytic domain (1 to 502) expressed in Escherichia coli BL21 (DE3) using ATP as subst... |
J Med Chem 56: 9569-85 (2014)
Article DOI: 10.1021/jm401049v
BindingDB Entry DOI: 10.7270/Q2BG2QFT |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(RAT) | BDBM50445229
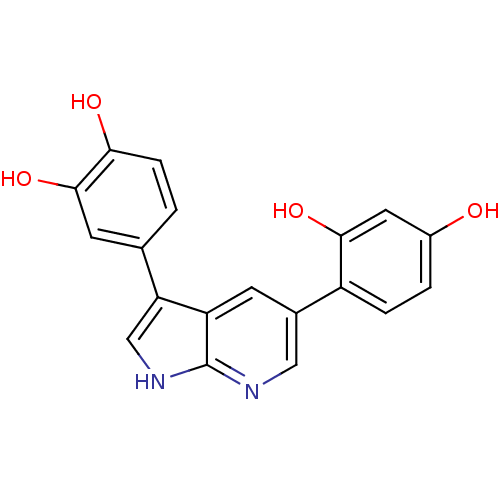
(CHEMBL3102953)Show SMILES Oc1ccc(c(O)c1)-c1cnc2[nH]cc(-c3ccc(O)c(O)c3)c2c1 Show InChI InChI=1S/C19H14N2O4/c22-12-2-3-13(17(24)7-12)11-5-14-15(9-21-19(14)20-8-11)10-1-4-16(23)18(25)6-10/h1-9,22-25H,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 2301, CNRS
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat recombinant 6xHis-tagged DYRK1A catalytic domain (1 to 502) expressed in Escherichia coli BL21 (DE3) using ATP as subst... |
J Med Chem 56: 9569-85 (2014)
Article DOI: 10.1021/jm401049v
BindingDB Entry DOI: 10.7270/Q2BG2QFT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204250

(CHEMBL3949176)Show SMILES [H][C@]12C[C@H](F)[C@]([H])(C1)[C@]1(C)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r| Show InChI InChI=1S/C17H16F4N2O2S/c1-16-13-4-10(5-15(13)18)14(16)8-23(26(16,24)25)11-3-2-9(7-22)12(6-11)17(19,20)21/h2-3,6,10,13-15H,4-5,8H2,1H3/t10-,13+,14+,15+,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204249

(CHEMBL3935242)Show SMILES [H][C@]12CC[C@]([H])(C1=O)[C@]1(C)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r,TLB:7:6:2.3:8.10| Show InChI InChI=1S/C17H15F3N2O3S/c1-16-12-5-4-11(15(12)23)14(16)8-22(26(16,24)25)10-3-2-9(7-21)13(6-10)17(18,19)20/h2-3,6,11-12,14H,4-5,8H2,1H3/t11-,12-,14+,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(RAT) | BDBM50445227
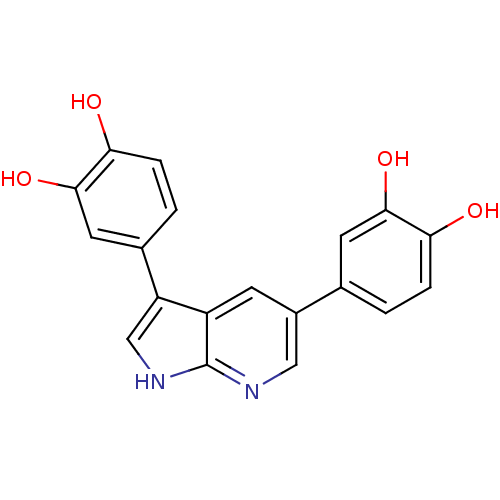
(CHEMBL3102955)Show SMILES Oc1ccc(cc1O)-c1c[nH]c2ncc(cc12)-c1ccc(O)c(O)c1 Show InChI InChI=1S/C19H14N2O4/c22-15-3-1-10(6-17(15)24)12-5-13-14(9-21-19(13)20-8-12)11-2-4-16(23)18(25)7-11/h1-9,22-25H,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 2301, CNRS
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat recombinant 6xHis-tagged DYRK1A catalytic domain (1 to 502) expressed in Escherichia coli BL21 (DE3) using ATP as subst... |
J Med Chem 56: 9569-85 (2014)
Article DOI: 10.1021/jm401049v
BindingDB Entry DOI: 10.7270/Q2BG2QFT |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50492518
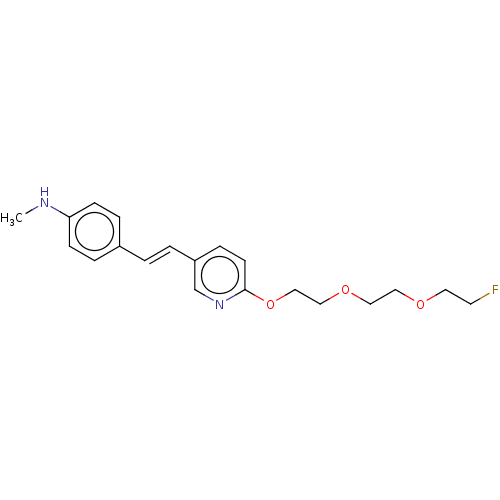
(Florbetapir | US10906900, AV45)Show InChI InChI=1S/C20H25FN2O3/c1-22-19-7-4-17(5-8-19)2-3-18-6-9-20(23-16-18)26-15-14-25-13-12-24-11-10-21/h2-9,16,22H,10-15H2,1H3/b3-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]BOB-4 from Amyloid beta (1 to 42) (unknown origin) |
Eur J Med Chem 104: 86-96 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.028
BindingDB Entry DOI: 10.7270/Q22R3VP8 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(RAT) | BDBM50445228

(CHEMBL3102954)Show SMILES Oc1ccc(O)c(c1)-c1cnc2[nH]cc(-c3ccc(O)c(O)c3)c2c1 Show InChI InChI=1S/C19H14N2O4/c22-12-2-4-16(23)13(7-12)11-5-14-15(9-21-19(14)20-8-11)10-1-3-17(24)18(25)6-10/h1-9,22-25H,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 2301, CNRS
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat recombinant 6xHis-tagged DYRK1A catalytic domain (1 to 502) expressed in Escherichia coli BL21 (DE3) using ATP as subst... |
J Med Chem 56: 9569-85 (2014)
Article DOI: 10.1021/jm401049v
BindingDB Entry DOI: 10.7270/Q2BG2QFT |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497739
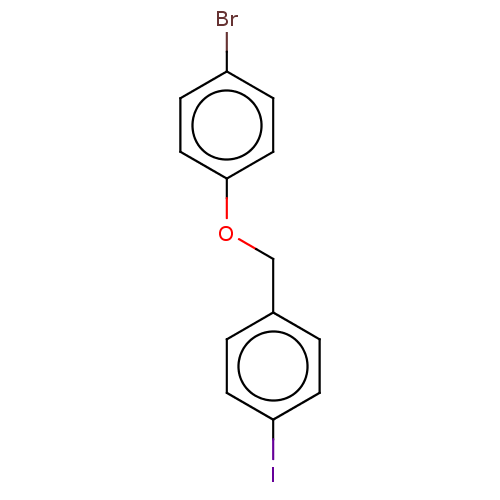
(CHEMBL3314330)Show InChI InChI=1S/C13H10BrIO/c14-11-3-7-13(8-4-11)16-9-10-1-5-12(15)6-2-10/h1-8H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497739

(CHEMBL3314330)Show InChI InChI=1S/C13H10BrIO/c14-11-3-7-13(8-4-11)16-9-10-1-5-12(15)6-2-10/h1-8H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204248
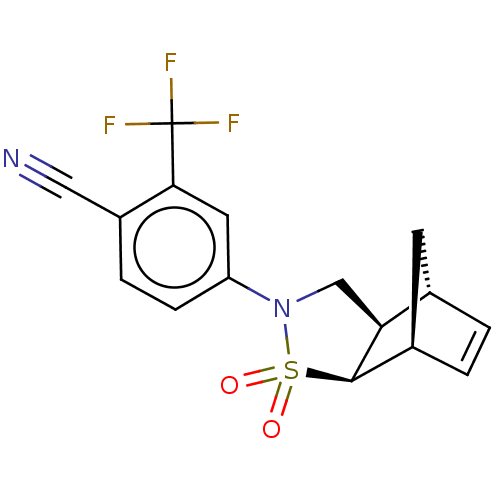
(CHEMBL3987109)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@]1([H])[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r,c:5| Show InChI InChI=1S/C16H13F3N2O2S/c17-16(18,19)14-6-12(4-3-11(14)7-20)21-8-13-9-1-2-10(5-9)15(13)24(21,22)23/h1-4,6,9-10,13,15H,5,8H2/t9-,10+,13-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50407800
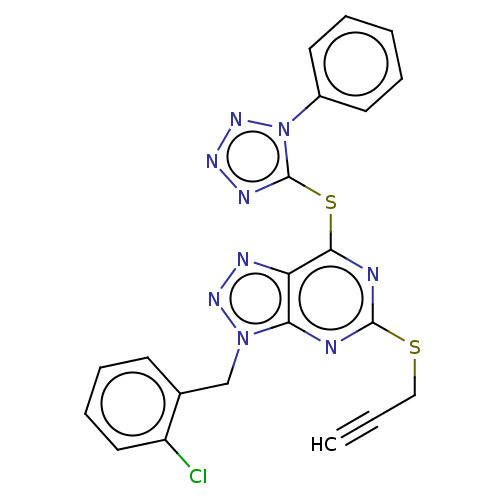
(CHEMBL5291138)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C(=O)[C@H](Cc2cccs2)NC(=O)[C@H](CCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C38H53N11O5S/c39-37(40)43-15-5-12-26-33(51)47-28(20-25-11-7-17-55-25)35(53)48-21-24-10-2-1-8-22(24)18-31(48)36(54)49-29-14-4-3-9-23(29)19-30(49)34(52)46-27(32(50)45-26)13-6-16-44-38(41)42/h1-2,7-8,10-11,17,23,26-31H,3-6,9,12-16,18-21H2,(H,45,50)(H,46,52)(H,47,51)(H4,39,40,43)(H4,41,42,44)/t23?,26-,27-,28-,29?,30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497743
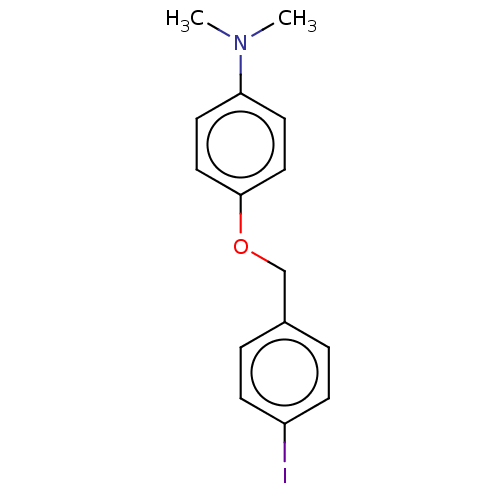
(CHEMBL3314340)Show InChI InChI=1S/C15H16INO/c1-17(2)14-7-9-15(10-8-14)18-11-12-3-5-13(16)6-4-12/h3-10H,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497743

(CHEMBL3314340)Show InChI InChI=1S/C15H16INO/c1-17(2)14-7-9-15(10-8-14)18-11-12-3-5-13(16)6-4-12/h3-10H,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497724
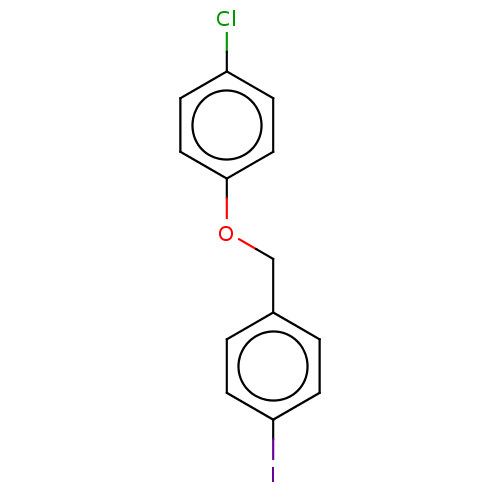
(CHEMBL3314329)Show InChI InChI=1S/C13H10ClIO/c14-11-3-7-13(8-4-11)16-9-10-1-5-12(15)6-2-10/h1-8H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497724

(CHEMBL3314329)Show InChI InChI=1S/C13H10ClIO/c14-11-3-7-13(8-4-11)16-9-10-1-5-12(15)6-2-10/h1-8H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Homo sapiens (Human)) | BDBM50773

(1-[4-(2-methoxyphenyl)-1-piperazinyl]-3-(1-naphtha...)Show InChI InChI=1S/C24H28N2O3/c1-28-24-11-5-4-10-22(24)26-15-13-25(14-16-26)17-20(27)18-29-23-12-6-8-19-7-2-3-9-21(19)23/h2-12,20,27H,13-18H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Displacement of [125I-HEAT from human alpha1B-adrenoreceptor expressed in CHOK1 cell membranes incubated for 60 mins |
Bioorg Med Chem Lett 28: 547-551 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.068
BindingDB Entry DOI: 10.7270/Q2057JHB |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50500796

(CHEMBL3760007)Show InChI InChI=1S/C17H20FNO2/c1-19(2)15-5-9-17(10-6-15)21-13-14-3-7-16(8-4-14)20-12-11-18/h3-10H,11-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]BOB-4 from Amyloid beta (1 to 42) (unknown origin) |
Eur J Med Chem 104: 86-96 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.028
BindingDB Entry DOI: 10.7270/Q22R3VP8 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497740
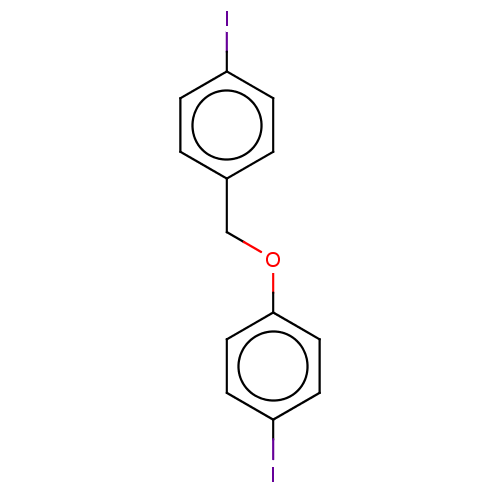
(CHEMBL3314331)Show InChI InChI=1S/C13H10I2O/c14-11-3-1-10(2-4-11)9-16-13-7-5-12(15)6-8-13/h1-8H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497740

(CHEMBL3314331)Show InChI InChI=1S/C13H10I2O/c14-11-3-1-10(2-4-11)9-16-13-7-5-12(15)6-8-13/h1-8H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497737
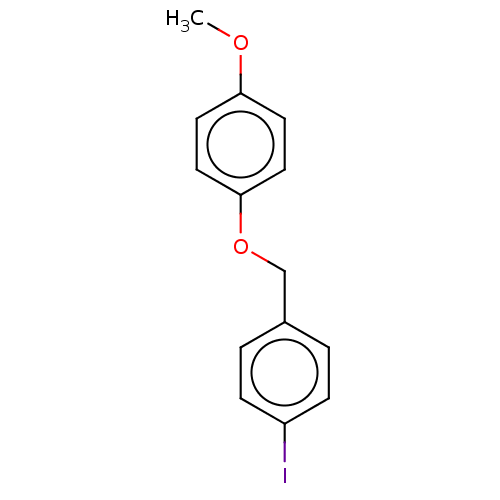
(CHEMBL3314322)Show InChI InChI=1S/C14H13IO2/c1-16-13-6-8-14(9-7-13)17-10-11-2-4-12(15)5-3-11/h2-9H,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497737

(CHEMBL3314322)Show InChI InChI=1S/C14H13IO2/c1-16-13-6-8-14(9-7-13)17-10-11-2-4-12(15)5-3-11/h2-9H,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50500797

(CHEMBL3758709)Show InChI InChI=1S/C17H20FNO2/c1-19(2)15-5-3-14(4-6-15)13-21-17-9-7-16(8-10-17)20-12-11-18/h3-10H,11-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]BOB-4 from Amyloid beta (1 to 42) (unknown origin) |
Eur J Med Chem 104: 86-96 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.028
BindingDB Entry DOI: 10.7270/Q22R3VP8 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204240

(CHEMBL3939272)Show SMILES [H][C@@]12C[C@@H](O)[C@@]([H])(C1)[C@]1([H])CN(c3ccc(C#N)c(c3)C(F)(F)F)S(=O)(=O)[C@]21C |r| Show InChI InChI=1S/C17H17F3N2O3S/c1-16-10-4-12(15(23)5-10)14(16)8-22(26(16,24)25)11-3-2-9(7-21)13(6-11)17(18,19)20/h2-3,6,10,12,14-15,23H,4-5,8H2,1H3/t10-,12-,14-,15+,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50122787
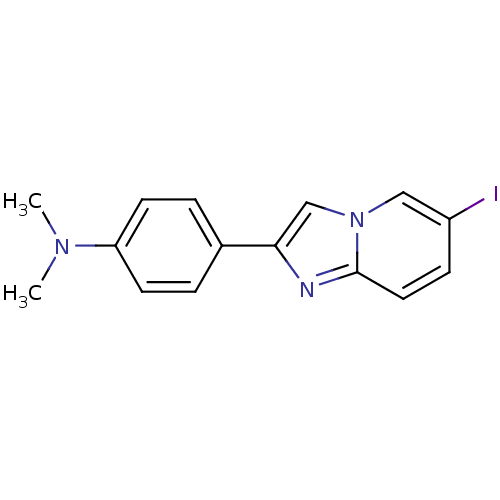
(2-(4'-dimethylaminophenyl)-6-iodoimidazo[1,2-a]pyr...)Show InChI InChI=1S/C15H14IN3/c1-18(2)13-6-3-11(4-7-13)14-10-19-9-12(16)5-8-15(19)17-14/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50122787

(2-(4'-dimethylaminophenyl)-6-iodoimidazo[1,2-a]pyr...)Show InChI InChI=1S/C15H14IN3/c1-18(2)13-6-3-11(4-7-13)14-10-19-9-12(16)5-8-15(19)17-14/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50122787

(2-(4'-dimethylaminophenyl)-6-iodoimidazo[1,2-a]pyr...)Show InChI InChI=1S/C15H14IN3/c1-18(2)13-6-3-11(4-7-13)14-10-19-9-12(16)5-8-15(19)17-14/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]BOB-4 from Amyloid beta (1 to 42) (unknown origin) |
Eur J Med Chem 104: 86-96 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.028
BindingDB Entry DOI: 10.7270/Q22R3VP8 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129793
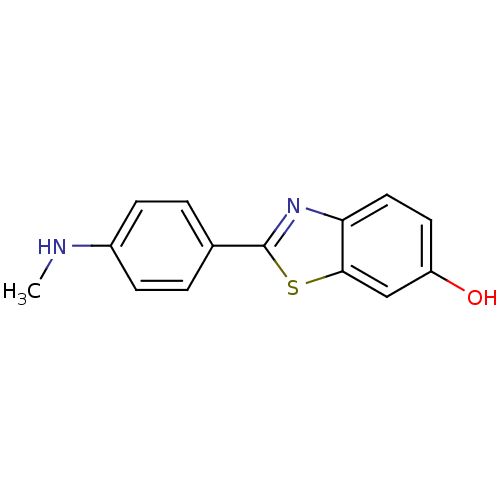
(2-(4''-methylaminophenyl)-6-hydroxybenzothiazole |...)Show InChI InChI=1S/C14H12N2OS/c1-15-10-4-2-9(3-5-10)14-16-12-7-6-11(17)8-13(12)18-14/h2-8,15,17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]BOB-4 from Amyloid beta (1 to 42) (unknown origin) |
Eur J Med Chem 104: 86-96 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.028
BindingDB Entry DOI: 10.7270/Q22R3VP8 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129793

(2-(4''-methylaminophenyl)-6-hydroxybenzothiazole |...)Show InChI InChI=1S/C14H12N2OS/c1-15-10-4-2-9(3-5-10)14-16-12-7-6-11(17)8-13(12)18-14/h2-8,15,17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129793

(2-(4''-methylaminophenyl)-6-hydroxybenzothiazole |...)Show InChI InChI=1S/C14H12N2OS/c1-15-10-4-2-9(3-5-10)14-16-12-7-6-11(17)8-13(12)18-14/h2-8,15,17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346870
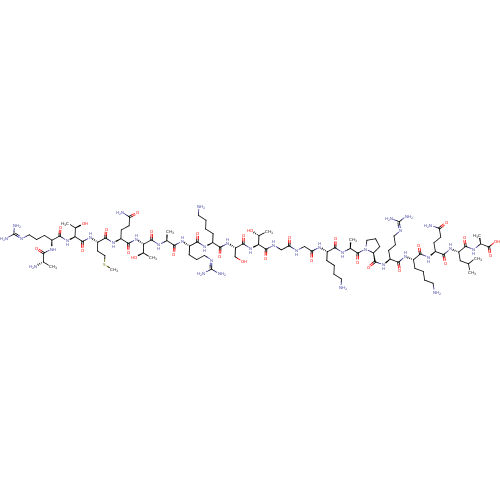
(CHEMBL1797647)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C93H169N35O28S/c1-45(2)41-62(83(148)113-49(6)90(155)156)123-79(144)59(28-30-65(98)133)119-75(140)54(22-12-15-34-95)117-77(142)57(25-18-37-107-92(102)103)121-85(150)64-27-20-39-128(64)89(154)48(5)112-74(139)53(21-11-14-33-94)114-68(136)43-109-67(135)42-110-86(151)69(50(7)130)125-84(149)63(44-129)124-78(143)55(23-13-16-35-96)118-76(141)56(24-17-36-106-91(100)101)116-73(138)47(4)111-87(152)70(51(8)131)126-82(147)60(29-31-66(99)134)120-80(145)61(32-40-157-10)122-88(153)71(52(9)132)127-81(146)58(115-72(137)46(3)97)26-19-38-108-93(104)105/h45-64,69-71,129-132H,11-44,94-97H2,1-10H3,(H2,98,133)(H2,99,134)(H,109,135)(H,110,151)(H,111,152)(H,112,139)(H,113,148)(H,114,136)(H,115,137)(H,116,138)(H,117,142)(H,118,141)(H,119,140)(H,120,145)(H,121,150)(H,122,153)(H,123,144)(H,124,143)(H,125,149)(H,126,147)(H,127,146)(H,155,156)(H4,100,101,106)(H4,102,103,107)(H4,104,105,108)/t46-,47-,48-,49-,50+,51+,52+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,69-,70-,71-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Blocking activity was assessed by antagonism of (-)-noradrenaline induced contraction of rat prostatic vas deferens (alpha1A adrenoceptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497725
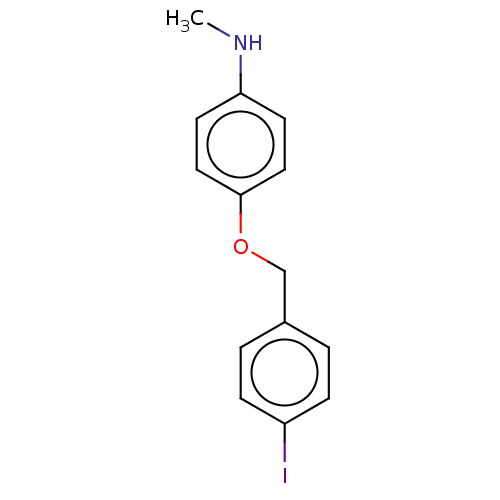
(CHEMBL3314337)Show InChI InChI=1S/C14H14INO/c1-16-13-6-8-14(9-7-13)17-10-11-2-4-12(15)5-3-11/h2-9,16H,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497725

(CHEMBL3314337)Show InChI InChI=1S/C14H14INO/c1-16-13-6-8-14(9-7-13)17-10-11-2-4-12(15)5-3-11/h2-9,16H,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497723
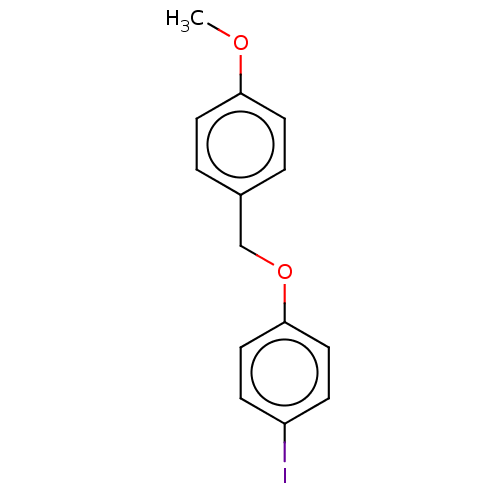
(CHEMBL3314342)Show InChI InChI=1S/C14H13IO2/c1-16-13-6-2-11(3-7-13)10-17-14-8-4-12(15)5-9-14/h2-9H,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497723

(CHEMBL3314342)Show InChI InChI=1S/C14H13IO2/c1-16-13-6-2-11(3-7-13)10-17-14-8-4-12(15)5-9-14/h2-9H,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50346870

(CHEMBL1797647)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C93H169N35O28S/c1-45(2)41-62(83(148)113-49(6)90(155)156)123-79(144)59(28-30-65(98)133)119-75(140)54(22-12-15-34-95)117-77(142)57(25-18-37-107-92(102)103)121-85(150)64-27-20-39-128(64)89(154)48(5)112-74(139)53(21-11-14-33-94)114-68(136)43-109-67(135)42-110-86(151)69(50(7)130)125-84(149)63(44-129)124-78(143)55(23-13-16-35-96)118-76(141)56(24-17-36-106-91(100)101)116-73(138)47(4)111-87(152)70(51(8)131)126-82(147)60(29-31-66(99)134)120-80(145)61(32-40-157-10)122-88(153)71(52(9)132)127-81(146)58(115-72(137)46(3)97)26-19-38-108-93(104)105/h45-64,69-71,129-132H,11-44,94-97H2,1-10H3,(H2,98,133)(H2,99,134)(H,109,135)(H,110,151)(H,111,152)(H,112,139)(H,113,148)(H,114,136)(H,115,137)(H,116,138)(H,117,142)(H,118,141)(H,119,140)(H,120,145)(H,121,150)(H,122,153)(H,123,144)(H,124,143)(H,125,149)(H,126,147)(H,127,146)(H,155,156)(H4,100,101,106)(H4,102,103,107)(H4,104,105,108)/t46-,47-,48-,49-,50+,51+,52+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,69-,70-,71-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497727
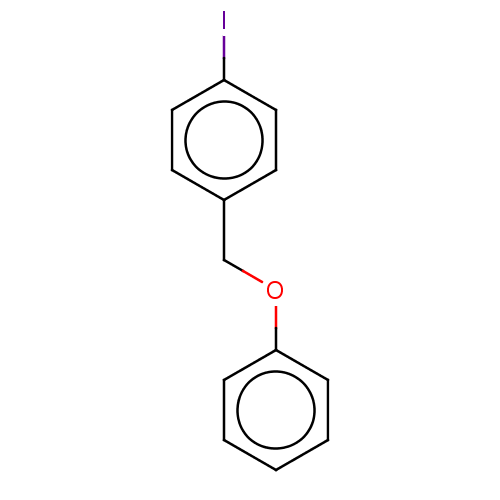
(CHEMBL3314332)Show InChI InChI=1S/C13H11IO/c14-12-8-6-11(7-9-12)10-15-13-4-2-1-3-5-13/h1-9H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497727

(CHEMBL3314332)Show InChI InChI=1S/C13H11IO/c14-12-8-6-11(7-9-12)10-15-13-4-2-1-3-5-13/h1-9H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204244

(CHEMBL3907304)Show SMILES [H][C@]12CC[C@]([H])(C1O)[C@]1(C)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r,TLB:7:6:2.3:8.10| Show InChI InChI=1S/C17H17F3N2O3S/c1-16-12-5-4-11(15(12)23)14(16)8-22(26(16,24)25)10-3-2-9(7-21)13(6-10)17(18,19)20/h2-3,6,11-12,14-15,23H,4-5,8H2,1H3/t11-,12-,14+,15?,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50204244

(CHEMBL3907304)Show SMILES [H][C@]12CC[C@]([H])(C1O)[C@]1(C)[C@@]2([H])CN(c2ccc(C#N)c(c2)C(F)(F)F)S1(=O)=O |r,TLB:7:6:2.3:8.10| Show InChI InChI=1S/C17H17F3N2O3S/c1-16-12-5-4-11(15(12)23)14(16)8-22(26(16,24)25)10-3-2-9(7-21)13(6-10)17(18,19)20/h2-3,6,11-12,14-15,23H,4-5,8H2,1H3/t11-,12-,14+,15?,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 26: 5707-5711 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.059
BindingDB Entry DOI: 10.7270/Q2PK0J43 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50497731
(CHEMBL3314328)Show InChI InChI=1S/C13H10FIO/c14-11-3-7-13(8-4-11)16-9-10-1-5-12(15)6-2-10/h1-8H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]1-Iodo-4-((4-methoxyphenoxy)methyl)benzene from amyloid beta42 (unknown origin) after 3 hrs by gamma counting |
J Med Chem 57: 6030-42 (2014)
Article DOI: 10.1021/jm5004396
BindingDB Entry DOI: 10.7270/Q2J1065N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data