

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50102780 (2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM13940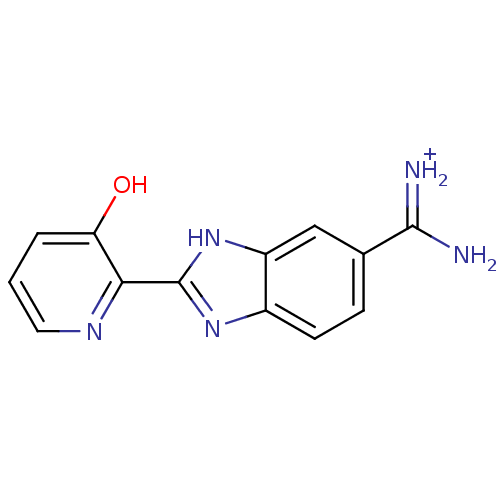 (2-(3-HYDROXY-PYRIDIN-2-YL)-1H-BENZOIMIDAZOLE-5-CAR...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Corporation | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 307: 1451-86 (2001) Article DOI: 10.1006/jmbi.2001.4516 BindingDB Entry DOI: 10.7270/Q2FX77PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50102790 (2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13940 (2-(3-HYDROXY-PYRIDIN-2-YL)-1H-BENZOIMIDAZOLE-5-CAR...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Corporation | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 307: 1451-86 (2001) Article DOI: 10.1006/jmbi.2001.4516 BindingDB Entry DOI: 10.7270/Q2FX77PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50102790 (2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human Serine Protease tissue type Plasminogen Activator (t-PA). | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50102790 (2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description ComInhibition of Human Serine Protease Urokinase Plasminogen Activator (u-PA). | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50120038 (2-{[2-({6-[1-(1-Carboxy-2-phosphono-ethylcarbamoyl...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of HCV serine protease NS3/NS4A in the presence of EDTA. | Bioorg Med Chem Lett 12: 3129-33 (2002) BindingDB Entry DOI: 10.7270/Q25D8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50102767 (2-(3-Bromo-2-hydroxy-phenyl)-1H-indole-5-carboxami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50102780 (2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inihibtion of Human Serine Protease tissue type Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50101866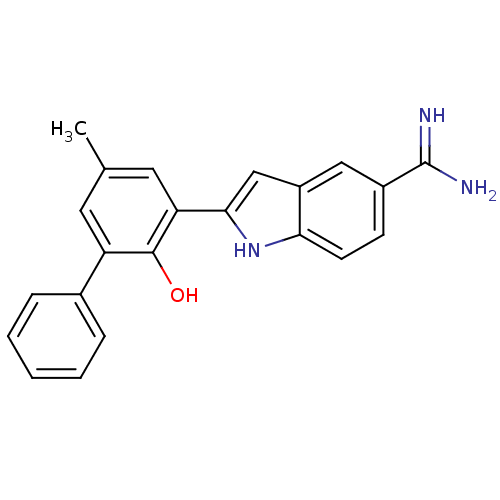 (2-(2-Hydroxy-5-methyl-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50102786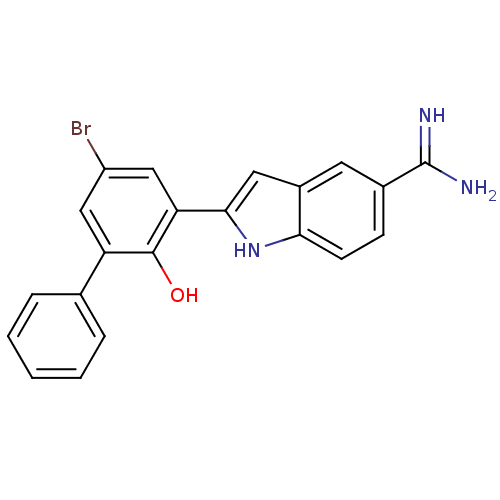 (2-(5-Bromo-2-hydroxy-biphenyl-3-yl)-1H-indole-5-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50102767 (2-(3-Bromo-2-hydroxy-phenyl)-1H-indole-5-carboxami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13940 (2-(3-HYDROXY-PYRIDIN-2-YL)-1H-BENZOIMIDAZOLE-5-CAR...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 43 | -41.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Axys Pharmaceuticals Corporation | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 307: 1451-86 (2001) Article DOI: 10.1006/jmbi.2001.4516 BindingDB Entry DOI: 10.7270/Q2FX77PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101873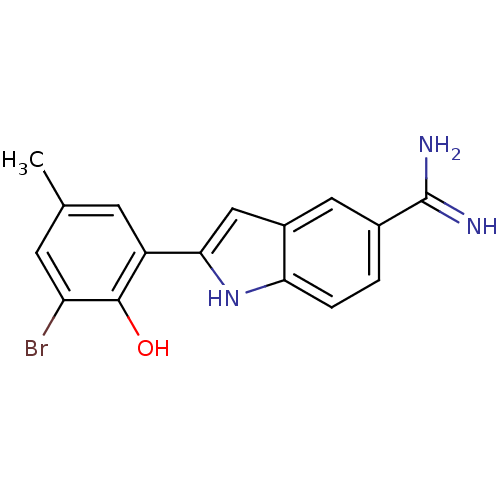 (2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50102778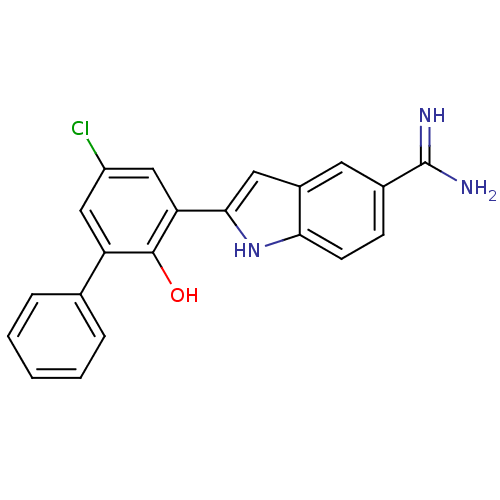 (2-(5-Chloro-2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50101873 (2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description ComInhibition of Human Serine Protease Urokinase Plasminogen Activator (u-PA). | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13937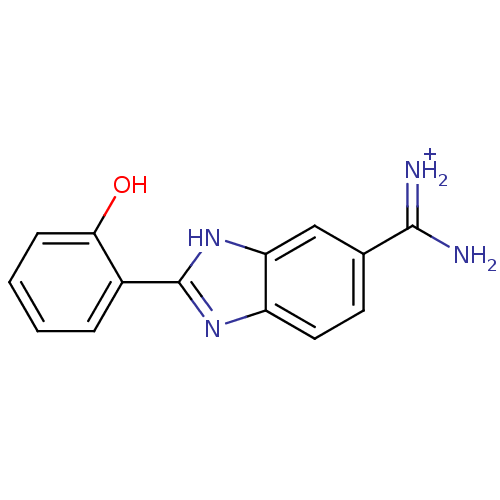 (2-(2-HYDROXY-PHENYL)-1H-BENZOIMIDAZOLE-5-CARBOXAMI...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 65 | -40.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Axys Pharmaceuticals Corporation | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 307: 1451-86 (2001) Article DOI: 10.1006/jmbi.2001.4516 BindingDB Entry DOI: 10.7270/Q2FX77PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50120023 (2-[(2-{[6-(1-Carboxy-2-phosphono-ethylcarbamoyl)-1...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of HCV serine protease NS3/NS4A in the presence of Zn | Bioorg Med Chem Lett 12: 3129-33 (2002) BindingDB Entry DOI: 10.7270/Q25D8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50102790 (2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human Serine Protease Trypsin. | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50102780 (2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50102792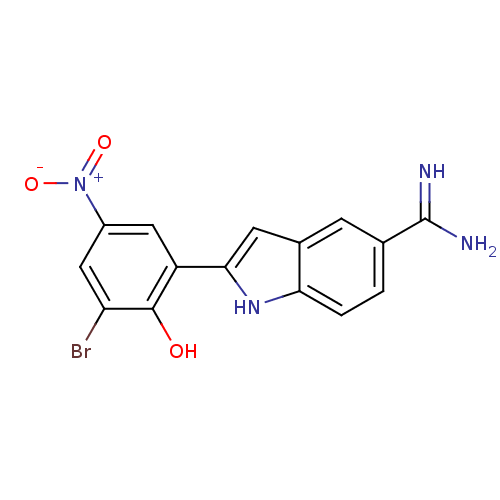 (2-(2-Hydroxy-3-bromo-5-nitro-phenyl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50102784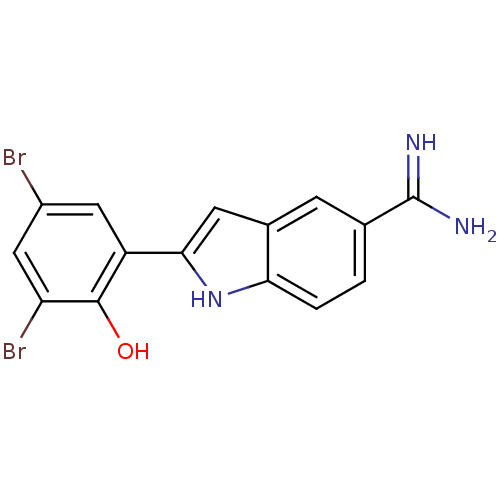 (2-(3,5-Dibromo-2-hydroxy-phenyl)-1H-indole-5-carbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50102786 (2-(5-Bromo-2-hydroxy-biphenyl-3-yl)-1H-indole-5-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inihibtion of Human Serine Protease tissue type Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50120050 (2-({2-[6-(1-Carboxy-2-phosphono-ethylcarbamoyl)-1-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of HCV serine protease NS3/NS4A in the presence of EDTA | Bioorg Med Chem Lett 12: 3129-33 (2002) BindingDB Entry DOI: 10.7270/Q25D8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50102780 (2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human Serine Protease Plasmin | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50102784 (2-(3,5-Dibromo-2-hydroxy-phenyl)-1H-indole-5-carbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50101866 (2-(2-Hydroxy-5-methyl-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inihibtion of Human Serine Protease tissue type Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50102790 (2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human Serine Protease Thrombin. | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50102767 (2-(3-Bromo-2-hydroxy-phenyl)-1H-indole-5-carboxami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Activity against Human Serine Protease Thrombin | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50102780 (2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Activity against Human Serine Protease Trypsin | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50101866 (2-(2-Hydroxy-5-methyl-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Activity against Human Serine Protease Trypsin | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50102778 (2-(5-Chloro-2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Activity against Human Serine Protease Trypsin | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50120012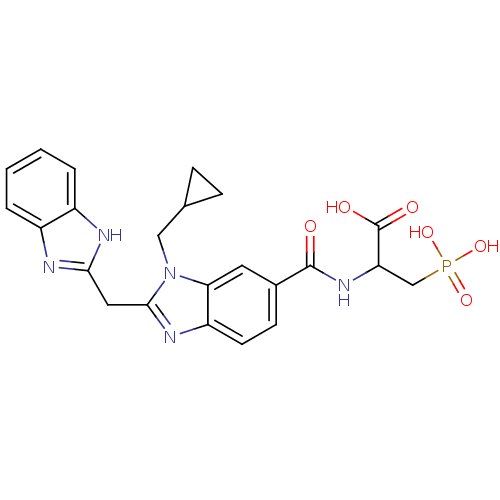 (2-{[2-(1H-Benzoimidazol-2-ylmethyl)-3-cyclopropylm...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of HCV serine protease NS3/NS4A in the presence of Zn | Bioorg Med Chem Lett 12: 3129-33 (2002) BindingDB Entry DOI: 10.7270/Q25D8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50102778 (2-(5-Chloro-2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inihibtion of Human Serine Protease tissue type Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM13937 (2-(2-HYDROXY-PHENYL)-1H-BENZOIMIDAZOLE-5-CARBOXAMI...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Corporation | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 307: 1451-86 (2001) Article DOI: 10.1006/jmbi.2001.4516 BindingDB Entry DOI: 10.7270/Q2FX77PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101873 (2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human Serine Protease Thrombin. | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50102790 (2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human Serine Protease Plasmin. | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50120036 (2-{[2-(5-Hydroxy-1H-benzoimidazol-2-ylmethyl)-3-me...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of HCV serine protease NS3/NS4A in the presence of Zn | Bioorg Med Chem Lett 12: 3129-33 (2002) BindingDB Entry DOI: 10.7270/Q25D8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50120027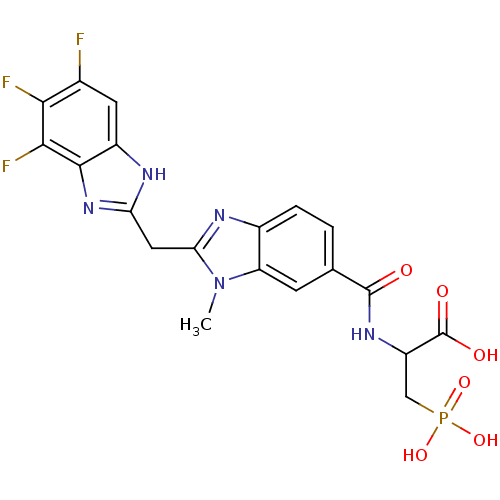 (2-{[3-Methyl-2-(5,6,7-trifluoro-1H-benzoimidazol-2...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of HCV serine protease NS3/NS4A in the presence of Zn | Bioorg Med Chem Lett 12: 3129-33 (2002) BindingDB Entry DOI: 10.7270/Q25D8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50103860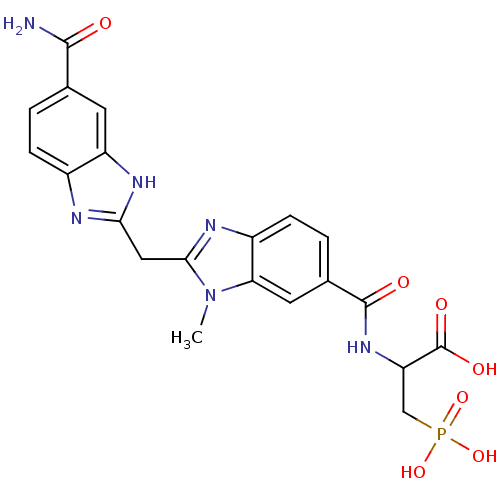 (2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of HCV serine protease NS3/NS4A in the presence of EDTA. | Bioorg Med Chem Lett 12: 3129-33 (2002) BindingDB Entry DOI: 10.7270/Q25D8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50120048 (2-{[2-(5-Methoxy-1H-benzoimidazol-2-ylmethyl)-3-me...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of HCV serine protease NS3/NS4A in the presence of Zn | Bioorg Med Chem Lett 12: 3129-33 (2002) BindingDB Entry DOI: 10.7270/Q25D8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101866 (2-(2-Hydroxy-5-methyl-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50102786 (2-(5-Bromo-2-hydroxy-biphenyl-3-yl)-1H-indole-5-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50120014 (2-{[2-(5,6-Difluoro-1H-benzoimidazol-2-ylmethyl)-3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of HCV serine protease NS3/NS4A in the presence of Zn | Bioorg Med Chem Lett 12: 3129-33 (2002) BindingDB Entry DOI: 10.7270/Q25D8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50120015 (2-({2-[1-(1H-Benzoimidazol-2-yl)-ethyl]-3-methyl-3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of HCV serine protease NS3/NS4A in the presence of Zn | Bioorg Med Chem Lett 12: 3129-33 (2002) BindingDB Entry DOI: 10.7270/Q25D8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50102792 (2-(2-Hydroxy-3-bromo-5-nitro-phenyl)-1H-indole-5-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human Serine Protease Thrombin. | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50120013 (2-{[2-(4,6-Difluoro-1H-benzoimidazol-2-ylmethyl)-3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of HCV serine protease NS3/NS4A in the presence of Zn | Bioorg Med Chem Lett 12: 3129-33 (2002) BindingDB Entry DOI: 10.7270/Q25D8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50120047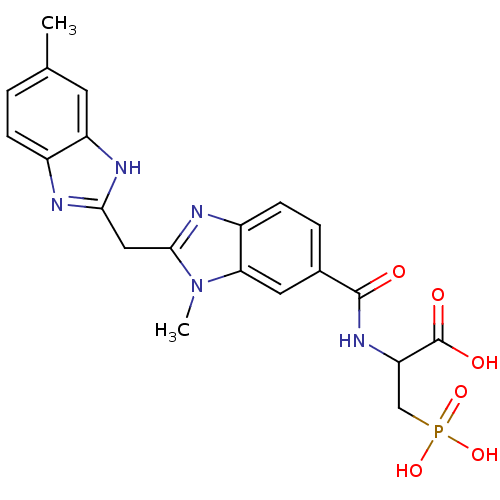 (2-{[3-Methyl-2-(5-methyl-1H-benzoimidazol-2-ylmeth...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of HCV serine protease NS3/NS4A in the presence of EDTA. | Bioorg Med Chem Lett 12: 3129-33 (2002) BindingDB Entry DOI: 10.7270/Q25D8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50102767 (2-(3-Bromo-2-hydroxy-phenyl)-1H-indole-5-carboxami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human Serine Protease Plasmin | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50120046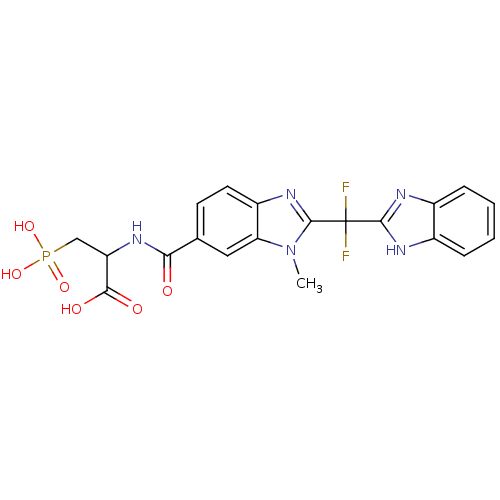 (2-({2-[(1H-Benzoimidazol-2-yl)-difluoro-methyl]-3-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of HCV serine protease NS3/NS4A in the presence of Zn | Bioorg Med Chem Lett 12: 3129-33 (2002) BindingDB Entry DOI: 10.7270/Q25D8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 338 total ) | Next | Last >> |