 Found 3688 hits with Last Name = 'maclean' and Initial = 'j'
Found 3688 hits with Last Name = 'maclean' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50418185
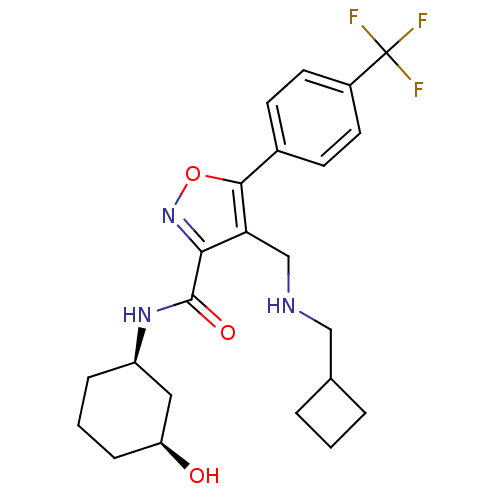
(CHEMBL1761695)Show SMILES O[C@H]1CCC[C@H](C1)NC(=O)c1noc(c1CNCC1CCC1)-c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C23H28F3N3O3/c24-23(25,26)16-9-7-15(8-10-16)21-19(13-27-12-14-3-1-4-14)20(29-32-21)22(31)28-17-5-2-6-18(30)11-17/h7-10,14,17-18,27,30H,1-6,11-13H2,(H,28,31)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 21: 2559-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.112
BindingDB Entry DOI: 10.7270/Q2BR8TDN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50418184

(CHEMBL1761694)Show SMILES O[C@H]1CCC[C@H](C1)NC(=O)c1noc(c1CNCC1CC1)-c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C22H26F3N3O3/c23-22(24,25)15-8-6-14(7-9-15)20-18(12-26-11-13-4-5-13)19(28-31-20)21(30)27-16-2-1-3-17(29)10-16/h6-9,13,16-17,26,29H,1-5,10-12H2,(H,27,30)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 21: 2559-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.112
BindingDB Entry DOI: 10.7270/Q2BR8TDN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50418183
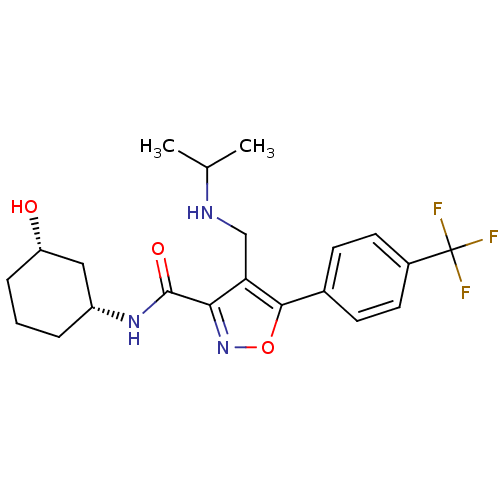
(CHEMBL1761688)Show SMILES CC(C)NCc1c(noc1-c1ccc(cc1)C(F)(F)F)C(=O)N[C@@H]1CCC[C@H](O)C1 |r| Show InChI InChI=1S/C21H26F3N3O3/c1-12(2)25-11-17-18(20(29)26-15-4-3-5-16(28)10-15)27-30-19(17)13-6-8-14(9-7-13)21(22,23)24/h6-9,12,15-16,25,28H,3-5,10-11H2,1-2H3,(H,26,29)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of labeled-dofetilide from human ERG |
Bioorg Med Chem Lett 21: 4652-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.051
BindingDB Entry DOI: 10.7270/Q2M32X1M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50418183

(CHEMBL1761688)Show SMILES CC(C)NCc1c(noc1-c1ccc(cc1)C(F)(F)F)C(=O)N[C@@H]1CCC[C@H](O)C1 |r| Show InChI InChI=1S/C21H26F3N3O3/c1-12(2)25-11-17-18(20(29)26-15-4-3-5-16(28)10-15)27-30-19(17)13-6-8-14(9-7-13)21(22,23)24/h6-9,12,15-16,25,28H,3-5,10-11H2,1-2H3,(H,26,29)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 21: 2559-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.112
BindingDB Entry DOI: 10.7270/Q2BR8TDN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM286224
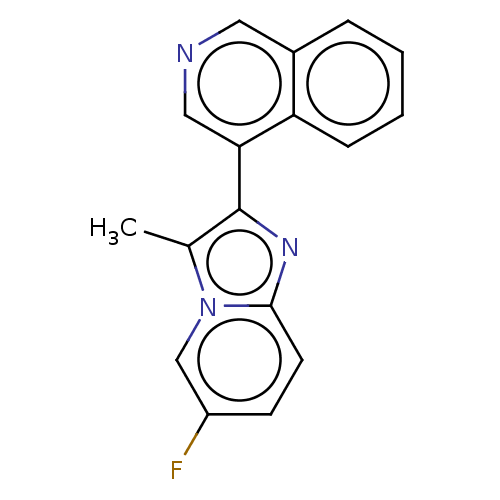
(4-(6-fluoro-3-ethylimidazo[1,2- a]pyridin-2-yl)iso...)Show InChI InChI=1S/C17H12FN3/c1-11-17(20-16-7-6-13(18)10-21(11)16)15-9-19-8-12-4-2-3-5-14(12)15/h2-10H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
US Patent
| Assay Description
V79 cell lines stably expressing the either the human CYP11B2 or the human CYP11B1 enzyme were generated using a standard transfection protocol. V79 ... |
US Patent US9518055 (2016)
BindingDB Entry DOI: 10.7270/Q23B625B |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50418189
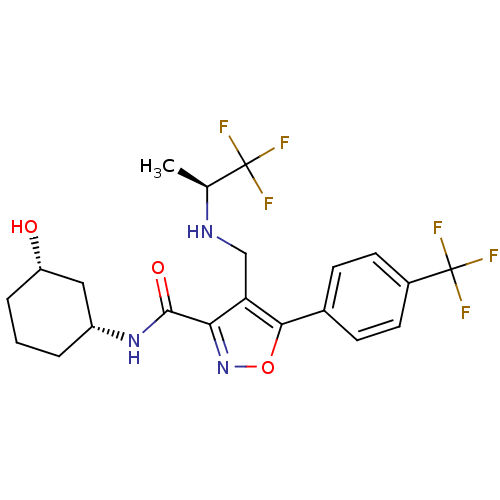
(CHEMBL1761696)Show SMILES C[C@H](NCc1c(noc1-c1ccc(cc1)C(F)(F)F)C(=O)N[C@@H]1CCC[C@H](O)C1)C(F)(F)F |r| Show InChI InChI=1S/C21H23F6N3O3/c1-11(20(22,23)24)28-10-16-17(19(32)29-14-3-2-4-15(31)9-14)30-33-18(16)12-5-7-13(8-6-12)21(25,26)27/h5-8,11,14-15,28,31H,2-4,9-10H2,1H3,(H,29,32)/t11-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ influx by FLIPR assay |
Bioorg Med Chem Lett 21: 2559-63 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.112
BindingDB Entry DOI: 10.7270/Q2BR8TDN |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50130661
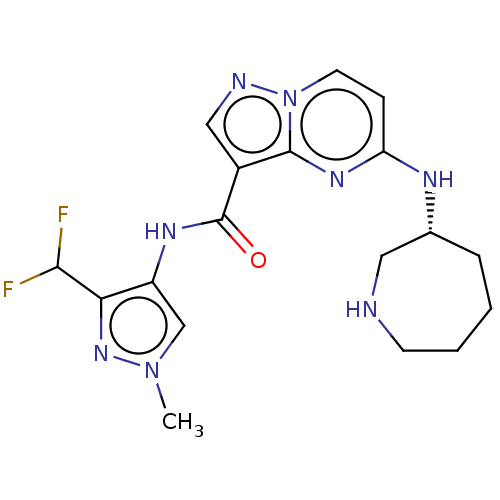
(CHEMBL3634510 | US10329294, Example 162)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCCNC4)nc23)c(n1)C(F)F |r| Show InChI InChI=1S/C18H22F2N8O/c1-27-10-13(15(26-27)16(19)20)24-18(29)12-9-22-28-7-5-14(25-17(12)28)23-11-4-2-3-6-21-8-11/h5,7,9-11,16,21H,2-4,6,8H2,1H3,(H,23,25)(H,24,29)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 (unknown origin) |
Bioorg Med Chem Lett 25: 5384-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.028
BindingDB Entry DOI: 10.7270/Q2WS8W3R |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095475
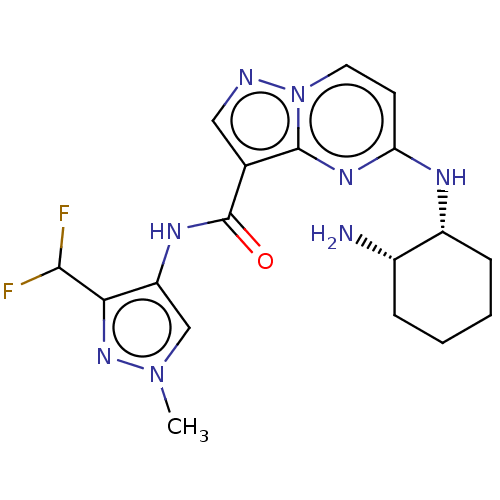
(CHEMBL3590479)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCC[C@@H]4N)nc23)c(n1)C(F)F |r| Show InChI InChI=1S/C23H28N4O3/c1-26(2)14-7-15-30-22-16-21(27(25-22)17-18-8-5-4-6-9-18)23(28)24-19-10-12-20(29-3)13-11-19/h4-6,8-13,16H,7,14-15,17H2,1-3H3,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Rattus norvegicus (rat)) | BDBM50437406
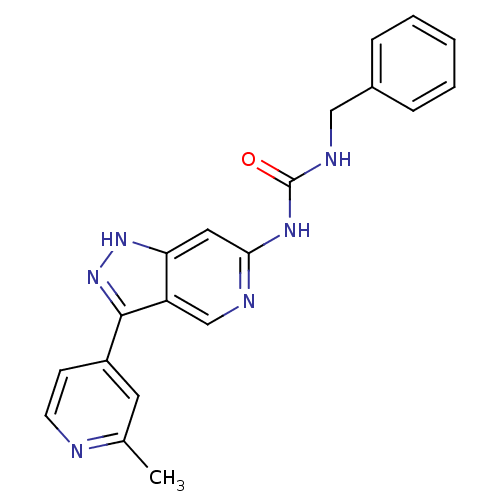
(CHEMBL2408788 | US9023865, 9)Show SMILES Cc1cc(ccn1)-c1n[nH]c2cc(NC(=O)NCc3ccccc3)ncc12 Show InChI InChI=1S/C20H18N6O/c1-13-9-15(7-8-21-13)19-16-12-22-18(10-17(16)25-26-19)24-20(27)23-11-14-5-3-2-4-6-14/h2-10,12H,11H2,1H3,(H,25,26)(H2,22,23,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50437407

(CHEMBL2408789 | US9023865, 6)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3ccnc(C)c3)c2cn1)c1ccccc1 |r| Show InChI InChI=1S/C21H20N6O/c1-13-10-16(8-9-22-13)20-17-12-23-19(11-18(17)26-27-20)25-21(28)24-14(2)15-6-4-3-5-7-15/h3-12,14H,1-2H3,(H,26,27)(H2,23,24,25,28)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Rattus norvegicus (rat)) | BDBM50437406

(CHEMBL2408788 | US9023865, 9)Show SMILES Cc1cc(ccn1)-c1n[nH]c2cc(NC(=O)NCc3ccccc3)ncc12 Show InChI InChI=1S/C20H18N6O/c1-13-9-15(7-8-21-13)19-16-12-22-18(10-17(16)25-26-19)24-20(27)23-11-14-5-3-2-4-6-14/h2-10,12H,11H2,1H3,(H,25,26)(H2,22,23,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50437407

(CHEMBL2408789 | US9023865, 6)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3ccnc(C)c3)c2cn1)c1ccccc1 |r| Show InChI InChI=1S/C21H20N6O/c1-13-10-16(8-9-22-13)20-17-12-23-19(11-18(17)26-27-20)25-21(28)24-14(2)15-6-4-3-5-7-15/h3-12,14H,1-2H3,(H,26,27)(H2,23,24,25,28)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50130681
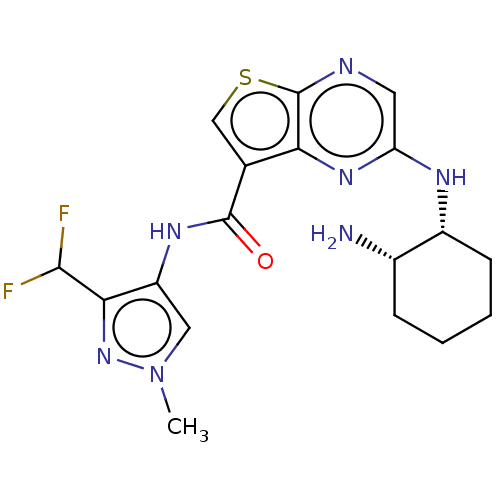
(CHEMBL3634383)Show SMILES Cn1cc(NC(=O)c2csc3ncc(N[C@@H]4CCCC[C@@H]4N)nc23)c(n1)C(F)F |r| Show InChI InChI=1S/C18H21F2N7OS/c1-27-7-12(15(26-27)16(19)20)24-17(28)9-8-29-18-14(9)25-13(6-22-18)23-11-5-3-2-4-10(11)21/h6-8,10-11,16H,2-5,21H2,1H3,(H,23,25)(H,24,28)/t10-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 (unknown origin) |
Bioorg Med Chem Lett 25: 5384-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.028
BindingDB Entry DOI: 10.7270/Q2WS8W3R |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095475

(CHEMBL3590479)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCC[C@@H]4N)nc23)c(n1)C(F)F |r| Show InChI InChI=1S/C23H28N4O3/c1-26(2)14-7-15-30-22-16-21(27(25-22)17-18-8-5-4-6-9-18)23(28)24-19-10-12-20(29-3)13-11-19/h4-6,8-13,16H,7,14-15,17H2,1-3H3,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 (unknown origin) |
Bioorg Med Chem Lett 25: 5384-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.028
BindingDB Entry DOI: 10.7270/Q2WS8W3R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092190
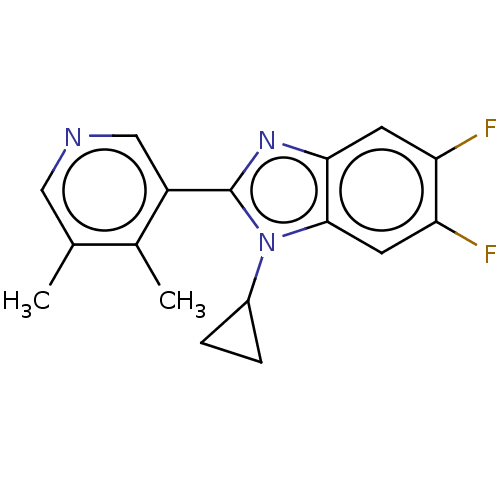
(CHEMBL3582482)Show InChI InChI=1S/C17H15F2N3/c1-9-7-20-8-12(10(9)2)17-21-15-5-13(18)14(19)6-16(15)22(17)11-3-4-11/h5-8,11H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50130668

(CHEMBL3634613)Show SMILES Cn1cc(NC(=O)c2csc3ncc(N[C@@H]4CCCC[C@@H]4N)nc23)c(Cl)n1 |r| Show InChI InChI=1S/C17H20ClN7OS/c1-25-7-12(15(18)24-25)22-16(26)9-8-27-17-14(9)23-13(6-20-17)21-11-5-3-2-4-10(11)19/h6-8,10-11H,2-5,19H2,1H3,(H,21,23)(H,22,26)/t10-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 (unknown origin) |
Bioorg Med Chem Lett 25: 5384-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.028
BindingDB Entry DOI: 10.7270/Q2WS8W3R |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095470
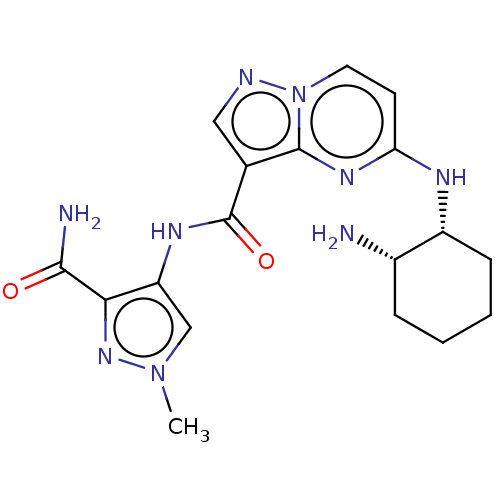
(CHEMBL3590474 | US10155765, Example 9)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCC[C@@H]4N)nc23)c(n1)C(N)=O |r| Show InChI InChI=1S/C21H25N3O/c1-23(2)14-9-15-25-21-16-20(19-12-7-4-8-13-19)24(22-21)17-18-10-5-3-6-11-18/h3-8,10-13,16H,9,14-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095474
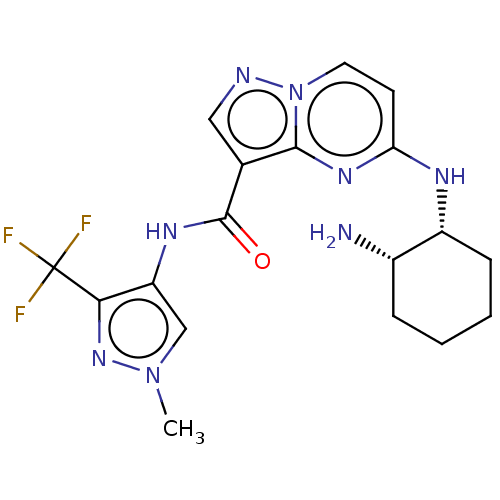
(CHEMBL3590478 | US10329294, Example 2)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCC[C@@H]4N)nc23)c(n1)C(F)(F)F |r| Show InChI InChI=1S/C20H23N3O/c1-22(2)14-9-15-24-20-16-19(17-10-5-3-6-11-17)23(21-20)18-12-7-4-8-13-18/h3-8,10-13,16H,9,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092142

(CHEMBL3582477)Show SMILES CC(C)(O)c1cncc(c1)-c1nc2cc(F)c(F)cc2n1C1CC1 Show InChI InChI=1S/C11H17NO8/c1-4(14)12-8-5(15)2-7(11(18)19)20-10(8)9(17)6(16)3-13/h2,5-6,8-10,13,15-17H,3H2,1H3,(H,12,14)(H,18,19)/t5-,6-,8+,9-,10+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157795

(US9023865, 636)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3ccnc(F)c3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157185
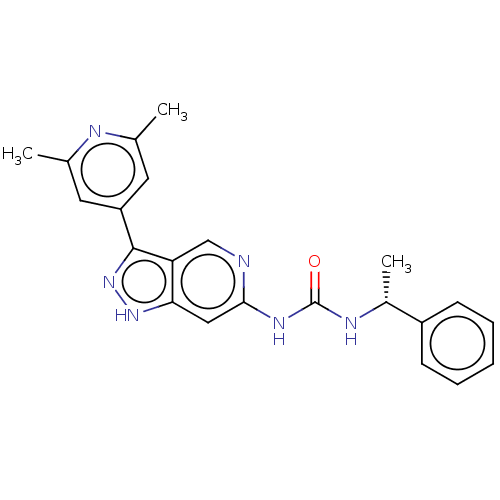
(US9023865, 31)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3cc(C)nc(C)c3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157795

(US9023865, 636)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3ccnc(F)c3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157185

(US9023865, 31)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3cc(C)nc(C)c3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157183

(US9023865, 29)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3cnn(C)c3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM157183

(US9023865, 29)Show SMILES C[C@@H](NC(=O)Nc1cc2[nH]nc(-c3cnn(C)c3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM286221
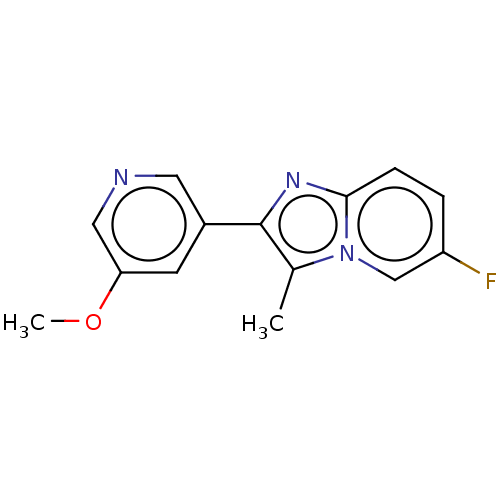
(6-fluoro-3-methyl-2-(5- methoxypyridin-3- yl)imida...)Show InChI InChI=1S/C14H12FN3O/c1-9-14(10-5-12(19-2)7-16-6-10)17-13-4-3-11(15)8-18(9)13/h3-8H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
US Patent
| Assay Description
V79 cell lines stably expressing the either the human CYP11B2 or the human CYP11B1 enzyme were generated using a standard transfection protocol. V79 ... |
US Patent US9518055 (2016)
BindingDB Entry DOI: 10.7270/Q23B625B |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095474

(CHEMBL3590478 | US10329294, Example 2)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCC[C@@H]4N)nc23)c(n1)C(F)(F)F |r| Show InChI InChI=1S/C20H23N3O/c1-22(2)14-9-15-24-20-16-19(17-10-5-3-6-11-17)23(21-20)18-12-7-4-8-13-18/h3-8,10-13,16H,9,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 (unknown origin) |
Bioorg Med Chem Lett 25: 5384-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.028
BindingDB Entry DOI: 10.7270/Q2WS8W3R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50130660
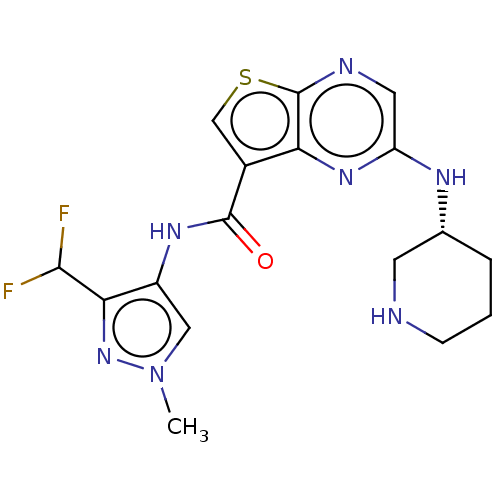
(CHEMBL3634509 | US10040802, Example 32)Show SMILES Cn1cc(NC(=O)c2csc3ncc(N[C@@H]4CCCNC4)nc23)c(n1)C(F)F |r| Show InChI InChI=1S/C17H19F2N7OS/c1-26-7-11(14(25-26)15(18)19)23-16(27)10-8-28-17-13(10)24-12(6-21-17)22-9-3-2-4-20-5-9/h6-9,15,20H,2-5H2,1H3,(H,22,24)(H,23,27)/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 (unknown origin) |
Bioorg Med Chem Lett 25: 5384-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.028
BindingDB Entry DOI: 10.7270/Q2WS8W3R |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50130674

(CHEMBL3634616)Show SMILES Cn1cc(NC(=O)c2csc3ncc(N[C@@H]4CCCC[C@@H]4N)nc23)c(n1)C#N |r| Show InChI InChI=1S/C18H20N8OS/c1-26-8-14(13(6-19)25-26)23-17(27)10-9-28-18-16(10)24-15(7-21-18)22-12-5-3-2-4-11(12)20/h7-9,11-12H,2-5,20H2,1H3,(H,22,24)(H,23,27)/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 (unknown origin) |
Bioorg Med Chem Lett 25: 5384-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.028
BindingDB Entry DOI: 10.7270/Q2WS8W3R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092182
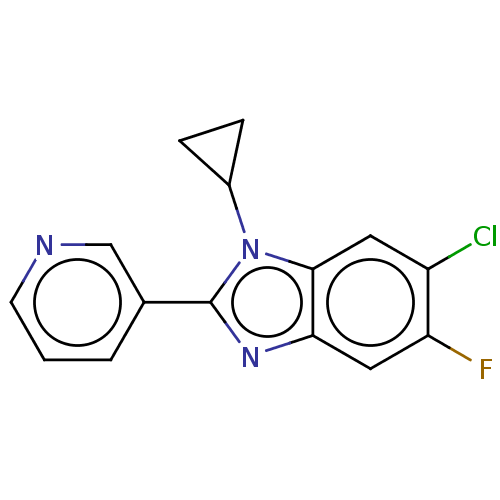
(CHEMBL3582470)Show InChI InChI=1S/C15H11ClFN3/c16-11-6-14-13(7-12(11)17)19-15(20(14)10-3-4-10)9-2-1-5-18-8-9/h1-2,5-8,10H,3-4H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092185
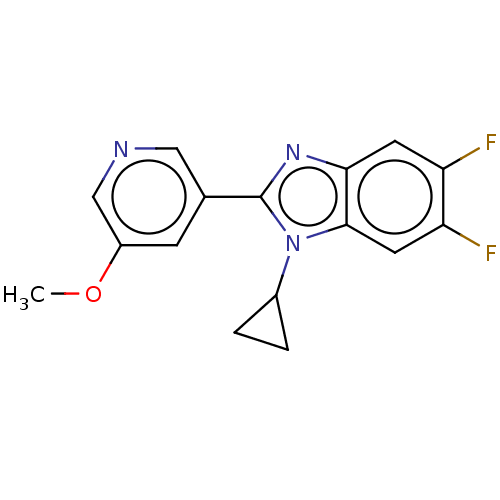
(CHEMBL3582474)Show InChI InChI=1S/C16H13F2N3O/c1-22-11-4-9(7-19-8-11)16-20-14-5-12(17)13(18)6-15(14)21(16)10-2-3-10/h4-8,10H,2-3H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM286222
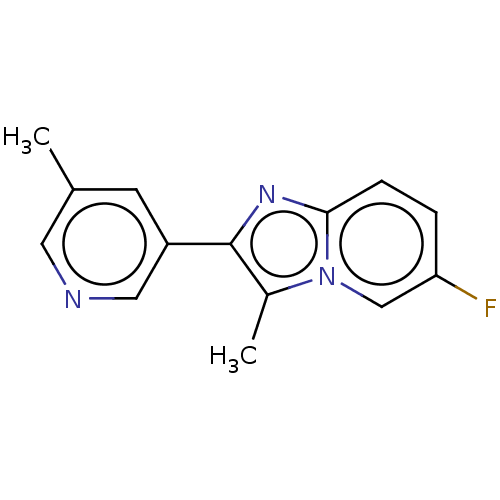
(6-fluoro-3-methyl-2-(5- methylpyridin-3-yl)imidazo...)Show InChI InChI=1S/C14H12FN3/c1-9-5-11(7-16-6-9)14-10(2)18-8-12(15)3-4-13(18)17-14/h3-8H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
US Patent
| Assay Description
V79 cell lines stably expressing the either the human CYP11B2 or the human CYP11B1 enzyme were generated using a standard transfection protocol. V79 ... |
US Patent US9518055 (2016)
BindingDB Entry DOI: 10.7270/Q23B625B |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50130679
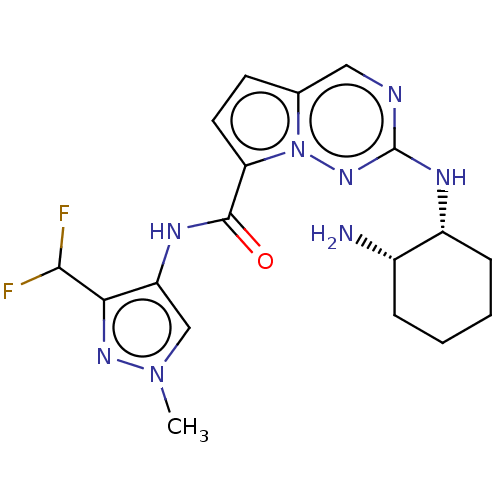
(CHEMBL3634381)Show SMILES Cn1cc(NC(=O)c2ccc3cnc(N[C@@H]4CCCC[C@@H]4N)nn23)c(n1)C(F)F |r| Show InChI InChI=1S/C18H22F2N8O/c1-27-9-13(15(25-27)16(19)20)23-17(29)14-7-6-10-8-22-18(26-28(10)14)24-12-5-3-2-4-11(12)21/h6-9,11-12,16H,2-5,21H2,1H3,(H,23,29)(H,24,26)/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 (unknown origin) |
Bioorg Med Chem Lett 25: 5384-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.028
BindingDB Entry DOI: 10.7270/Q2WS8W3R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50206963
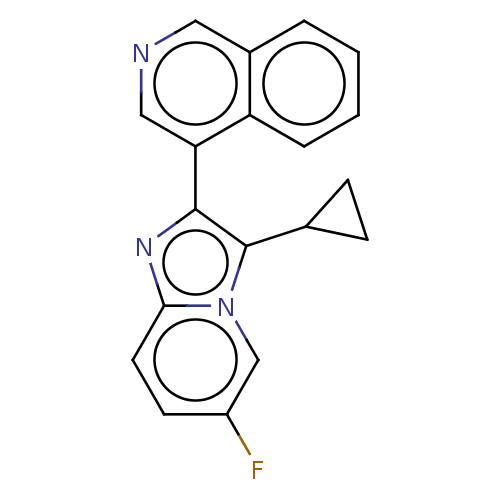
(CHEMBL3948337 | US9518055, Example 41)Show InChI InChI=1S/C19H14FN3/c20-14-7-8-17-22-18(19(12-5-6-12)23(17)11-14)16-10-21-9-13-3-1-2-4-15(13)16/h1-4,7-12H,5-6H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
US Patent
| Assay Description
V79 cell lines stably expressing the either the human CYP11B2 or the human CYP11B1 enzyme were generated using a standard transfection protocol. V79 ... |
US Patent US9518055 (2016)
BindingDB Entry DOI: 10.7270/Q23B625B |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50130689

(CHEMBL3634506 | US10329294, Example 91)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCNC4)nc23)c(n1)C(F)F |r| Show InChI InChI=1S/C17H20F2N8O/c1-26-9-12(14(25-26)15(18)19)23-17(28)11-8-21-27-6-4-13(24-16(11)27)22-10-3-2-5-20-7-10/h4,6,8-10,15,20H,2-3,5,7H2,1H3,(H,22,24)(H,23,28)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 (unknown origin) |
Bioorg Med Chem Lett 25: 5384-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.028
BindingDB Entry DOI: 10.7270/Q2WS8W3R |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50130664

(CHEMBL3634512 | US10329294, Example 149)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCC[C@@H]4N)nc23)c(Cl)n1 |r| Show InChI InChI=1S/C17H21ClN8O/c1-25-9-13(15(18)24-25)22-17(27)10-8-20-26-7-6-14(23-16(10)26)21-12-5-3-2-4-11(12)19/h6-9,11-12H,2-5,19H2,1H3,(H,21,23)(H,22,27)/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 (unknown origin) |
Bioorg Med Chem Lett 25: 5384-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.028
BindingDB Entry DOI: 10.7270/Q2WS8W3R |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50130684

(CHEMBL3634502 | US10329294, Example 9)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCNC4)nc23)c(n1)C(F)(F)F |r| Show InChI InChI=1S/C17H19F3N8O/c1-27-9-12(14(26-27)17(18,19)20)24-16(29)11-8-22-28-6-4-13(25-15(11)28)23-10-3-2-5-21-7-10/h4,6,8-10,21H,2-3,5,7H2,1H3,(H,23,25)(H,24,29)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 (unknown origin) |
Bioorg Med Chem Lett 25: 5384-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.028
BindingDB Entry DOI: 10.7270/Q2WS8W3R |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095532
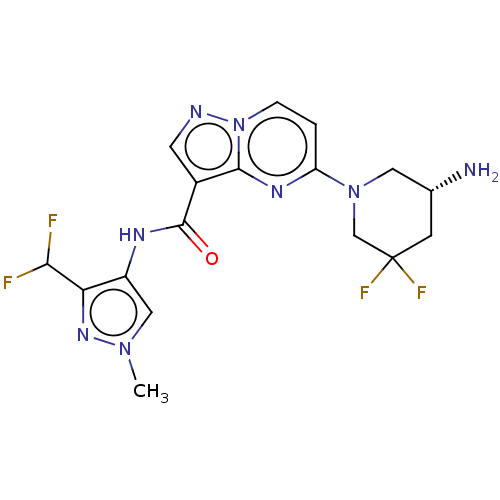
(CHEMBL3590515 | US10329294, Example 173)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(nc23)N2C[C@H](N)CC(F)(F)C2)c(n1)C(F)F |r| Show InChI InChI=1S/C15H18N2O2/c18-15-13(10-11-4-2-1-3-5-11)14(19-17-15)12-6-8-16-9-7-12/h1-5,12,16H,6-10H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095534

(CHEMBL3590516 | US10329294, Example 252)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(nc23)N2C[C@H](N)C[C@@H](F)C2)c(n1)C(F)(F)F |r| Show InChI InChI=1S/C9H14N2O2/c1-6-8(13-11-9(6)12)7-2-4-10-5-3-7/h7,10H,2-5H2,1H3,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092137
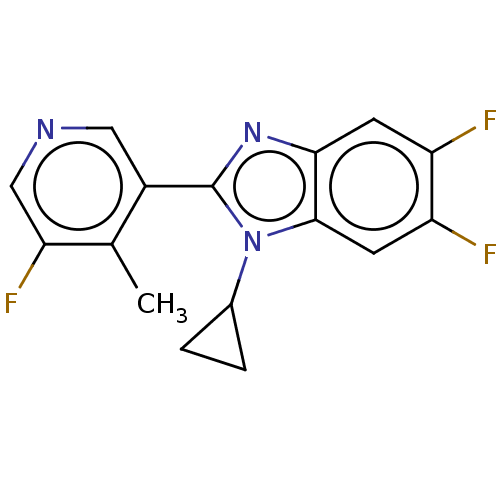
(CHEMBL3582481)Show InChI InChI=1S/C16H12F3N3/c1-8-10(6-20-7-13(8)19)16-21-14-4-11(17)12(18)5-15(14)22(16)9-2-3-9/h4-7,9H,2-3H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Rattus norvegicus (rat)) | BDBM157161
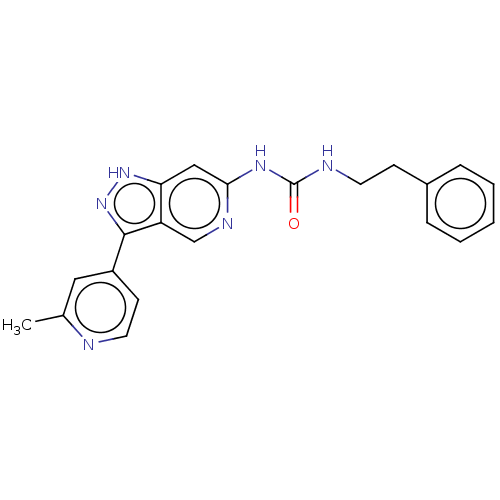
(US9023865, 5)Show SMILES Cc1cc(ccn1)-c1n[nH]c2cc(NC(=O)NCCc3ccccc3)ncc12 Show InChI InChI=1S/C21H20N6O/c1-14-11-16(8-10-22-14)20-17-13-24-19(12-18(17)26-27-20)25-21(28)23-9-7-15-5-3-2-4-6-15/h2-6,8,10-13H,7,9H2,1H3,(H,26,27)(H2,23,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM394063
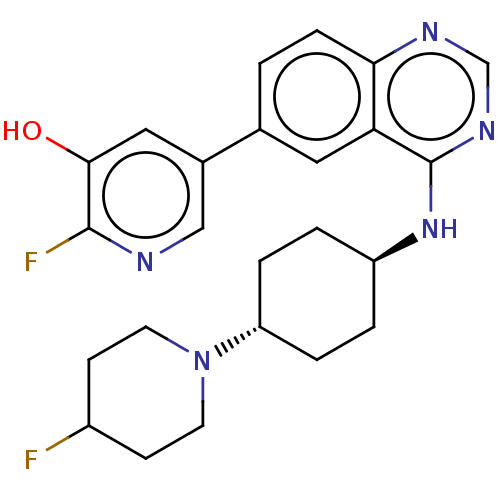
(2-fluoro-5-(4-{[trans-4- (4-fluoropiperidin-1- yl)...)Show SMILES Oc1cc(cnc1F)-c1ccc2ncnc(N[C@H]3CC[C@@H](CC3)N3CCC(F)CC3)c2c1 |r,wU:20.24,wD:17.17,(1.93,6.67,;.38,6.67,;-.38,5.33,;-1.93,5.33,;-2.69,6.67,;-1.93,8,;-.38,8,;.38,9.34,;-2.69,4,;-4.23,4,;-5,2.67,;-4.23,1.33,;-5,,;-4.23,-1.33,;-2.69,-1.33,;-1.93,,;-.38,,;.38,-1.33,;-.39,-2.67,;.38,-4,;1.92,-4,;2.69,-2.67,;1.92,-1.33,;2.69,-5.33,;1.92,-6.67,;2.69,-8,;4.23,-8,;5,-9.34,;5,-6.67,;4.23,-5.33,;-2.69,1.33,;-1.93,2.67,)| Show InChI InChI=1S/C24H27F2N5O/c25-17-7-9-31(10-8-17)19-4-2-18(3-5-19)30-24-20-11-15(1-6-21(20)28-14-29-24)16-12-22(32)23(26)27-13-16/h1,6,11-14,17-19,32H,2-5,7-10H2,(H,28,29,30)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... |
J Med Chem 52: 1873-84 (2009)
BindingDB Entry DOI: 10.7270/Q2CN767V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM394067
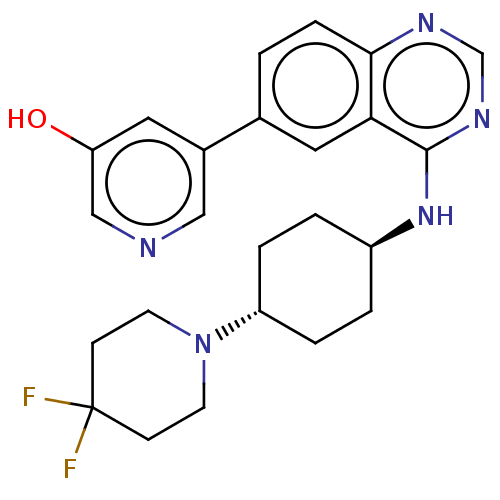
(5-(4-{[trans-4-(4,4- difluoropiperidin-1- yl)cyclo...)Show SMILES Oc1cncc(c1)-c1ccc2ncnc(N[C@H]3CC[C@@H](CC3)N3CCC(F)(F)CC3)c2c1 |r,wU:16.16,wD:19.23,(1.64,7.44,;.1,7.44,;-.67,8.77,;-2.21,8.77,;-2.98,7.44,;-2.21,6.1,;-.67,6.1,;-2.98,4.77,;-4.52,4.77,;-5.29,3.44,;-4.52,2.1,;-5.29,.77,;-4.52,-.56,;-2.98,-.56,;-2.21,.77,;-.67,.77,;.1,-.56,;1.64,-.56,;2.41,-1.9,;1.64,-3.23,;.1,-3.23,;-.67,-1.9,;2.41,-4.56,;1.64,-5.9,;2.41,-7.23,;3.95,-7.23,;3.95,-8.77,;5.29,-8,;4.72,-5.9,;3.95,-4.56,;-2.98,2.1,;-2.21,3.44,)| Show InChI InChI=1S/C24H27F2N5O/c25-24(26)7-9-31(10-8-24)19-4-2-18(3-5-19)30-23-21-12-16(1-6-22(21)28-15-29-23)17-11-20(32)14-27-13-17/h1,6,11-15,18-19,32H,2-5,7-10H2,(H,28,29,30)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... |
J Med Chem 52: 1873-84 (2009)
BindingDB Entry DOI: 10.7270/Q2CN767V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM394068

(5-(4-{[trans-4-(4- fluoropiperidin-1- yl)cyclohexy...)Show SMILES Oc1cncc(c1)-c1ccc2ncnc(N[C@H]3CC[C@@H](CC3)N3CCC(F)CC3)c2c1 |r,wU:16.16,wD:19.23,(1.93,7.34,;.38,7.34,;-.38,8.67,;-1.93,8.67,;-2.69,7.34,;-1.93,6,;-.38,6,;-2.69,4.67,;-4.23,4.67,;-5,3.33,;-4.23,2,;-5,.67,;-4.23,-.67,;-2.69,-.67,;-1.93,.67,;-.38,.67,;.38,-.67,;1.92,-.67,;2.69,-2,;1.92,-3.33,;.38,-3.33,;-.39,-2,;2.69,-4.67,;1.92,-6,;2.69,-7.34,;4.23,-7.34,;5,-8.67,;5,-6,;4.23,-4.67,;-2.69,2,;-1.93,3.33,)| Show InChI InChI=1S/C24H28FN5O/c25-18-7-9-30(10-8-18)20-4-2-19(3-5-20)29-24-22-12-16(1-6-23(22)27-15-28-24)17-11-21(31)14-26-13-17/h1,6,11-15,18-20,31H,2-5,7-10H2,(H,27,28,29)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... |
J Med Chem 52: 1873-84 (2009)
BindingDB Entry DOI: 10.7270/Q2CN767V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Rattus norvegicus (rat)) | BDBM157161

(US9023865, 5)Show SMILES Cc1cc(ccn1)-c1n[nH]c2cc(NC(=O)NCCc3ccccc3)ncc12 Show InChI InChI=1S/C21H20N6O/c1-14-11-16(8-10-22-14)20-17-13-24-19(12-18(17)26-27-20)25-21(28)23-9-7-15-5-3-2-4-6-15/h2-6,8,10-13H,7,9H2,1H3,(H,26,27)(H2,23,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse ERK2 preincubated for 15 mins followed by addition of IMAP peptide substrate and ATP measured after 60 mins by IMAP-FP assay |
J Med Chem 59: 6501-11 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00708
BindingDB Entry DOI: 10.7270/Q2J969VW |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM393974
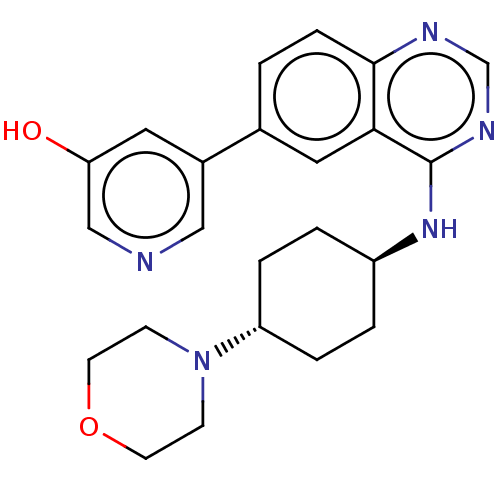
(5-(4-{[trans-4- (morpholin-4- yl)cyclohexyl]amino}...)Show SMILES Oc1cncc(c1)-c1ccc2ncnc(N[C@H]3CC[C@@H](CC3)N3CCOCC3)c2c1 |r,wU:16.16,wD:19.23,(1.92,6.67,;.39,6.67,;-.38,8,;-1.92,8,;-2.69,6.67,;-1.92,5.33,;-.38,5.33,;-2.69,4,;-4.23,4,;-5,2.67,;-4.23,1.33,;-5,,;-4.23,-1.33,;-2.69,-1.33,;-1.92,,;-.38,,;.39,-1.33,;1.93,-1.33,;2.7,-2.67,;1.93,-4,;.39,-4,;-.38,-2.67,;2.7,-5.33,;1.92,-6.67,;2.7,-8,;4.23,-8,;5,-6.67,;4.23,-5.33,;-2.69,1.33,;-1.92,2.67,)| Show InChI InChI=1S/C23H27N5O2/c29-20-11-17(13-24-14-20)16-1-6-22-21(12-16)23(26-15-25-22)27-18-2-4-19(5-3-18)28-7-9-30-10-8-28/h1,6,11-15,18-19,29H,2-5,7-10H2,(H,25,26,27)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... |
J Med Chem 52: 1873-84 (2009)
BindingDB Entry DOI: 10.7270/Q2CN767V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM394042
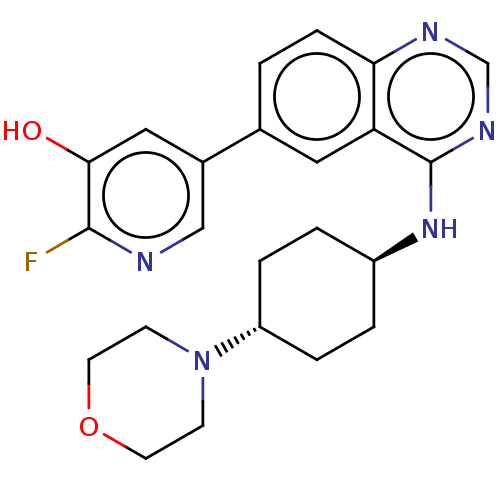
(2-fluoro-5-(4-{[trans-4- (morpholin-4- yl)cyclohex...)Show SMILES Oc1cc(cnc1F)-c1ccc2ncnc(N[C@H]3CC[C@@H](CC3)N3CCOCC3)c2c1 |r,wU:20.24,wD:17.17,(1.93,6,;.38,6,;-.38,4.67,;-1.93,4.67,;-2.69,6,;-1.93,7.34,;-.38,7.34,;.38,8.67,;-2.69,3.33,;-4.23,3.33,;-5,2,;-4.23,.67,;-5,-.67,;-4.23,-2,;-2.69,-2,;-1.93,-.67,;-.38,-.67,;.38,-2,;-.39,-3.33,;.38,-4.67,;1.92,-4.67,;2.69,-3.33,;1.92,-2,;2.69,-6,;1.92,-7.34,;2.69,-8.67,;4.23,-8.67,;5,-7.34,;4.23,-6,;-2.69,.67,;-1.93,2,)| Show InChI InChI=1S/C23H26FN5O2/c24-22-21(30)12-16(13-25-22)15-1-6-20-19(11-15)23(27-14-26-20)28-17-2-4-18(5-3-17)29-7-9-31-10-8-29/h1,6,11-14,17-18,30H,2-5,7-10H2,(H,26,27,28)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... |
J Med Chem 52: 1873-84 (2009)
BindingDB Entry DOI: 10.7270/Q2CN767V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM394051
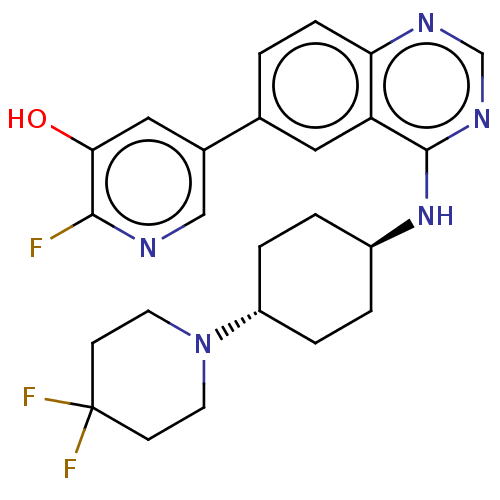
(US9969749, Example 5-1)Show SMILES Oc1cc(cnc1F)-c1ccc2ncnc(N[C@H]3CC[C@@H](CC3)N3CCC(F)(F)CC3)c2c1 |r,wU:20.24,wD:17.17,(1.64,6.77,;.1,6.77,;-.67,5.44,;-2.21,5.44,;-2.98,6.77,;-2.21,8.11,;-.67,8.11,;.1,9.44,;-2.98,4.1,;-4.52,4.1,;-5.29,2.77,;-4.52,1.44,;-5.29,.1,;-4.52,-1.23,;-2.98,-1.23,;-2.21,.1,;-.67,.1,;.1,-1.23,;-.67,-2.56,;.1,-3.9,;1.64,-3.9,;2.41,-2.56,;1.64,-1.23,;2.41,-5.23,;1.64,-6.57,;2.41,-7.9,;3.95,-7.9,;3.95,-9.44,;5.29,-8.67,;4.72,-6.57,;3.95,-5.23,;-2.98,1.44,;-2.21,2.77,)| Show InChI InChI=1S/C24H26F3N5O/c25-22-21(33)12-16(13-28-22)15-1-6-20-19(11-15)23(30-14-29-20)31-17-2-4-18(5-3-17)32-9-7-24(26,27)8-10-32/h1,6,11-14,17-18,33H,2-5,7-10H2,(H,29,30,31)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
The kinase activity of IRAK4 is determined by its ability to catalyze the phosphorylation of a fluorescent polypeptide substrate. The extent of phosp... |
J Med Chem 52: 1873-84 (2009)
BindingDB Entry DOI: 10.7270/Q2CN767V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data