

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50206074 ((+/-)-N-{2-[2-(1-methyl-pyrrolidin-2-yl)-ethylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106707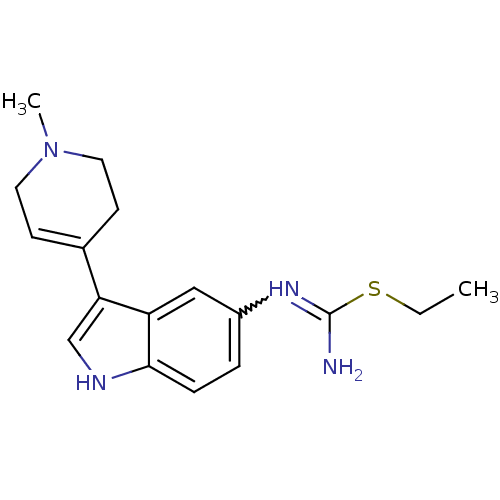 (CHEMBL1957358 | US8586620, 67) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106699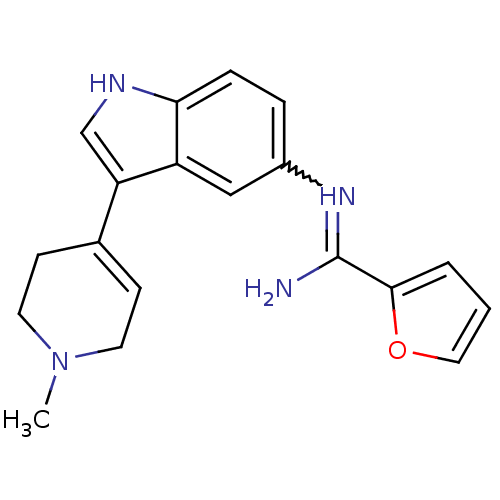 (CHEMBL1957350 | US8586620, 46) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50206073 (CHEMBL233652 | N-[2-(2-pyridin-2-yl-ethylamino)-be...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106698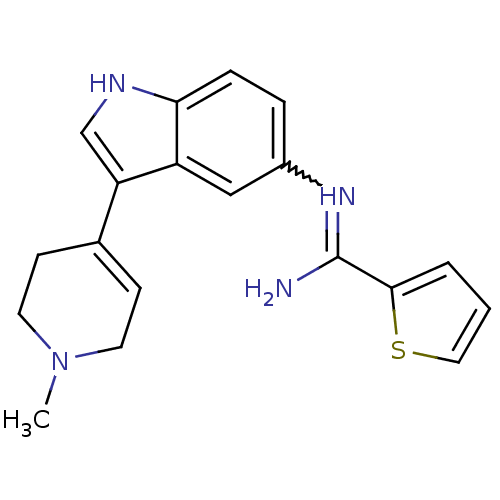 (CHEMBL1957349 | US8586620, 42) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106706 (CHEMBL1957356 | US8586620, 64) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50206064 (CHEMBL233857 | N-[2-(1-benzyl-piperidin-4-ylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50206065 (CHEMBL233655 | N-{2-[1-(3-methoxy-benzyl)-piperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106715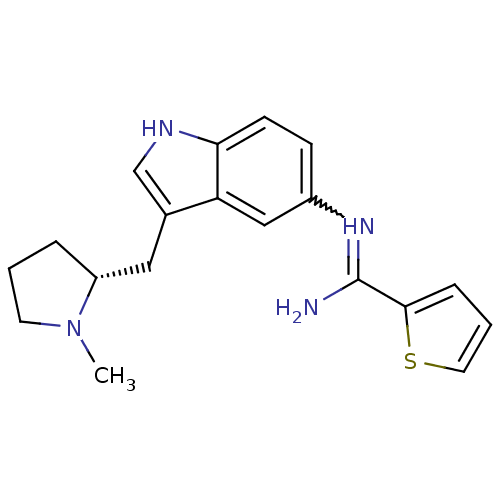 (US8586620, 97) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM106698 (CHEMBL1957349 | US8586620, 42) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50206070 (CHEMBL412797 | N-[2-(2-morpholin-4-yl-ethylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50206080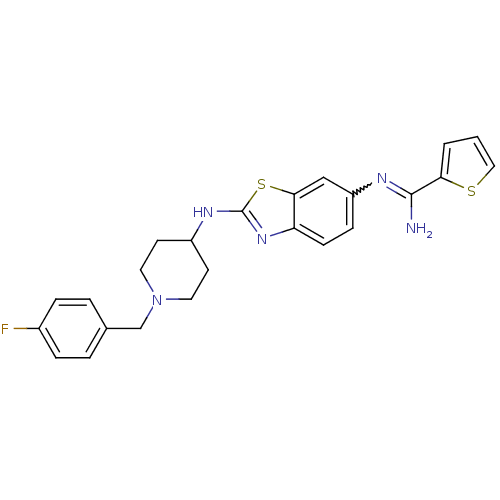 (CHEMBL233444 | N-{2-[1-(4-fluoro-benzyl)-piperidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50206082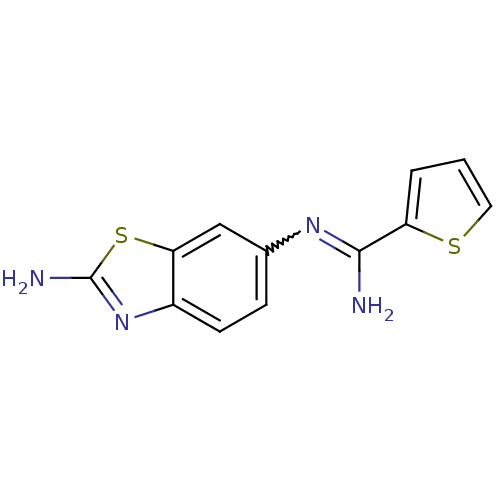 (CHEMBL397506 | N-(2-amino-benzothiazol-6-yl)-thiop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50206076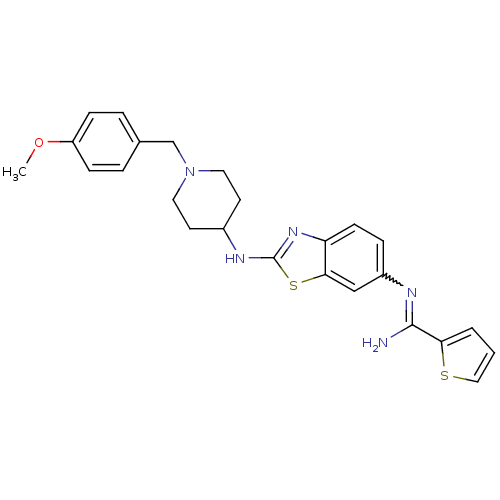 (CHEMBL234498 | N-{2-[1-(4-methoxy-benzyl)-piperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106737 (CHEMBL1823366 | US8586620, 139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50206067 (CHEMBL233228 | N-(2-amino-benzothiazol-5-yl)-thiop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106701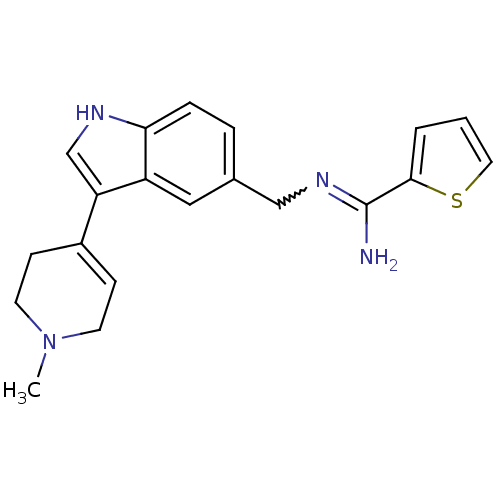 (CHEMBL1957352 | US8586620, 51) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106735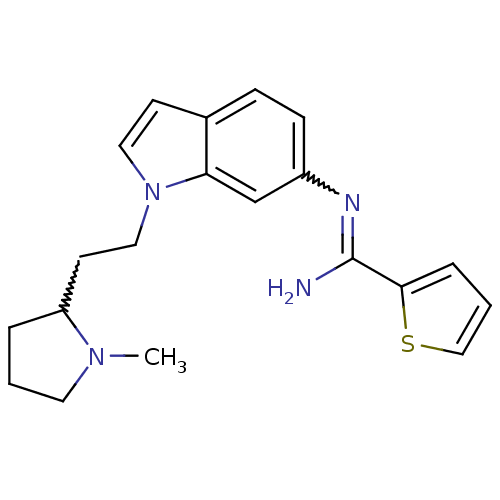 (US8586620, 137) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106746 (CHEMBL1823375 | US8586620, 168) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106700 (CHEMBL1957354 | US8586620, 47) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM106717 (US8586620, 105) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106704 (US8586620, 59) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50206075 (4-(4-{6-[(thiophene-2-carboximidoyl)-amino]-benzot...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50206066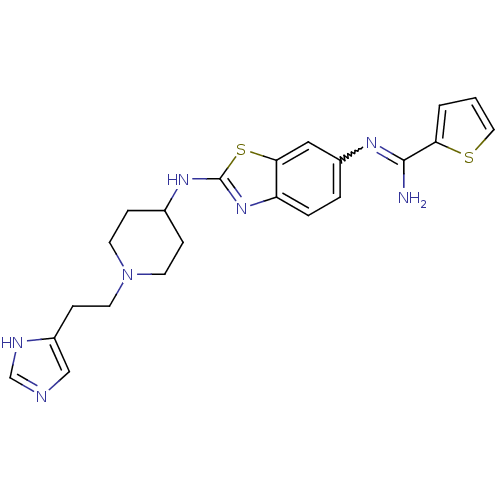 (CHEMBL233653 | N-(2-{1-[2-(3H-imidazol-4-yl)-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM106715 (US8586620, 97) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106736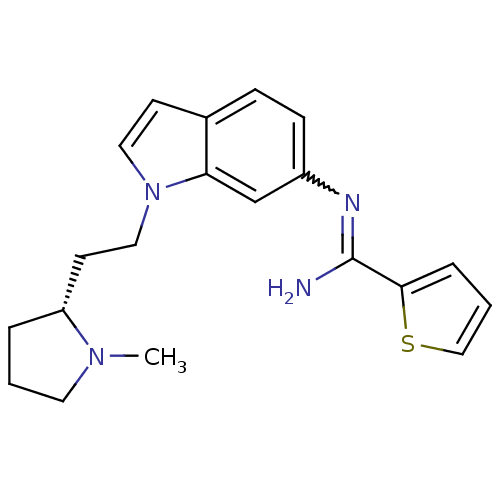 (CHEMBL1823365 | US8586620, 138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106720 (US8586620, 110) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106696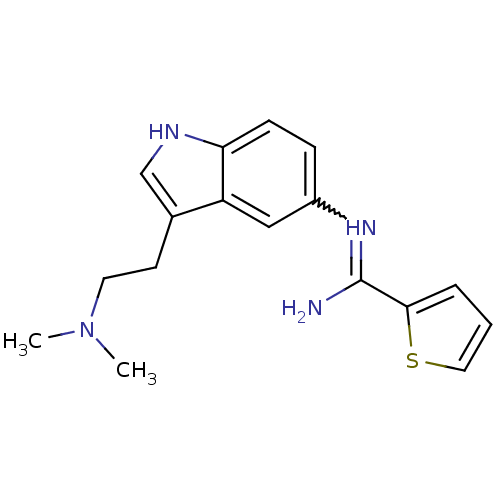 (US8586620, 56) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50206079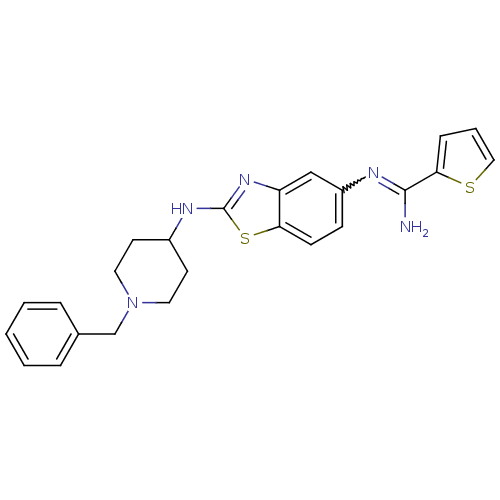 (CHEMBL233229 | N-[2-(1-benzyl-piperidin-4-ylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106703 (US8586620, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106720 (US8586620, 110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106734 (CHEMBL1823363 | US8586620, 136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106712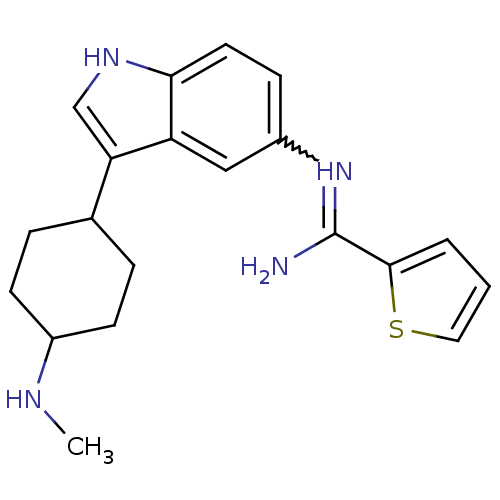 (US8586620, 84) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM106720 (US8586620, 110) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50206064 (CHEMBL233857 | N-[2-(1-benzyl-piperidin-4-ylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106721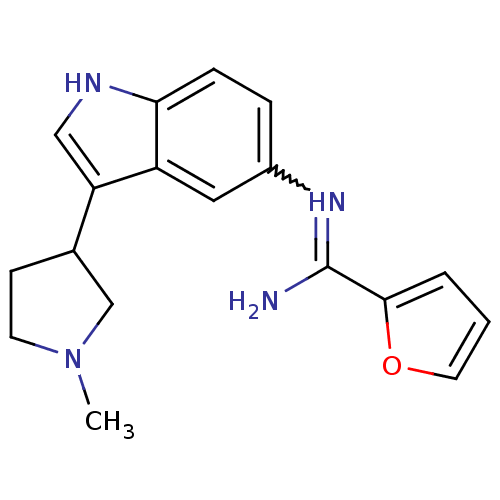 (US8586620, 111) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106697 (CHEMBL1957353 | US8586620, 43) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106740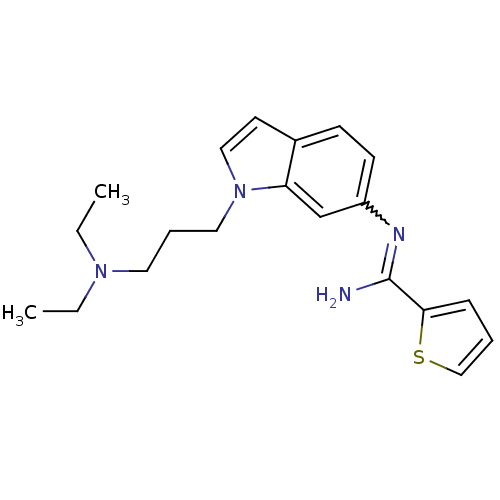 (CHEMBL1823369 | US8586620, 158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106705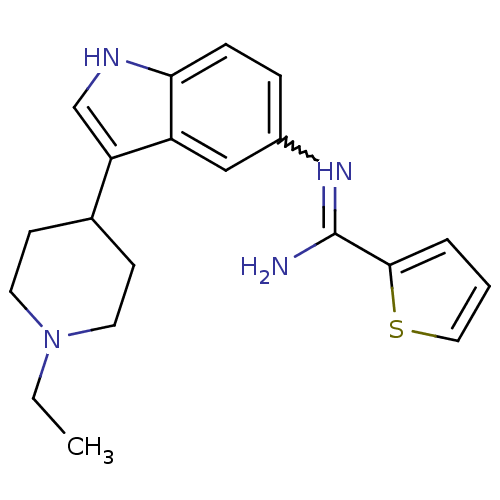 (US8586620, 62) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106739 (CHEMBL1823371 | US8586620, 157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106724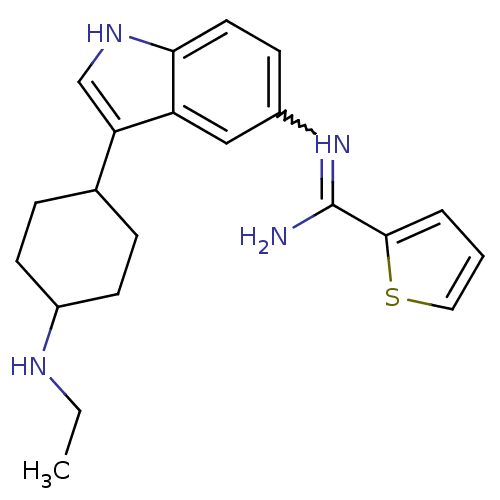 (US8586620, 121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50206066 (CHEMBL233653 | N-(2-{1-[2-(3H-imidazol-4-yl)-ethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human endothelial NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106711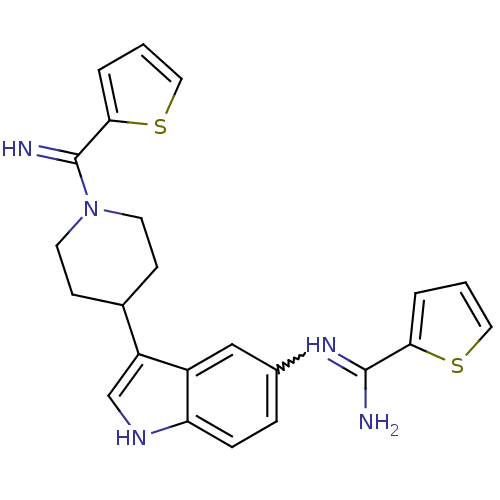 (US8586620, 77) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 717 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50206070 (CHEMBL412797 | N-[2-(2-morpholin-4-yl-ethylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50206071 (CHEMBL233648 | N-{2-[1-(4-fluoro-benzyl)-piperidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106717 (US8586620, 105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106715 (US8586620, 97) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106738 (CHEMBL1823367 | US8586620, 156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 211 total ) | Next | Last >> |