 Found 820 hits with Last Name = 'shang' and Initial = 'j'
Found 820 hits with Last Name = 'shang' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125977
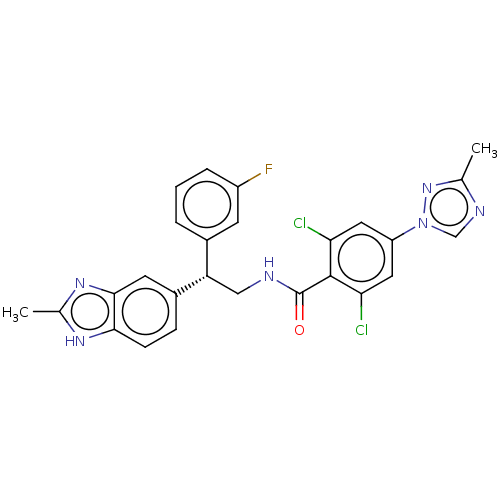
(CHEMBL3627897)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2ccc3[nH]c(C)nc3c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H21Cl2FN6O/c1-14-31-13-35(34-14)19-10-21(27)25(22(28)11-19)26(36)30-12-20(16-4-3-5-18(29)8-16)17-6-7-23-24(9-17)33-15(2)32-23/h3-11,13,20H,12H2,1-2H3,(H,30,36)(H,32,33)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human factor 9a using CH3SO2-DCHG-Gly-Arg-AFC.AcOH as substrate preinubated for 30 mins followed by substrate addition measured after 1... |
J Med Chem 59: 1818-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01293
BindingDB Entry DOI: 10.7270/Q29Z96S8 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50151405

(CHEMBL3775211 | US10189819, Example 79)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2cc(F)c3[nH]c(C)nc3c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H20Cl2F2N6O/c1-13-32-12-36(35-13)18-9-20(27)24(21(28)10-18)26(37)31-11-19(15-4-3-5-17(29)6-15)16-7-22(30)25-23(8-16)33-14(2)34-25/h3-10,12,19H,11H2,1-2H3,(H,31,37)(H,33,34)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human factor 9a using CH3SO2-DCHG-Gly-Arg-AFC.AcOH as substrate preinubated for 30 mins followed by substrate addition measured after 1... |
J Med Chem 59: 1818-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01293
BindingDB Entry DOI: 10.7270/Q29Z96S8 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50204495

(CHEMBL3921061)Show InChI InChI=1S/C18H17NO4/c1-19(2)18(22)23-16-5-3-4-14(12-16)17(21)11-8-13-6-9-15(20)10-7-13/h3-12,20H,1-2H3/b11-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
South China University of Technology
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human serum BChE using S-Butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition... |
Bioorg Med Chem 25: 360-371 (2017)
Article DOI: 10.1016/j.bmc.2016.11.002
BindingDB Entry DOI: 10.7270/Q2NK3H15 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486243
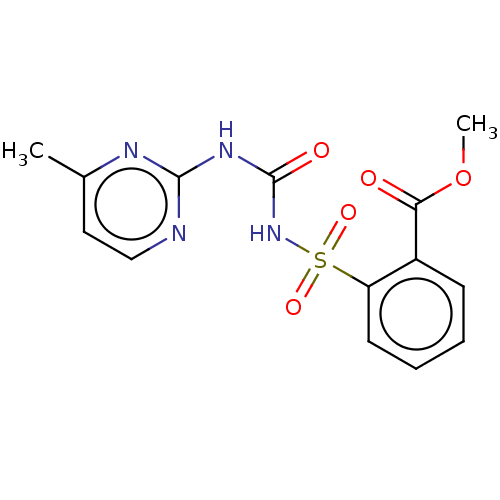
(MONOSULFURON ESTER)Show InChI InChI=1S/C14H14N4O5S/c1-9-7-8-15-13(16-9)17-14(20)18-24(21,22)11-6-4-3-5-10(11)12(19)23-2/h3-8H,1-2H3,(H2,15,16,17,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetohydroxyacid synthase colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50103521
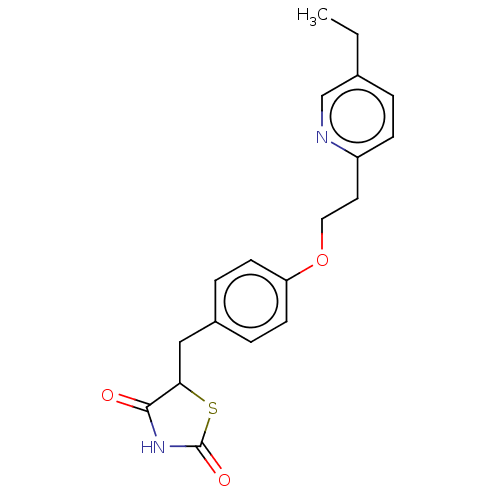
(Actos | CHEBI:8228 | Duetact | Pioglitazone | US10...)Show InChI InChI=1S/C19H20N2O3S/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Displacement of fluormone PanPPAR green tracer ligand from human 6His-tagged PPARgamma isoform 1 LBD (203 to 477 residues) expressed in Escherichia c... |
J Med Chem 62: 2008-2023 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01573
BindingDB Entry DOI: 10.7270/Q2TH8R5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50103521

(Actos | CHEBI:8228 | Duetact | Pioglitazone | US10...)Show InChI InChI=1S/C19H20N2O3S/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Displacement of fluormone PanPPAR green tracer ligand from human 6His-tagged PPARgamma isoform 1 LBD (203 to 477 residues) expressed in Escherichia c... |
J Med Chem 62: 2008-2023 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01573
BindingDB Entry DOI: 10.7270/Q2TH8R5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50204495

(CHEMBL3921061)Show InChI InChI=1S/C18H17NO4/c1-19(2)18(22)23-16-5-3-4-14(12-16)17(21)11-8-13-6-9-15(20)10-7-13/h3-12,20H,1-2H3/b11-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
South China University of Technology
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addit... |
Bioorg Med Chem 25: 360-371 (2017)
Article DOI: 10.1016/j.bmc.2016.11.002
BindingDB Entry DOI: 10.7270/Q2NK3H15 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50530214

(CHEBI:82937 | Leriglitazone | Min-102)Show InChI InChI=1S/C19H20N2O4S/c1-12(22)14-4-5-15(20-11-14)8-9-25-16-6-2-13(3-7-16)10-17-18(23)21-19(24)26-17/h2-7,11-12,17,22H,8-10H2,1H3,(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Displacement of fluormone PanPPAR green tracer ligand from human 6His-tagged PPARgamma isoform 1 LBD (203 to 477 residues) expressed in Escherichia c... |
J Med Chem 62: 2008-2023 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01573
BindingDB Entry DOI: 10.7270/Q2TH8R5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50530214

(CHEBI:82937 | Leriglitazone | Min-102)Show InChI InChI=1S/C19H20N2O4S/c1-12(22)14-4-5-15(20-11-14)8-9-25-16-6-2-13(3-7-16)10-17-18(23)21-19(24)26-17/h2-7,11-12,17,22H,8-10H2,1H3,(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Displacement of fluormone PanPPAR green tracer ligand from human 6His-tagged PPARgamma isoform 1 LBD (203 to 477 residues) expressed in Escherichia c... |
J Med Chem 62: 2008-2023 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01573
BindingDB Entry DOI: 10.7270/Q2TH8R5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486242

(CHEMBL2230130)Show InChI InChI=1S/C8H7N3S2/c1-2-4-7(5-3-1)12-13-8-9-6-10-11-8/h1-6H,(H,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetohydroxyacid synthase colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125977

(CHEMBL3627897)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2ccc3[nH]c(C)nc3c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H21Cl2FN6O/c1-14-31-13-35(34-14)19-10-21(27)25(22(28)11-19)26(36)30-12-20(16-4-3-5-18(29)8-16)17-6-7-23-24(9-17)33-15(2)32-23/h3-11,13,20H,12H2,1-2H3,(H,30,36)(H,32,33)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using n-Acetyl-KPR-AFC as substrate preinubated for 30 mins followed by substrate addition measured after 1 hr by fluo... |
J Med Chem 59: 1818-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01293
BindingDB Entry DOI: 10.7270/Q29Z96S8 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486241

(CHEBI:5869 | IMAZAQUIN)Show SMILES CC(C)C1(C)N=C(NC1=O)c1nc2ccccc2cc1C(O)=O |c:5| Show InChI InChI=1S/C17H17N3O3/c1-9(2)17(3)16(23)19-14(20-17)13-11(15(21)22)8-10-6-4-5-7-12(10)18-13/h4-9H,1-3H3,(H,21,22)(H,19,20,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetohydroxyacid synthase colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486245
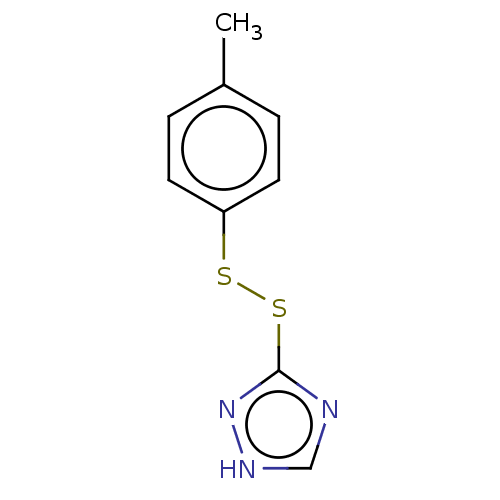
(CHEMBL2230131)Show InChI InChI=1S/C9H9N3S2/c1-7-2-4-8(5-3-7)13-14-9-10-6-11-12-9/h2-6H,1H3,(H,10,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 4.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetohydroxyacid synthase colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486244
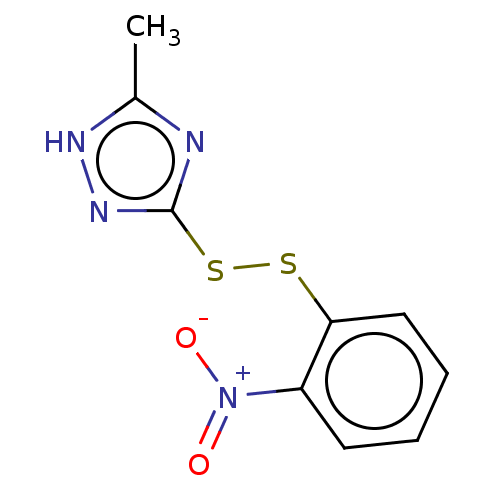
(CHEMBL2229985)Show InChI InChI=1S/C9H8N4O2S2/c1-6-10-9(12-11-6)17-16-8-5-3-2-4-7(8)13(14)15/h2-5H,1H3,(H,10,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 5.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetohydroxyacid synthase colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486243

(MONOSULFURON ESTER)Show InChI InChI=1S/C14H14N4O5S/c1-9-7-8-15-13(16-9)17-14(20)18-24(21,22)11-6-4-3-5-10(11)12(19)23-2/h3-8H,1-2H3,(H2,15,16,17,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 8.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana acetohydroxyacid synthase W574L mutant colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486242

(CHEMBL2230130)Show InChI InChI=1S/C8H7N3S2/c1-2-4-7(5-3-1)12-13-8-9-6-10-11-8/h1-6H,(H,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana acetohydroxyacid synthase W574L mutant colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486245

(CHEMBL2230131)Show InChI InChI=1S/C9H9N3S2/c1-7-2-4-8(5-3-7)13-14-9-10-6-11-12-9/h2-6H,1H3,(H,10,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana acetohydroxyacid synthase W574L mutant colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486244

(CHEMBL2229985)Show InChI InChI=1S/C9H8N4O2S2/c1-6-10-9(12-11-6)17-16-8-5-3-2-4-7(8)13(14)15/h2-5H,1H3,(H,10,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana acetohydroxyacid synthase W574L mutant colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486241

(CHEBI:5869 | IMAZAQUIN)Show SMILES CC(C)C1(C)N=C(NC1=O)c1nc2ccccc2cc1C(O)=O |c:5| Show InChI InChI=1S/C17H17N3O3/c1-9(2)17(3)16(23)19-14(20-17)13-11(15(21)22)8-10-6-4-5-7-12(10)18-13/h4-9H,1-3H3,(H,21,22)(H,19,20,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana acetohydroxyacid synthase W574L mutant colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468144
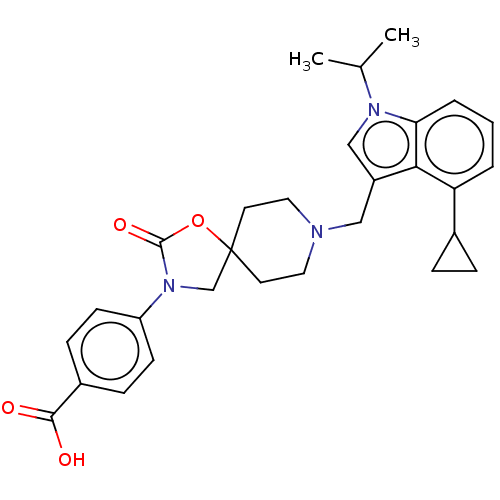
(CHEMBL4286244)Show SMILES CC(C)n1cc(CN2CCC3(CN(C(=O)O3)c3ccc(cc3)C(O)=O)CC2)c2c(cccc12)C1CC1 Show InChI InChI=1S/C29H33N3O4/c1-19(2)31-17-22(26-24(20-6-7-20)4-3-5-25(26)31)16-30-14-12-29(13-15-30)18-32(28(35)36-29)23-10-8-21(9-11-23)27(33)34/h3-5,8-11,17,19-20H,6-7,12-16,18H2,1-2H3,(H,33,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468160
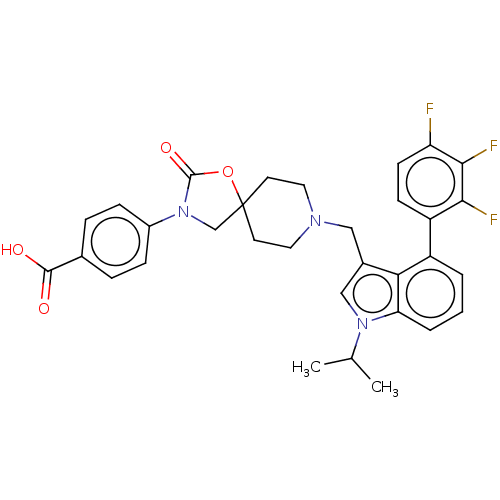
(CHEMBL4293458)Show SMILES CC(C)n1cc(CN2CCC3(CN(C(=O)O3)c3ccc(cc3)C(O)=O)CC2)c2c(cccc12)-c1ccc(F)c(F)c1F Show InChI InChI=1S/C32H30F3N3O4/c1-19(2)37-17-21(27-23(4-3-5-26(27)37)24-10-11-25(33)29(35)28(24)34)16-36-14-12-32(13-15-36)18-38(31(41)42-32)22-8-6-20(7-9-22)30(39)40/h3-11,17,19H,12-16,18H2,1-2H3,(H,39,40) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468136
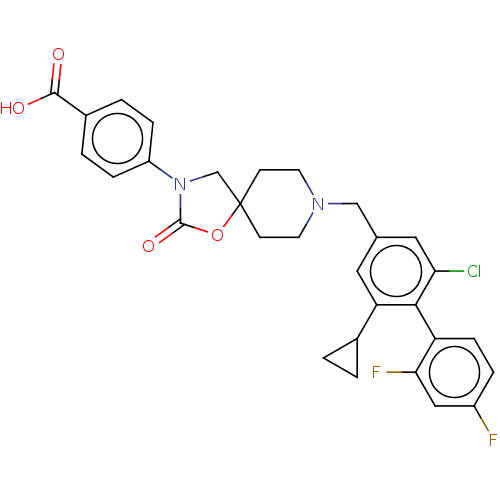
(CHEMBL4287248)Show SMILES OC(=O)c1ccc(cc1)N1CC2(CCN(Cc3cc(Cl)c(c(c3)C3CC3)-c3ccc(F)cc3F)CC2)OC1=O |(21.02,-19.09,;21.18,-20.63,;22.58,-21.25,;19.93,-21.53,;20.1,-23.06,;18.86,-23.97,;17.45,-23.35,;17.28,-21.81,;18.53,-20.9,;16.22,-24.26,;14.73,-23.79,;13.83,-25.05,;13.06,-26.37,;11.51,-26.37,;10.73,-25.04,;9.19,-25.04,;8.42,-23.71,;6.88,-23.71,;6.11,-22.39,;4.58,-22.39,;6.86,-21.06,;8.41,-21.06,;9.2,-22.38,;9.17,-19.71,;9.17,-18.18,;10.5,-18.93,;6.09,-19.73,;6.85,-18.39,;6.08,-17.07,;4.54,-17.07,;3.77,-15.74,;3.76,-18.41,;4.55,-19.73,;3.79,-21.07,;11.51,-23.69,;13.06,-23.69,;14.75,-26.3,;16.22,-25.81,;17.47,-26.71,)| Show InChI InChI=1S/C30H27ClF2N2O4/c31-25-14-18(13-24(19-1-2-19)27(25)23-8-5-21(32)15-26(23)33)16-34-11-9-30(10-12-34)17-35(29(38)39-30)22-6-3-20(4-7-22)28(36)37/h3-8,13-15,19H,1-2,9-12,16-17H2,(H,36,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50374938
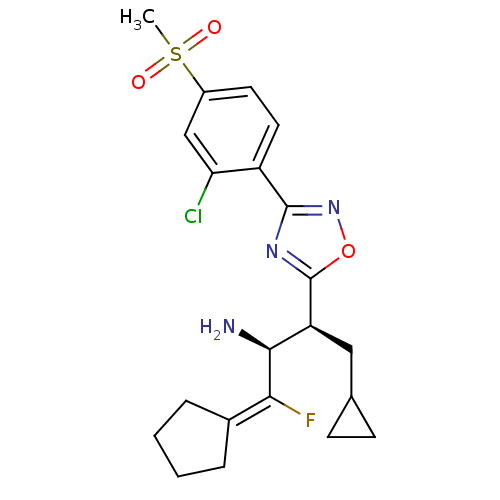
(CHEMBL402163)Show SMILES [#6]S(=O)(=O)c1ccc(-c2noc(n2)-[#6@@H](-[#6]-[#6]-2-[#6]-[#6]-2)-[#6@H](-[#7])-[#6](\F)=[#6]-2/[#6]-[#6]-[#6]-[#6]-2)c(Cl)c1 |r| Show InChI InChI=1S/C21H25ClFN3O3S/c1-30(27,28)14-8-9-15(17(22)11-14)20-25-21(29-26-20)16(10-12-6-7-12)19(24)18(23)13-4-2-3-5-13/h8-9,11-12,16,19H,2-7,10,24H2,1H3/t16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 expressed in insect cell |
Bioorg Med Chem Lett 18: 2409-13 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.050
BindingDB Entry DOI: 10.7270/Q2HD7WJ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468144

(CHEMBL4286244)Show SMILES CC(C)n1cc(CN2CCC3(CN(C(=O)O3)c3ccc(cc3)C(O)=O)CC2)c2c(cccc12)C1CC1 Show InChI InChI=1S/C29H33N3O4/c1-19(2)31-17-22(26-24(20-6-7-20)4-3-5-25(26)31)16-30-14-12-29(13-15-30)18-32(28(35)36-29)23-10-8-21(9-11-23)27(33)34/h3-5,8-11,17,19-20H,6-7,12-16,18H2,1-2H3,(H,33,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM123216
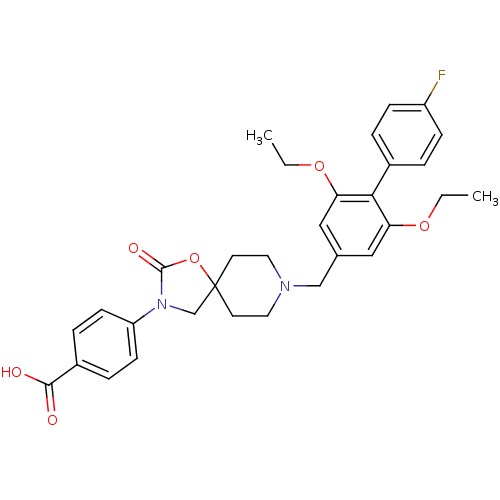
(US8742110, 3-1)Show SMILES CCOc1cc(CN2CCC3(CN(C(=O)O3)c3ccc(cc3)C(O)=O)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C31H33FN2O6/c1-3-38-26-17-21(18-27(39-4-2)28(26)22-5-9-24(32)10-6-22)19-33-15-13-31(14-16-33)20-34(30(37)40-31)25-11-7-23(8-12-25)29(35)36/h5-12,17-18H,3-4,13-16,19-20H2,1-2H3,(H,35,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468136

(CHEMBL4287248)Show SMILES OC(=O)c1ccc(cc1)N1CC2(CCN(Cc3cc(Cl)c(c(c3)C3CC3)-c3ccc(F)cc3F)CC2)OC1=O |(21.02,-19.09,;21.18,-20.63,;22.58,-21.25,;19.93,-21.53,;20.1,-23.06,;18.86,-23.97,;17.45,-23.35,;17.28,-21.81,;18.53,-20.9,;16.22,-24.26,;14.73,-23.79,;13.83,-25.05,;13.06,-26.37,;11.51,-26.37,;10.73,-25.04,;9.19,-25.04,;8.42,-23.71,;6.88,-23.71,;6.11,-22.39,;4.58,-22.39,;6.86,-21.06,;8.41,-21.06,;9.2,-22.38,;9.17,-19.71,;9.17,-18.18,;10.5,-18.93,;6.09,-19.73,;6.85,-18.39,;6.08,-17.07,;4.54,-17.07,;3.77,-15.74,;3.76,-18.41,;4.55,-19.73,;3.79,-21.07,;11.51,-23.69,;13.06,-23.69,;14.75,-26.3,;16.22,-25.81,;17.47,-26.71,)| Show InChI InChI=1S/C30H27ClF2N2O4/c31-25-14-18(13-24(19-1-2-19)27(25)23-8-5-21(32)15-26(23)33)16-34-11-9-30(10-12-34)17-35(29(38)39-30)22-6-3-20(4-7-22)28(36)37/h3-8,13-15,19H,1-2,9-12,16-17H2,(H,36,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468146
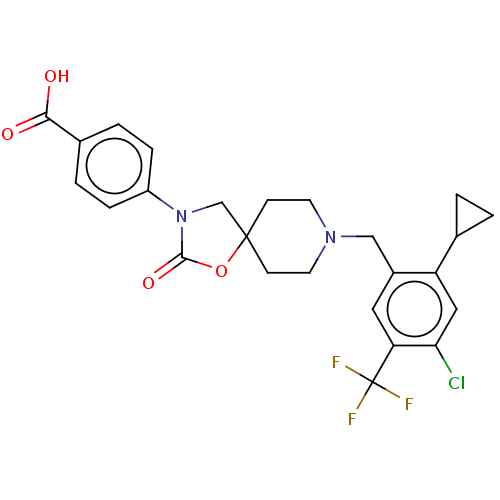
(CHEMBL4285917)Show SMILES OC(=O)c1ccc(cc1)N1CC2(CCN(Cc3cc(c(Cl)cc3C3CC3)C(F)(F)F)CC2)OC1=O Show InChI InChI=1S/C25H24ClF3N2O4/c26-21-12-19(15-1-2-15)17(11-20(21)25(27,28)29)13-30-9-7-24(8-10-30)14-31(23(34)35-24)18-5-3-16(4-6-18)22(32)33/h3-6,11-12,15H,1-2,7-10,13-14H2,(H,32,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468157
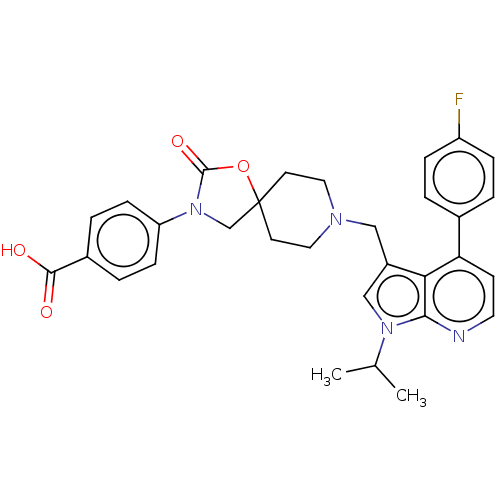
(CHEMBL4289661)Show SMILES CC(C)n1cc(CN2CCC3(CN(C(=O)O3)c3ccc(cc3)C(O)=O)CC2)c2c(ccnc12)-c1ccc(F)cc1 Show InChI InChI=1S/C31H31FN4O4/c1-20(2)35-18-23(27-26(11-14-33-28(27)35)21-3-7-24(32)8-4-21)17-34-15-12-31(13-16-34)19-36(30(39)40-31)25-9-5-22(6-10-25)29(37)38/h3-11,14,18,20H,12-13,15-17,19H2,1-2H3,(H,37,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468157

(CHEMBL4289661)Show SMILES CC(C)n1cc(CN2CCC3(CN(C(=O)O3)c3ccc(cc3)C(O)=O)CC2)c2c(ccnc12)-c1ccc(F)cc1 Show InChI InChI=1S/C31H31FN4O4/c1-20(2)35-18-23(27-26(11-14-33-28(27)35)21-3-7-24(32)8-4-21)17-34-15-12-31(13-16-34)19-36(30(39)40-31)25-9-5-22(6-10-25)29(37)38/h3-11,14,18,20H,12-13,15-17,19H2,1-2H3,(H,37,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468160

(CHEMBL4293458)Show SMILES CC(C)n1cc(CN2CCC3(CN(C(=O)O3)c3ccc(cc3)C(O)=O)CC2)c2c(cccc12)-c1ccc(F)c(F)c1F Show InChI InChI=1S/C32H30F3N3O4/c1-19(2)37-17-21(27-23(4-3-5-26(27)37)24-10-11-25(33)29(35)28(24)34)16-36-14-12-32(13-15-36)18-38(31(41)42-32)22-8-6-20(7-9-22)30(39)40/h3-11,17,19H,12-16,18H2,1-2H3,(H,39,40) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50468129
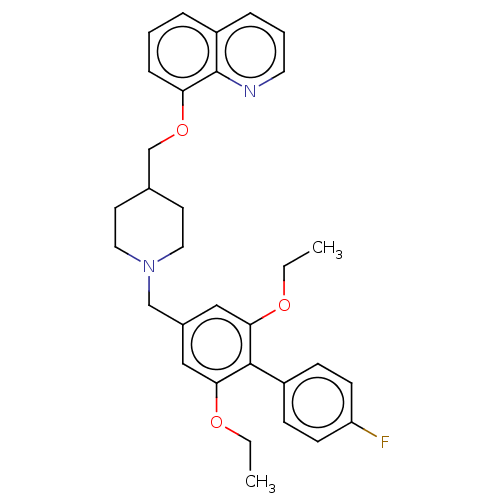
(CHEMBL4288848)Show SMILES CCOc1cc(CN2CCC(COc3cccc4cccnc34)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O3/c1-3-36-29-19-24(20-30(37-4-2)31(29)25-10-12-27(33)13-11-25)21-35-17-14-23(15-18-35)22-38-28-9-5-7-26-8-6-16-34-32(26)28/h5-13,16,19-20,23H,3-4,14-15,17-18,21-22H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468153

(CHEMBL4279392)Show SMILES CC1(C)CCSc2ccc(CN3CCC4(CN(C(=O)O4)c4ccc(cc4)C(O)=O)CC3)cc12 Show InChI InChI=1S/C26H30N2O4S/c1-25(2)11-14-33-22-8-3-18(15-21(22)25)16-27-12-9-26(10-13-27)17-28(24(31)32-26)20-6-4-19(5-7-20)23(29)30/h3-8,15H,9-14,16-17H2,1-2H3,(H,29,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468133
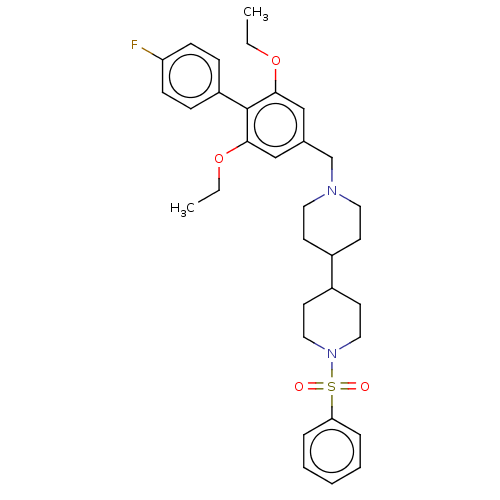
(CHEMBL4278161)Show SMILES CCOc1cc(CN2CCC(CC2)C2CCN(CC2)S(=O)(=O)c2ccccc2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C33H41FN2O4S/c1-3-39-31-22-25(23-32(40-4-2)33(31)28-10-12-29(34)13-11-28)24-35-18-14-26(15-19-35)27-16-20-36(21-17-27)41(37,38)30-8-6-5-7-9-30/h5-13,22-23,26-27H,3-4,14-21,24H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468159

(CHEMBL4278328)Show SMILES CC(C)n1cc(CN2CCC3(CN(C(=O)C3)c3ccc(cc3)C(O)=O)CC2)c2c(cccc12)C1CC1 Show InChI InChI=1S/C30H35N3O3/c1-20(2)32-18-23(28-25(21-6-7-21)4-3-5-26(28)32)17-31-14-12-30(13-15-31)16-27(34)33(19-30)24-10-8-22(9-11-24)29(35)36/h3-5,8-11,18,20-21H,6-7,12-17,19H2,1-2H3,(H,35,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468146

(CHEMBL4285917)Show SMILES OC(=O)c1ccc(cc1)N1CC2(CCN(Cc3cc(c(Cl)cc3C3CC3)C(F)(F)F)CC2)OC1=O Show InChI InChI=1S/C25H24ClF3N2O4/c26-21-12-19(15-1-2-15)17(11-20(21)25(27,28)29)13-30-9-7-24(8-10-30)14-31(23(34)35-24)18-5-3-16(4-6-18)22(32)33/h3-6,11-12,15H,1-2,7-10,13-14H2,(H,32,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM123216

(US8742110, 3-1)Show SMILES CCOc1cc(CN2CCC3(CN(C(=O)O3)c3ccc(cc3)C(O)=O)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C31H33FN2O6/c1-3-38-26-17-21(18-27(39-4-2)28(26)22-5-9-24(32)10-6-22)19-33-15-13-31(14-16-33)20-34(30(37)40-31)25-11-7-23(8-12-25)29(35)36/h5-12,17-18H,3-4,13-16,19-20H2,1-2H3,(H,35,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50468133

(CHEMBL4278161)Show SMILES CCOc1cc(CN2CCC(CC2)C2CCN(CC2)S(=O)(=O)c2ccccc2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C33H41FN2O4S/c1-3-39-31-22-25(23-32(40-4-2)33(31)28-10-12-29(34)13-11-28)24-35-18-14-26(15-19-35)27-16-20-36(21-17-27)41(37,38)30-8-6-5-7-9-30/h5-13,22-23,26-27H,3-4,14-21,24H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468150
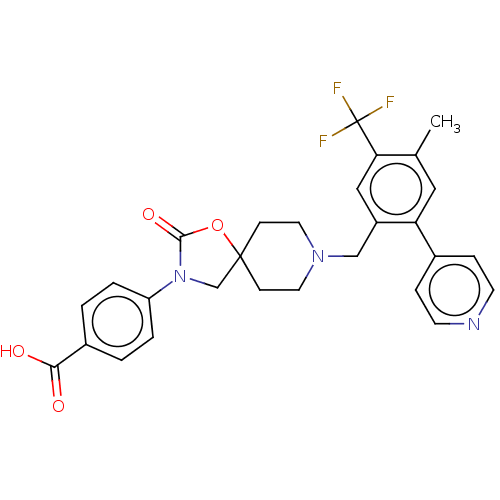
(CHEMBL4277006)Show SMILES Cc1cc(c(CN2CCC3(CN(C(=O)O3)c3ccc(cc3)C(O)=O)CC2)cc1C(F)(F)F)-c1ccncc1 Show InChI InChI=1S/C28H26F3N3O4/c1-18-14-23(19-6-10-32-11-7-19)21(15-24(18)28(29,30)31)16-33-12-8-27(9-13-33)17-34(26(37)38-27)22-4-2-20(3-5-22)25(35)36/h2-7,10-11,14-15H,8-9,12-13,16-17H2,1H3,(H,35,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468159

(CHEMBL4278328)Show SMILES CC(C)n1cc(CN2CCC3(CN(C(=O)C3)c3ccc(cc3)C(O)=O)CC2)c2c(cccc12)C1CC1 Show InChI InChI=1S/C30H35N3O3/c1-20(2)32-18-23(28-25(21-6-7-21)4-3-5-26(28)32)17-31-14-12-30(13-15-31)16-27(34)33(19-30)24-10-8-22(9-11-24)29(35)36/h3-5,8-11,18,20-21H,6-7,12-17,19H2,1-2H3,(H,35,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50468128

(CHEMBL4282052)Show SMILES OC(=O)C(F)(F)F.CCOc1cc(CN2CCC3(CN(C(=O)C3)c3ccc(cc3)C(O)=O)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O5/c1-3-39-27-17-22(18-28(40-4-2)30(27)23-5-9-25(33)10-6-23)20-34-15-13-32(14-16-34)19-29(36)35(21-32)26-11-7-24(8-12-26)31(37)38/h5-12,17-18H,3-4,13-16,19-21H2,1-2H3,(H,37,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse SSTR5 expressed in CHO-K1 cell membranes assessed as reduction in SST-28-induced inhibition of forskolin-stimulated cAMP... |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50468139
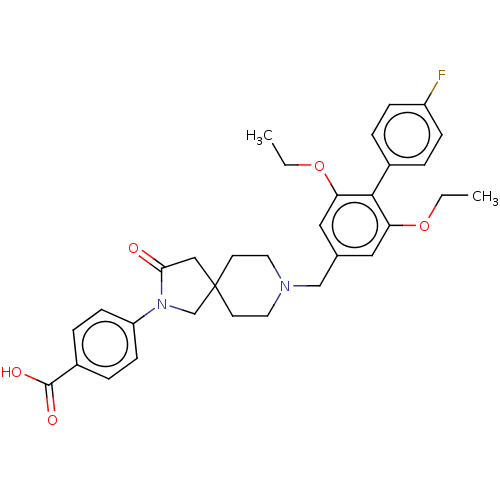
(CHEMBL4288391)Show SMILES CCOc1cc(CN2CCC3(CN(C(=O)C3)c3ccc(cc3)C(O)=O)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O5/c1-3-39-27-17-22(18-28(40-4-2)30(27)23-5-9-25(33)10-6-23)20-34-15-13-32(14-16-34)19-29(36)35(21-32)26-11-7-24(8-12-26)31(37)38/h5-12,17-18H,3-4,13-16,19-21H2,1-2H3,(H,37,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50468160

(CHEMBL4293458)Show SMILES CC(C)n1cc(CN2CCC3(CN(C(=O)O3)c3ccc(cc3)C(O)=O)CC2)c2c(cccc12)-c1ccc(F)c(F)c1F Show InChI InChI=1S/C32H30F3N3O4/c1-19(2)37-17-21(27-23(4-3-5-26(27)37)24-10-11-25(33)29(35)28(24)34)16-36-14-12-32(13-15-36)18-38(31(41)42-32)22-8-6-20(7-9-22)30(39)40/h3-11,17,19H,12-16,18H2,1-2H3,(H,39,40) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Mus musculus) | BDBM50322981
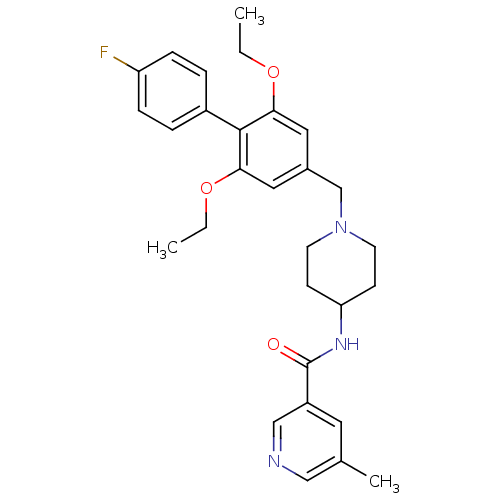
(CHEMBL1210207 | N-(1-((2,6-diethoxy-4'-fluorobiphe...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C29H34FN3O3/c1-4-35-26-15-21(16-27(36-5-2)28(26)22-6-8-24(30)9-7-22)19-33-12-10-25(11-13-33)32-29(34)23-14-20(3)17-31-18-23/h6-9,14-18,25H,4-5,10-13,19H2,1-3H3,(H,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from mouse SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468145

(CHEMBL4278915)Show SMILES Cn1cc(cn1)-c1ccc(cc1CN1CCC2(CN(C(=O)O2)c2ccc(cc2)C(O)=O)CC1)C(F)(F)F Show InChI InChI=1S/C26H25F3N4O4/c1-31-14-19(13-30-31)22-7-4-20(26(27,28)29)12-18(22)15-32-10-8-25(9-11-32)16-33(24(36)37-25)21-5-2-17(3-6-21)23(34)35/h2-7,12-14H,8-11,15-16H2,1H3,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468139

(CHEMBL4288391)Show SMILES CCOc1cc(CN2CCC3(CN(C(=O)C3)c3ccc(cc3)C(O)=O)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O5/c1-3-39-27-17-22(18-28(40-4-2)30(27)23-5-9-25(33)10-6-23)20-34-15-13-32(14-16-34)19-29(36)35(21-32)26-11-7-24(8-12-26)31(37)38/h5-12,17-18H,3-4,13-16,19-21H2,1-2H3,(H,37,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM123274
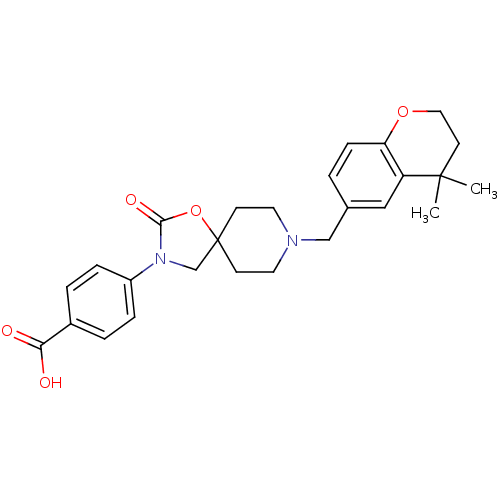
(US8742110, 5-10)Show SMILES CC1(C)CCOc2ccc(CN3CCC4(CN(C(=O)O4)c4ccc(cc4)C(O)=O)CC3)cc12 Show InChI InChI=1S/C26H30N2O5/c1-25(2)11-14-32-22-8-3-18(15-21(22)25)16-27-12-9-26(10-13-27)17-28(24(31)33-26)20-6-4-19(5-7-20)23(29)30/h3-8,15H,9-14,16-17H2,1-2H3,(H,29,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468128

(CHEMBL4282052)Show SMILES OC(=O)C(F)(F)F.CCOc1cc(CN2CCC3(CN(C(=O)C3)c3ccc(cc3)C(O)=O)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O5/c1-3-39-27-17-22(18-28(40-4-2)30(27)23-5-9-25(33)10-6-23)20-34-15-13-32(14-16-34)19-29(36)35(21-32)26-11-7-24(8-12-26)31(37)38/h5-12,17-18H,3-4,13-16,19-21H2,1-2H3,(H,37,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSTR5 expressed in CHO-K1 cell membranes assessed as reduction in SST-28-induced inhibition of forskolin-stimulated cAMP... |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468128

(CHEMBL4282052)Show SMILES OC(=O)C(F)(F)F.CCOc1cc(CN2CCC3(CN(C(=O)C3)c3ccc(cc3)C(O)=O)CC2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C32H35FN2O5/c1-3-39-27-17-22(18-28(40-4-2)30(27)23-5-9-25(33)10-6-23)20-34-15-13-32(14-16-34)19-29(36)35(21-32)26-11-7-24(8-12-26)31(37)38/h5-12,17-18H,3-4,13-16,19-21H2,1-2H3,(H,37,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of (3-125I-Tyr11)-SRIF-28 from human SSTR5 expressed in CHO-K1 cell membranes by filtration binding assay |
ACS Med Chem Lett 9: 1082-1087 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00305
BindingDB Entry DOI: 10.7270/Q2ZP48TS |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cSrc by ELISA |
Bioorg Med Chem 16: 5890-8 (2008)
Article DOI: 10.1016/j.bmc.2008.04.065
BindingDB Entry DOI: 10.7270/Q2Z60PZ6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50468154
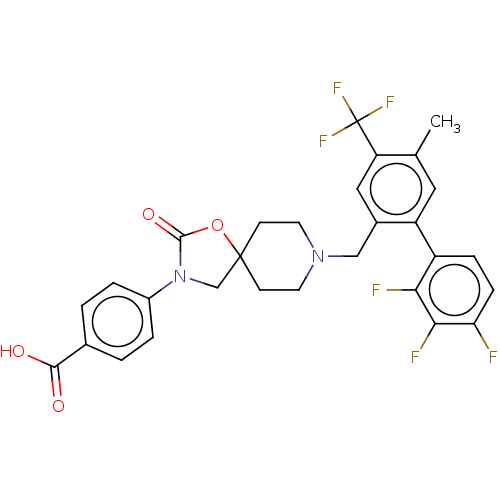
(CHEMBL4284956)Show SMILES Cc1cc(c(CN2CCC3(CN(C(=O)O3)c3ccc(cc3)C(O)=O)CC2)cc1C(F)(F)F)-c1ccc(F)c(F)c1F Show InChI InChI=1S/C29H24F6N2O4/c1-16-12-21(20-6-7-23(30)25(32)24(20)31)18(13-22(16)29(33,34)35)14-36-10-8-28(9-11-36)15-37(27(40)41-28)19-4-2-17(3-5-19)26(38)39/h2-7,12-13H,8-11,14-15H2,1H3,(H,38,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes |
ACS Med Chem Lett 9: 1088-1093 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00306
BindingDB Entry DOI: 10.7270/Q2V127H3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data