

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21130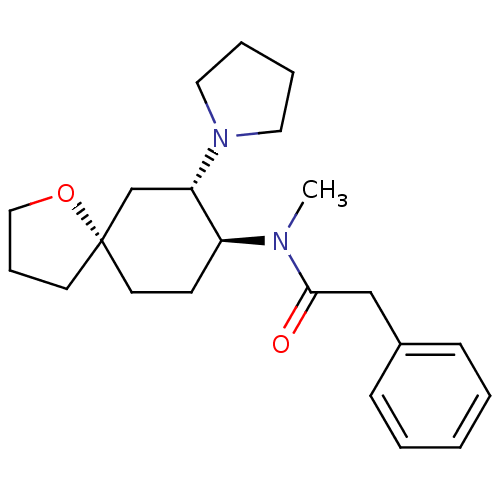 (N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 11.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University Curated by PDSP Ki Database | Biochemistry 44: 8643-51 (2005) Article DOI: 10.1021/bi050490d BindingDB Entry DOI: 10.7270/Q2QN65BH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21130 (N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 23.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University Curated by PDSP Ki Database | Biochemistry 44: 8643-51 (2005) Article DOI: 10.1021/bi050490d BindingDB Entry DOI: 10.7270/Q2QN65BH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM86548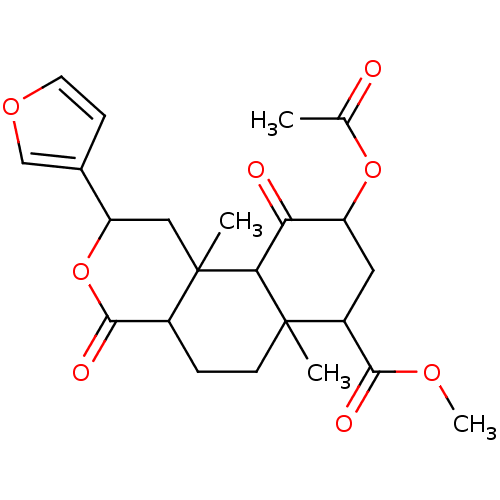 (CAS_83729-01-5 | NSC_128563 | Salvinorin A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University Curated by PDSP Ki Database | Biochemistry 44: 8643-51 (2005) Article DOI: 10.1021/bi050490d BindingDB Entry DOI: 10.7270/Q2QN65BH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM86548 (CAS_83729-01-5 | NSC_128563 | Salvinorin A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 45.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University Curated by PDSP Ki Database | Biochemistry 44: 8643-51 (2005) Article DOI: 10.1021/bi050490d BindingDB Entry DOI: 10.7270/Q2QN65BH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM224024 (BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University Curated by PDSP Ki Database | Biochemistry 44: 8643-51 (2005) Article DOI: 10.1021/bi050490d BindingDB Entry DOI: 10.7270/Q2QN65BH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50303652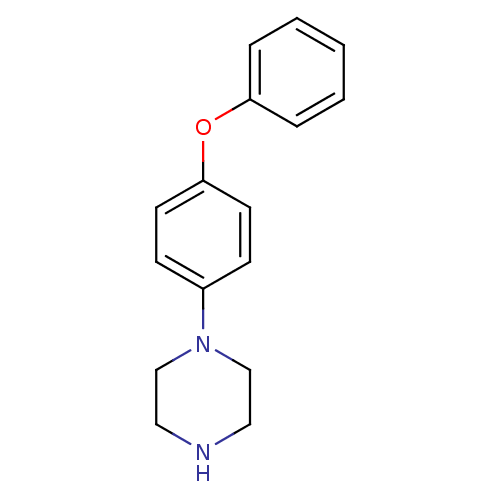 (1-(4-Phenoxyphenyl)piperazine | CHEMBL576512) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4H hydrolysis assessed as inhibition of LTB4 formation by LC-MS/MS | J Med Chem 53: 573-85 (2010) Checked by Author Article DOI: 10.1021/jm900838g BindingDB Entry DOI: 10.7270/Q22F7NJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50096154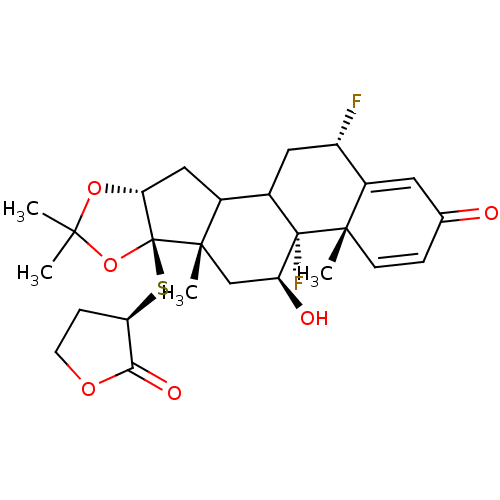 (4b,12-Difluoro-5-hydroxy-4a,6a,8,8-tetramethyl-6b-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Affinity for human glucocorticoid receptor | J Med Chem 44: 602-12 (2001) BindingDB Entry DOI: 10.7270/Q2GM86JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50303656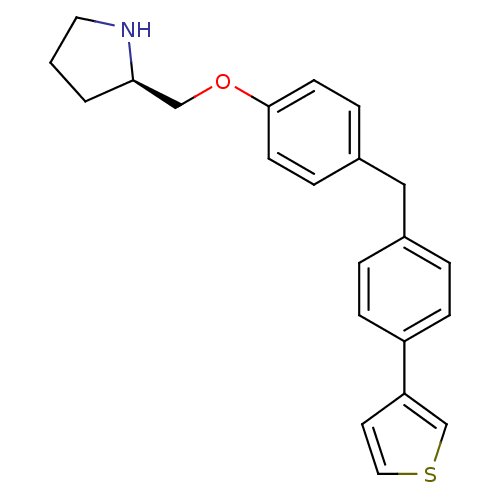 ((R)-2-[4-(4-Thiophen-3-yl-benzyl)phenoxymethyl]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4H hydrolysis assessed as inhibition of LTB4 formation by LC-MS/MS | J Med Chem 53: 573-85 (2010) Checked by Author Article DOI: 10.1021/jm900838g BindingDB Entry DOI: 10.7270/Q22F7NJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50096153 (4b,12-Difluoro-5-hydroxy-4a,6a-dimethyl-6b-(2-oxo-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Affinity for human glucocorticoid receptor | J Med Chem 44: 602-12 (2001) BindingDB Entry DOI: 10.7270/Q2GM86JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50303657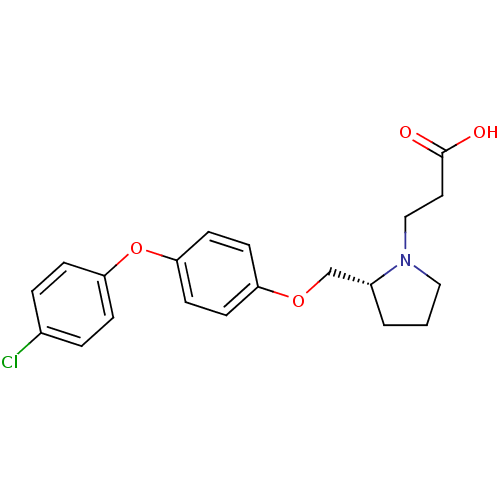 (3-{(R)-2-[4-(4-Chlorophenoxy)phenoxymethyl]pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Inhibition of human LTA4H hydrolysis assessed as inhibition of Ca2+ ionophore-stimulated LTB4 formation in human whole blood by ELISA | J Med Chem 53: 573-85 (2010) Checked by Author Article DOI: 10.1021/jm900838g BindingDB Entry DOI: 10.7270/Q22F7NJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50303650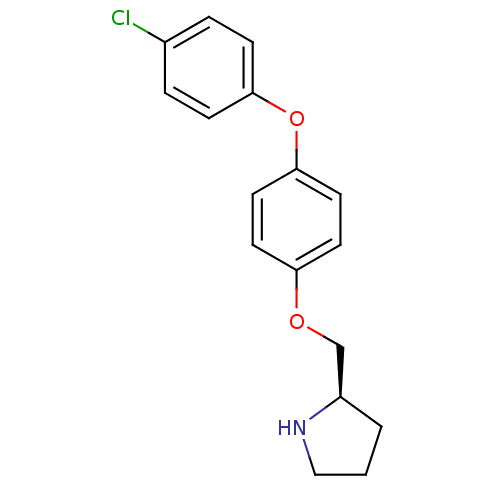 ((R)-2-[4-(4-Chlorophenoxy)phenoxymethyl]pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4H hydrolysis assessed as inhibition of LTB4 formation by LC-MS/MS | J Med Chem 53: 573-85 (2010) Checked by Author Article DOI: 10.1021/jm900838g BindingDB Entry DOI: 10.7270/Q22F7NJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50294161 ((R)-4-(2-((4-(4-chlorophenoxy)phenoxy)methyl)pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Inhibition of human LTA4H hydrolysis assessed as inhibition of Ca2+ ionophore-stimulated LTB4 formation in human whole blood by ELISA | J Med Chem 53: 573-85 (2010) Checked by Author Article DOI: 10.1021/jm900838g BindingDB Entry DOI: 10.7270/Q22F7NJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50303649 (4-{(S)-2-[4-(4-Chlorophenoxy)phenoxymethyl]pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Inhibition of human LTA4H hydrolysis assessed as inhibition of Ca2+ ionophore-stimulated LTB4 formation in human whole blood by ELISA | J Med Chem 53: 573-85 (2010) Checked by Author Article DOI: 10.1021/jm900838g BindingDB Entry DOI: 10.7270/Q22F7NJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50303649 (4-{(S)-2-[4-(4-Chlorophenoxy)phenoxymethyl]pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4H hydrolysis assessed as inhibition of LTB4 formation by LC-MS/MS | J Med Chem 53: 573-85 (2010) Checked by Author Article DOI: 10.1021/jm900838g BindingDB Entry DOI: 10.7270/Q22F7NJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50294161 ((R)-4-(2-((4-(4-chlorophenoxy)phenoxy)methyl)pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE biostructures, Inc. Curated by ChEMBL | Assay Description Inhibition of peptidase activity of human recombinant LTA4H expressed in Escherichia coli BL21-AI/pRARE | J Med Chem 52: 4694-715 (2009) Article DOI: 10.1021/jm900259h BindingDB Entry DOI: 10.7270/Q2F47P6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50294161 ((R)-4-(2-((4-(4-chlorophenoxy)phenoxy)methyl)pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE biostructures, Inc. Curated by ChEMBL | Assay Description Inhibition of hydrolase activity of human recombinant LTA4H expressed in Escherichia coli BL21-AI/pRARE assessed as LTB4 formation by tandem quadrupo... | J Med Chem 52: 4694-715 (2009) Article DOI: 10.1021/jm900259h BindingDB Entry DOI: 10.7270/Q2F47P6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50294162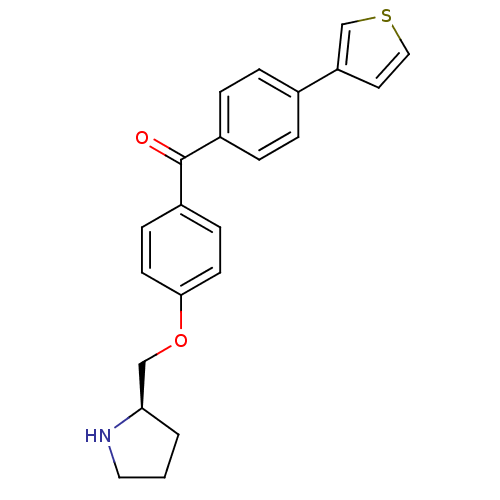 ((R)-(4-(pyrrolidin-2-ylmethoxy)phenyl)(4-(thiophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE biostructures, Inc. Curated by ChEMBL | Assay Description Inhibition of peptidase activity of human recombinant LTA4H expressed in Escherichia coli BL21-AI/pRARE | J Med Chem 52: 4694-715 (2009) Article DOI: 10.1021/jm900259h BindingDB Entry DOI: 10.7270/Q2F47P6N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50303648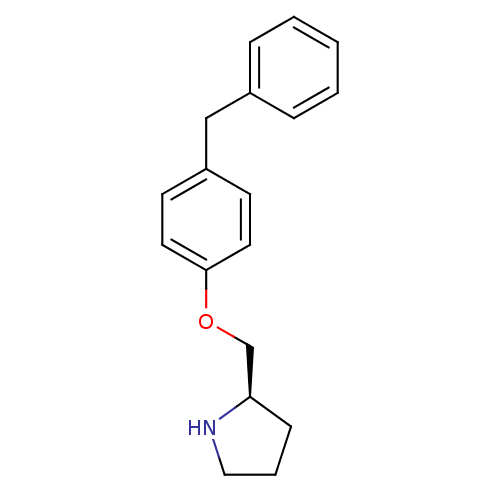 ((2R)-2-[(4-benzylphenoxy)methyl]pyrrolidine | (R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4H hydrolysis assessed as inhibition of LTB4 formation by LC-MS/MS | J Med Chem 53: 573-85 (2010) Checked by Author Article DOI: 10.1021/jm900838g BindingDB Entry DOI: 10.7270/Q22F7NJD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50294163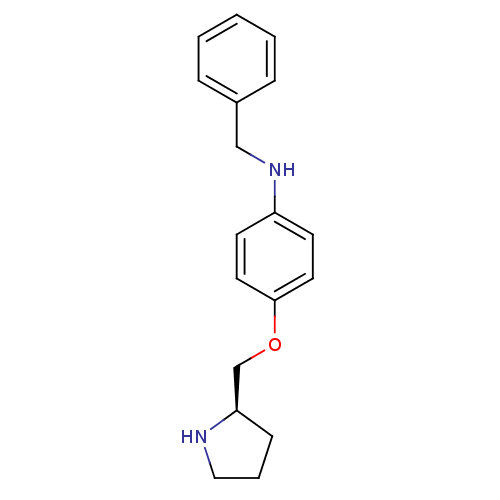 ((R)-N-benzyl-4-(pyrrolidin-2-ylmethoxy)aniline | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE biostructures, Inc. Curated by ChEMBL | Assay Description Inhibition of hydrolase activity of human recombinant LTA4H expressed in Escherichia coli BL21-AI/pRARE assessed as LTB4 formation by tandem quadrupo... | J Med Chem 52: 4694-715 (2009) Article DOI: 10.1021/jm900259h BindingDB Entry DOI: 10.7270/Q2F47P6N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50294162 ((R)-(4-(pyrrolidin-2-ylmethoxy)phenyl)(4-(thiophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE biostructures, Inc. Curated by ChEMBL | Assay Description Inhibition of hydrolase activity of human recombinant LTA4H expressed in Escherichia coli BL21-AI/pRARE assessed as LTB4 formation by tandem quadrupo... | J Med Chem 52: 4694-715 (2009) Article DOI: 10.1021/jm900259h BindingDB Entry DOI: 10.7270/Q2F47P6N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50294163 ((R)-N-benzyl-4-(pyrrolidin-2-ylmethoxy)aniline | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE biostructures, Inc. Curated by ChEMBL | Assay Description Inhibition of peptidase activity of human recombinant LTA4H expressed in Escherichia coli BL21-AI/pRARE | J Med Chem 52: 4694-715 (2009) Article DOI: 10.1021/jm900259h BindingDB Entry DOI: 10.7270/Q2F47P6N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50303658 ((2S)-2-[(4-Benzylphenoxy)methyl]pyrrolidine | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 244 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4H hydrolysis assessed as inhibition of LTB4 formation by LC-MS/MS | J Med Chem 53: 573-85 (2010) Checked by Author Article DOI: 10.1021/jm900838g BindingDB Entry DOI: 10.7270/Q22F7NJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426317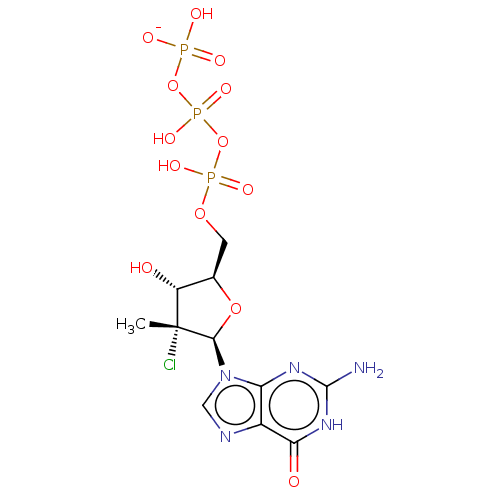 (US10513534, Compound 405) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426315 (US10513534, Compound 403) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426319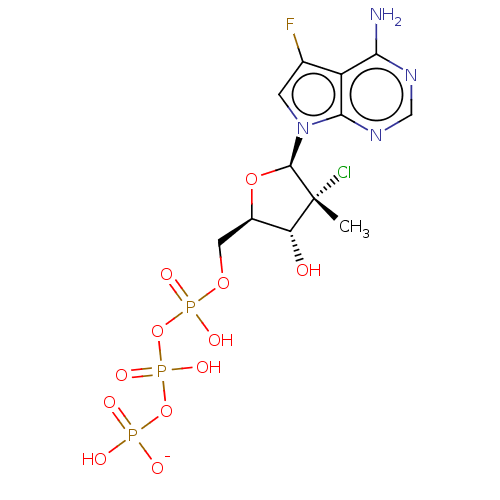 (US10513534, Compound 407) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426318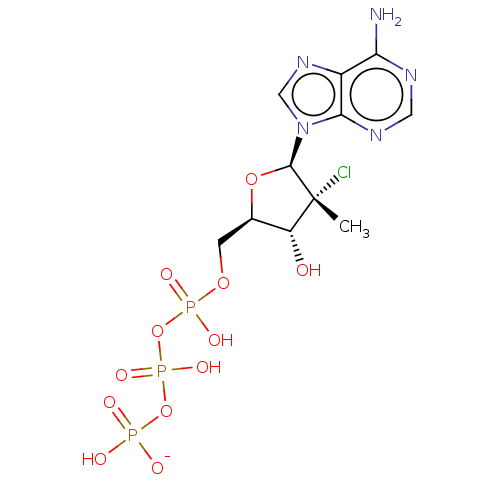 (US10513534, Compound 406) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426312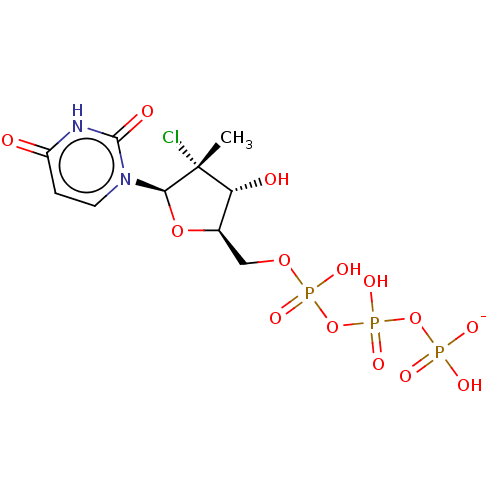 (US10513534, Compound 401) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50096155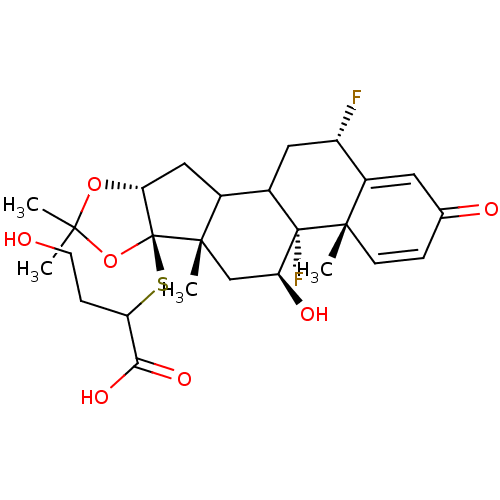 (2-(4b,12-Difluoro-5-hydroxy-4a,6a,8,8-tetramethyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 289 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Affinity for human glucocorticoid receptor | J Med Chem 44: 602-12 (2001) BindingDB Entry DOI: 10.7270/Q2GM86JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50303651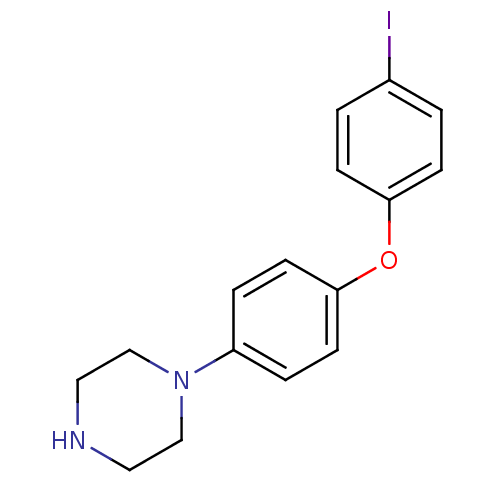 (1-[4-(4-Iodophenoxy)phenyl]piperazine | CHEMBL5672...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4H hydrolysis assessed as inhibition of LTB4 formation by LC-MS/MS | J Med Chem 53: 573-85 (2010) Checked by Author Article DOI: 10.1021/jm900838g BindingDB Entry DOI: 10.7270/Q22F7NJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50096156 (2-(4b,12-Difluoro-5-hydroxy-4a,6a-dimethyl-2-oxo-8...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Affinity for human glucocorticoid receptor | J Med Chem 44: 602-12 (2001) BindingDB Entry DOI: 10.7270/Q2GM86JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50303648 ((2R)-2-[(4-benzylphenoxy)methyl]pyrrolidine | (R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 449 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Inhibition of human LTA4H hydrolysis assessed as inhibition of Ca2+ ionophore-stimulated LTB4 formation in human whole blood by ELISA | J Med Chem 53: 573-85 (2010) Checked by Author Article DOI: 10.1021/jm900838g BindingDB Entry DOI: 10.7270/Q22F7NJD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50303650 ((R)-2-[4-(4-Chlorophenoxy)phenoxymethyl]pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 533 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Inhibition of human LTA4H hydrolysis assessed as inhibition of Ca2+ ionophore-stimulated LTB4 formation in human whole blood by ELISA | J Med Chem 53: 573-85 (2010) Checked by Author Article DOI: 10.1021/jm900838g BindingDB Entry DOI: 10.7270/Q22F7NJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426314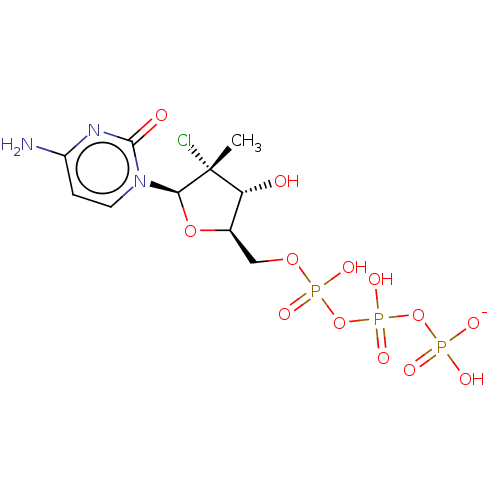 (US10513534, Compound 402) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426322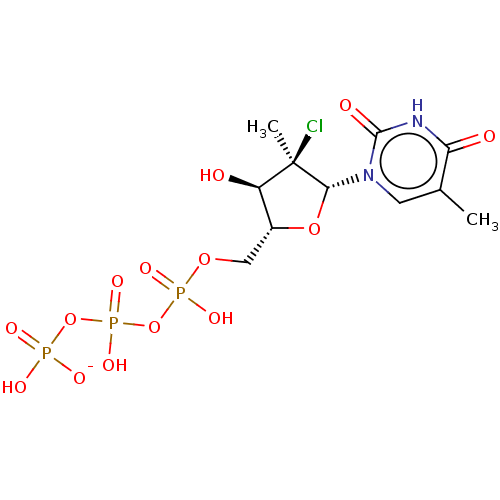 (US10513534, Compound 410) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426323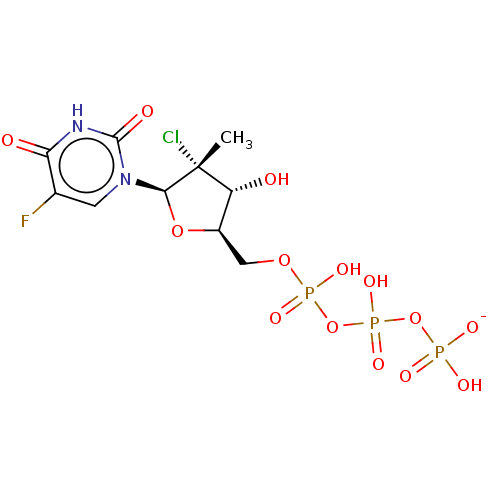 (US10513534, Compound 411) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426312 (US10513534, Compound 401) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426315 (US10513534, Compound 403) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426317 (US10513534, Compound 405) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426318 (US10513534, Compound 406) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426319 (US10513534, Compound 407) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426320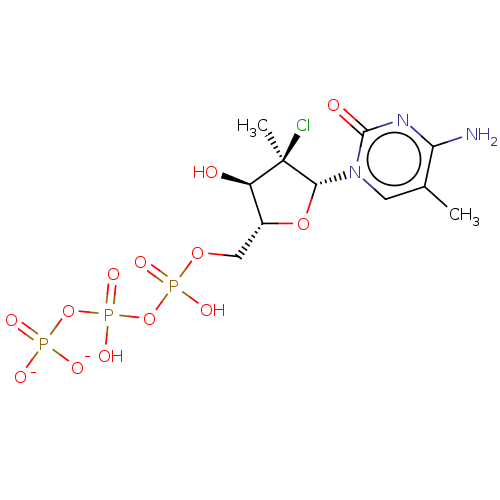 (US10513534, Compound 408) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426321 (US10513534, Compound 409) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50303653 (4-[2-(4-Benzylphenoxy)ethyl]pyridine | CHEMBL57813...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4H hydrolysis assessed as inhibition of LTB4 formation by LC-MS/MS | J Med Chem 53: 573-85 (2010) Checked by Author Article DOI: 10.1021/jm900838g BindingDB Entry DOI: 10.7270/Q22F7NJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426316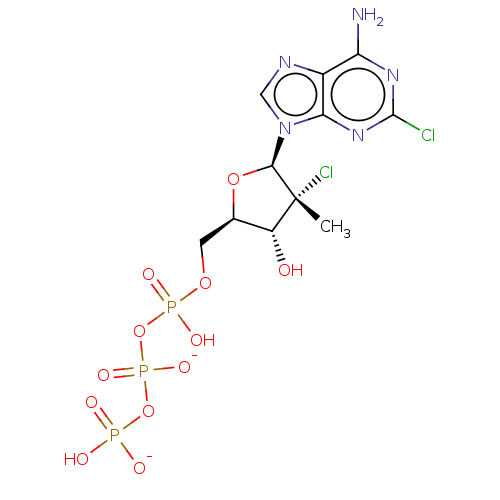 (US10513534, Compound 404) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426316 (US10513534, Compound 404) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50294164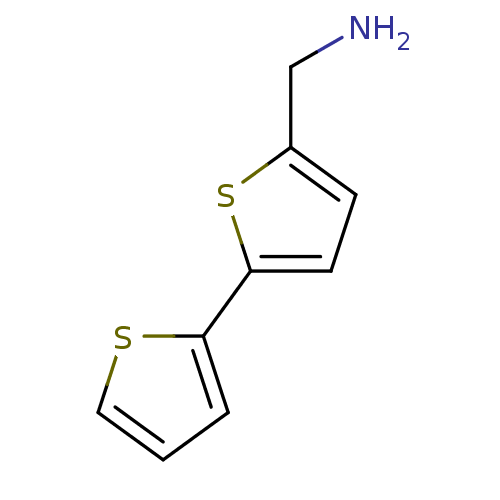 (1-(2,2'-bithiophen-5-yl)methanamine | 2,2'-bithiop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE biostructures, Inc. Curated by ChEMBL | Assay Description Inhibition of peptidase activity of human recombinant LTA4H expressed in Escherichia coli BL21-AI/pRARE | J Med Chem 52: 4694-715 (2009) Article DOI: 10.1021/jm900259h BindingDB Entry DOI: 10.7270/Q2F47P6N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50294164 (1-(2,2'-bithiophen-5-yl)methanamine | 2,2'-bithiop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 7.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE biostructures, Inc. Curated by ChEMBL | Assay Description Inhibition of hydrolase activity of human recombinant LTA4H expressed in Escherichia coli BL21-AI/pRARE assessed as LTB4 formation by tandem quadrupo... | J Med Chem 52: 4694-715 (2009) Article DOI: 10.1021/jm900259h BindingDB Entry DOI: 10.7270/Q2F47P6N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50294165 ((4-(thiophen-2-yl)phenyl)methanamine | 1-(4-thioph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4H hydrolysis assessed as inhibition of LTB4 formation by LC-MS/MS | J Med Chem 53: 573-85 (2010) Checked by Author Article DOI: 10.1021/jm900838g BindingDB Entry DOI: 10.7270/Q22F7NJD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50294165 ((4-(thiophen-2-yl)phenyl)methanamine | 1-(4-thioph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE biostructures, Inc. Curated by ChEMBL | Assay Description Inhibition of hydrolase activity of human recombinant LTA4H expressed in Escherichia coli BL21-AI/pRARE assessed as LTB4 formation by tandem quadrupo... | J Med Chem 52: 4694-715 (2009) Article DOI: 10.1021/jm900259h BindingDB Entry DOI: 10.7270/Q2F47P6N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50294165 ((4-(thiophen-2-yl)phenyl)methanamine | 1-(4-thioph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE biostructures, Inc. Curated by ChEMBL | Assay Description Inhibition of peptidase activity of human recombinant LTA4H expressed in Escherichia coli BL21-AI/pRARE | J Med Chem 52: 4694-715 (2009) Article DOI: 10.1021/jm900259h BindingDB Entry DOI: 10.7270/Q2F47P6N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 87 total ) | Next | Last >> |