 Found 906 hits with Last Name = 'chiba' and Initial = 'k'
Found 906 hits with Last Name = 'chiba' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Plasmepsin I
(Plasmodium falciparum) | BDBM8005
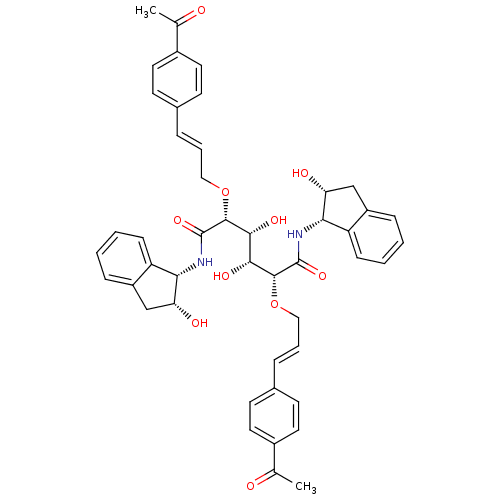
((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(4-acetylphenyl)pro...)Show SMILES CC(=O)c1ccc(\C=C\CO[C@H]([C@H](O)[C@@H](O)[C@@H](OC\C=C\c2ccc(cc2)C(C)=O)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)cc1 |r| Show InChI InChI=1S/C46H48N2O10/c1-27(49)31-19-15-29(16-20-31)9-7-23-57-43(45(55)47-39-35-13-5-3-11-33(35)25-37(39)51)41(53)42(54)44(58-24-8-10-30-17-21-32(22-18-30)28(2)50)46(56)48-40-36-14-6-4-12-34(36)26-38(40)52/h3-22,37-44,51-54H,23-26H2,1-2H3,(H,47,55)(H,48,56)/b9-7+,10-8+/t37-,38-,39+,40+,41-,42-,43-,44-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zambia
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Plasmodium falciparum Plasmepsin 1 |
J Med Chem 63: 4445-4467 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01622
BindingDB Entry DOI: 10.7270/Q2RR22K7 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM22202

(S1P | [32P]S1P | [33P]S1P | sphingosine-1-phosphat...)Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.920 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM22202

(S1P | [32P]S1P | [33P]S1P | sphingosine-1-phosphat...)Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | -47.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367879

(LISINOPRIL)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACE C-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.10 | -46.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.20 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM22202

(S1P | [32P]S1P | [33P]S1P | sphingosine-1-phosphat...)Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14- | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 2
(Homo sapiens (Human)) | BDBM22202

(S1P | [32P]S1P | [33P]S1P | sphingosine-1-phosphat...)Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | -44.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.10 | -44.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.20 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367879

(LISINOPRIL)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACE N-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM22202

(S1P | [32P]S1P | [33P]S1P | sphingosine-1-phosphat...)Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 5.90 | -43.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasmepsin II
(Plasmodium falciparum) | BDBM8005

((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(4-acetylphenyl)pro...)Show SMILES CC(=O)c1ccc(\C=C\CO[C@H]([C@H](O)[C@@H](O)[C@@H](OC\C=C\c2ccc(cc2)C(C)=O)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)cc1 |r| Show InChI InChI=1S/C46H48N2O10/c1-27(49)31-19-15-29(16-20-31)9-7-23-57-43(45(55)47-39-35-13-5-3-11-33(35)25-37(39)51)41(53)42(54)44(58-24-8-10-30-17-21-32(22-18-30)28(2)50)46(56)48-40-36-14-6-4-12-34(36)26-38(40)52/h3-22,37-44,51-54H,23-26H2,1-2H3,(H,47,55)(H,48,56)/b9-7+,10-8+/t37-,38-,39+,40+,41-,42-,43-,44-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zambia
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Plasmodium falciparum Plasmepsin 2 |
J Med Chem 63: 4445-4467 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01622
BindingDB Entry DOI: 10.7270/Q2RR22K7 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50189452
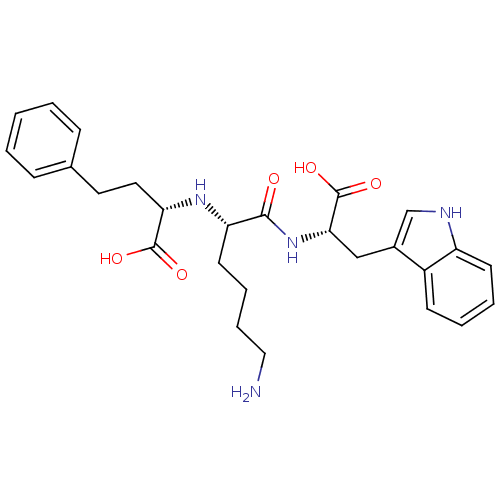
((S)-2-((S)-6-amino-1-((S)-1-carboxy-2-(1H-indol-3-...)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C27H34N4O5/c28-15-7-6-12-22(30-23(26(33)34)14-13-18-8-2-1-3-9-18)25(32)31-24(27(35)36)16-19-17-29-21-11-5-4-10-20(19)21/h1-5,8-11,17,22-24,29-30H,6-7,12-16,28H2,(H,31,32)(H,33,34)(H,35,36)/t22-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACE C-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 13 | -41.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 23 | -40.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50583450

(CHEMBL5077796)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACE C-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcium-dependent protein kinase 1
(Plasmodium Falciparum) | BDBM50321310

(CHEMBL4175786 | US10688093, Compound 229_0226_0284...)Show InChI InChI=1S/C15H16N4O/c1-2-8-16-14-6-7-15-17-10-13(19(15)18-14)11-4-3-5-12(20)9-11/h3-7,9-10,20H,2,8H2,1H3,(H,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant CDPK1 expressed in Escherichia coli expression system after 1 hr in presence of [gamma33P]ATP by scin... |
J Med Chem 61: 8061-8077 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00329
BindingDB Entry DOI: 10.7270/Q24170NC |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 37 | -39.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50017125
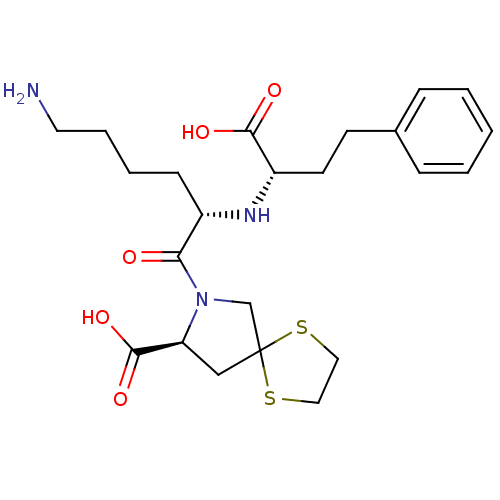
(1-[6-Amino-2-(1-carboxy-3-phenyl-propylamino)-hexa...)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CC2(C[C@H]1C(O)=O)SCCS2 Show InChI InChI=1S/C23H33N3O5S2/c24-11-5-4-8-17(25-18(21(28)29)10-9-16-6-2-1-3-7-16)20(27)26-15-23(32-12-13-33-23)14-19(26)22(30)31/h1-3,6-7,17-19,25H,4-5,8-15,24H2,(H,28,29)(H,30,31)/t17-,18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of rabbit testis recombinant ACE C domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50017125

(1-[6-Amino-2-(1-carboxy-3-phenyl-propylamino)-hexa...)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CC2(C[C@H]1C(O)=O)SCCS2 Show InChI InChI=1S/C23H33N3O5S2/c24-11-5-4-8-17(25-18(21(28)29)10-9-16-6-2-1-3-7-16)20(27)26-15-23(32-12-13-33-23)14-19(26)22(30)31/h1-3,6-7,17-19,25H,4-5,8-15,24H2,(H,28,29)(H,30,31)/t17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of testis ACE C domain |
Bioorg Med Chem Lett 16: 4616-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.004
BindingDB Entry DOI: 10.7270/Q2KP81SJ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50583449
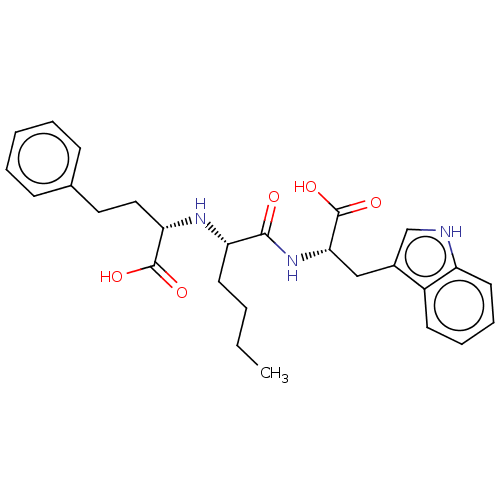
(CHEMBL5075705)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACE C-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM23164
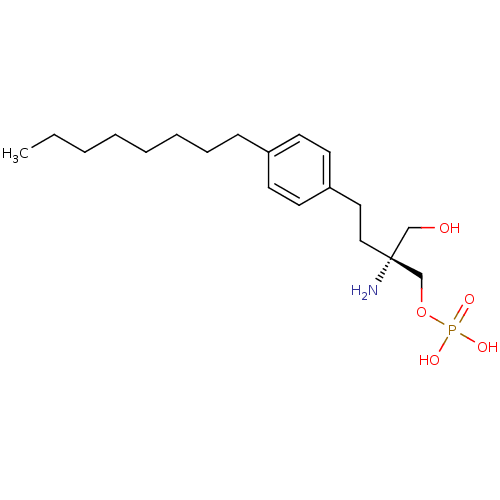
(CHEMBL190006 | FTY720-phosphate, (R)-2 | [(2R)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 105 | -37.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50583450

(CHEMBL5077796)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACE N-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM23164

(CHEMBL190006 | FTY720-phosphate, (R)-2 | [(2R)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 121 | -36.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50017125

(1-[6-Amino-2-(1-carboxy-3-phenyl-propylamino)-hexa...)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CC2(C[C@H]1C(O)=O)SCCS2 Show InChI InChI=1S/C23H33N3O5S2/c24-11-5-4-8-17(25-18(21(28)29)10-9-16-6-2-1-3-7-16)20(27)26-15-23(32-12-13-33-23)14-19(26)22(30)31/h1-3,6-7,17-19,25H,4-5,8-15,24H2,(H,28,29)(H,30,31)/t17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ACE N domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50017125

(1-[6-Amino-2-(1-carboxy-3-phenyl-propylamino)-hexa...)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CC2(C[C@H]1C(O)=O)SCCS2 Show InChI InChI=1S/C23H33N3O5S2/c24-11-5-4-8-17(25-18(21(28)29)10-9-16-6-2-1-3-7-16)20(27)26-15-23(32-12-13-33-23)14-19(26)22(30)31/h1-3,6-7,17-19,25H,4-5,8-15,24H2,(H,28,29)(H,30,31)/t17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of somatic ACE N domain |
Bioorg Med Chem Lett 16: 4616-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.004
BindingDB Entry DOI: 10.7270/Q2KP81SJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM8005

((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(4-acetylphenyl)pro...)Show SMILES CC(=O)c1ccc(\C=C\CO[C@H]([C@H](O)[C@@H](O)[C@@H](OC\C=C\c2ccc(cc2)C(C)=O)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)cc1 |r| Show InChI InChI=1S/C46H48N2O10/c1-27(49)31-19-15-29(16-20-31)9-7-23-57-43(45(55)47-39-35-13-5-3-11-33(35)25-37(39)51)41(53)42(54)44(58-24-8-10-30-17-21-32(22-18-30)28(2)50)46(56)48-40-36-14-6-4-12-34(36)26-38(40)52/h3-22,37-44,51-54H,23-26H2,1-2H3,(H,47,55)(H,48,56)/b9-7+,10-8+/t37-,38-,39+,40+,41-,42-,43-,44-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zambia
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
J Med Chem 63: 4445-4467 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01622
BindingDB Entry DOI: 10.7270/Q2RR22K7 |
More data for this
Ligand-Target Pair | |
Calcium-dependent protein kinase 1
(Plasmodium Falciparum) | BDBM50321309

(CHEMBL4165173)Show InChI InChI=1S/C17H15N3O2/c1-10-5-7-11(8-6-10)16(21)15-14(18)13(17(19)22)12-4-2-3-9-20(12)15/h2-9H,18H2,1H3,(H2,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 262 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant CDPK1 expressed in Escherichia coli expression system after 1 hr in presence of [gamma33P]ATP by scin... |
J Med Chem 61: 8061-8077 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00329
BindingDB Entry DOI: 10.7270/Q24170NC |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23164

(CHEMBL190006 | FTY720-phosphate, (R)-2 | [(2R)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 277 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50583451
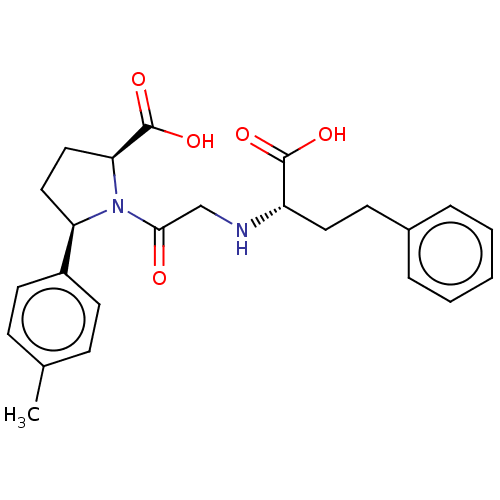
(CHEMBL5075686)Show SMILES Cc1ccc(cc1)[C@H]1CC[C@H](N1C(=O)CN[C@@H](CCc1ccccc1)C(O)=O)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACE C-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM23164

(CHEMBL190006 | FTY720-phosphate, (R)-2 | [(2R)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 586 | -33.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50583450

(CHEMBL5077796)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hGH-8His-NEP expressed in CHO cells preincubated for 15 mins followed by MCA-RPPGFDAFK-(Dnp)-OH peptide substrate addition by fluoresce... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50189517

((S)-2-((S)-5-benzamido-4-oxo-6-phenylhexanamido)-3...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C30H29N3O5/c34-27(25(17-20-9-3-1-4-10-20)33-29(36)21-11-5-2-6-12-21)15-16-28(35)32-26(30(37)38)18-22-19-31-24-14-8-7-13-23(22)24/h1-14,19,25-26,31H,15-18H2,(H,32,35)(H,33,36)(H,37,38)/t25-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of rabbit testis recombinant ACE C domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 2
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+3 | >-31.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 2
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | >-31.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 2
(Homo sapiens (Human)) | BDBM23164

(CHEMBL190006 | FTY720-phosphate, (R)-2 | [(2R)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | >-31.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Mitsubishi Pharma Corporation
| Assay Description
Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated ... |
Bioorg Med Chem 13: 425-32 (2005)
Article DOI: 10.1016/j.bmc.2004.10.008
BindingDB Entry DOI: 10.7270/Q257199Q |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50189452

((S)-2-((S)-6-amino-1-((S)-1-carboxy-2-(1H-indol-3-...)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C27H34N4O5/c28-15-7-6-12-22(30-23(26(33)34)14-13-18-8-2-1-3-9-18)25(32)31-24(27(35)36)16-19-17-29-21-11-5-4-10-20(19)21/h1-5,8-11,17,22-24,29-30H,6-7,12-16,28H2,(H,31,32)(H,33,34)(H,35,36)/t22-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACE N-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50027344
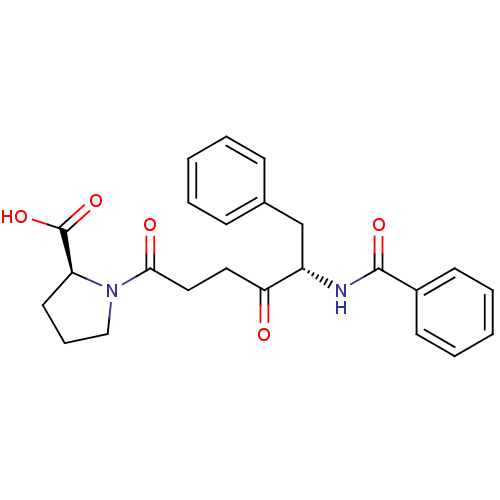
((S)-1-((S)-5-benzamido-4-oxo-6-phenylhexanoyl)pyrr...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C24H26N2O5/c27-21(13-14-22(28)26-15-7-12-20(26)24(30)31)19(16-17-8-3-1-4-9-17)25-23(29)18-10-5-2-6-11-18/h1-6,8-11,19-20H,7,12-16H2,(H,25,29)(H,30,31)/t19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of rabbit testis recombinant ACE C domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50583451

(CHEMBL5075686)Show SMILES Cc1ccc(cc1)[C@H]1CC[C@H](N1C(=O)CN[C@@H](CCc1ccccc1)C(O)=O)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACE N-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50583449

(CHEMBL5075705)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACE N-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50189452

((S)-2-((S)-6-amino-1-((S)-1-carboxy-2-(1H-indol-3-...)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C27H34N4O5/c28-15-7-6-12-22(30-23(26(33)34)14-13-18-8-2-1-3-9-18)25(32)31-24(27(35)36)16-19-17-29-21-11-5-4-10-20(19)21/h1-5,8-11,17,22-24,29-30H,6-7,12-16,28H2,(H,31,32)(H,33,34)(H,35,36)/t22-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of testis ACE C domain |
Bioorg Med Chem Lett 16: 4616-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.004
BindingDB Entry DOI: 10.7270/Q2KP81SJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50189520
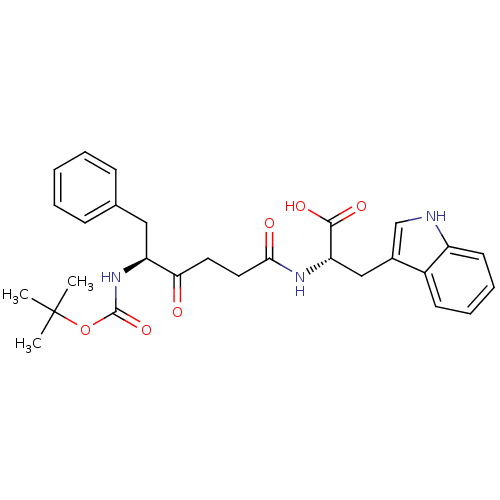
((S)-2-((S)-5-(tert-butoxycarbonyl)-4-oxo-6-phenylh...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)CCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C28H33N3O6/c1-28(2,3)37-27(36)31-22(15-18-9-5-4-6-10-18)24(32)13-14-25(33)30-23(26(34)35)16-19-17-29-21-12-8-7-11-20(19)21/h4-12,17,22-23,29H,13-16H2,1-3H3,(H,30,33)(H,31,36)(H,34,35)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ACE N domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50583449

(CHEMBL5075705)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hGH-8His-NEP expressed in CHO cells preincubated for 15 mins followed by MCA-RPPGFDAFK-(Dnp)-OH peptide substrate addition by fluoresce... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50189519
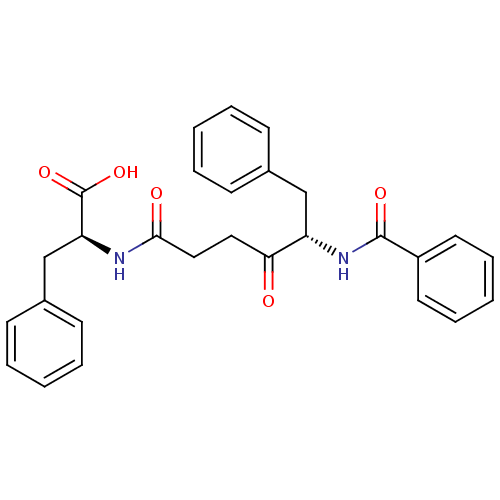
((S)-2-((S)-5-benzamido-4-oxo-6-phenylhexanamido)-3...)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)CCC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C28H28N2O5/c31-25(16-17-26(32)29-24(28(34)35)19-21-12-6-2-7-13-21)23(18-20-10-4-1-5-11-20)30-27(33)22-14-8-3-9-15-22/h1-15,23-24H,16-19H2,(H,29,32)(H,30,33)(H,34,35)/t23-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of rabbit testis recombinant ACE C domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50189454

((R)-2-((S)-6-amino-1-((S)-1-carboxy-2-(1H-indol-3-...)Show SMILES NCCCC[C@H](N[C@H](CCc1ccccc1)C(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C27H34N4O5/c28-15-7-6-12-22(30-23(26(33)34)14-13-18-8-2-1-3-9-18)25(32)31-24(27(35)36)16-19-17-29-21-11-5-4-10-20(19)21/h1-5,8-11,17,22-24,29-30H,6-7,12-16,28H2,(H,31,32)(H,33,34)(H,35,36)/t22-,23+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of testis ACE C domain |
Bioorg Med Chem Lett 16: 4616-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.004
BindingDB Entry DOI: 10.7270/Q2KP81SJ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50189518
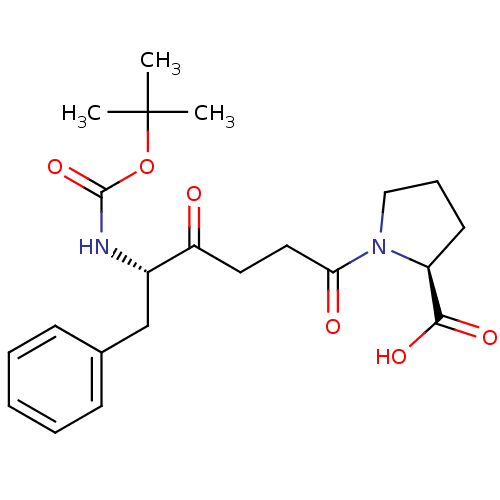
((S)-1-((S)-5-(tert-butoxycarbonyl)-4-oxo-6-phenylh...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)CCC(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C22H30N2O6/c1-22(2,3)30-21(29)23-16(14-15-8-5-4-6-9-15)18(25)11-12-19(26)24-13-7-10-17(24)20(27)28/h4-6,8-9,16-17H,7,10-14H2,1-3H3,(H,23,29)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ACE N domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50189521
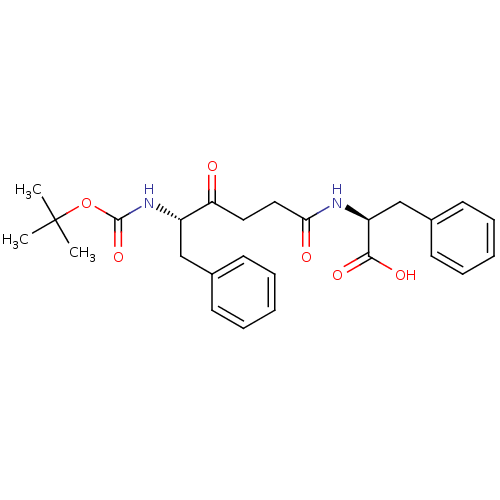
((S)-2-((S)-5-(tert-butoxycarbonyl)-4-oxo-6-phenylh...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)CCC(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C26H32N2O6/c1-26(2,3)34-25(33)28-20(16-18-10-6-4-7-11-18)22(29)14-15-23(30)27-21(24(31)32)17-19-12-8-5-9-13-19/h4-13,20-21H,14-17H2,1-3H3,(H,27,30)(H,28,33)(H,31,32)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ACE N domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50027344

((S)-1-((S)-5-benzamido-4-oxo-6-phenylhexanoyl)pyrr...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C24H26N2O5/c27-21(13-14-22(28)26-15-7-12-20(26)24(30)31)19(16-17-8-3-1-4-9-17)25-23(29)18-10-5-2-6-11-18/h1-6,8-11,19-20H,7,12-16H2,(H,25,29)(H,30,31)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ACE N domain |
Bioorg Med Chem Lett 16: 4612-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.003
BindingDB Entry DOI: 10.7270/Q2B56JC8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data