

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM13760 ((1R,3R,4S,7S)-4-[2-(4-chlorophenoxy)-2-methylpropa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM13768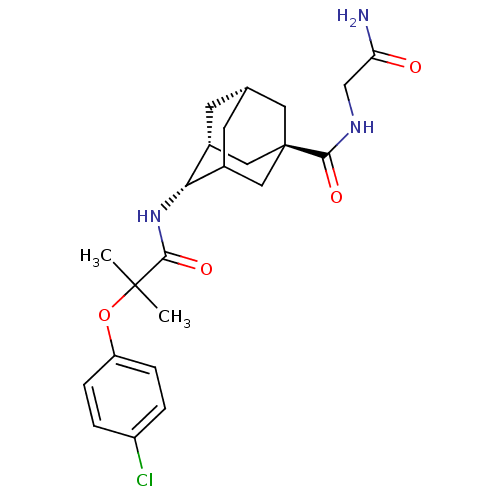 (N-[(1R,2S,5R,7S)-5-[(carbamoylmethyl)carbamoyl]ada...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM13756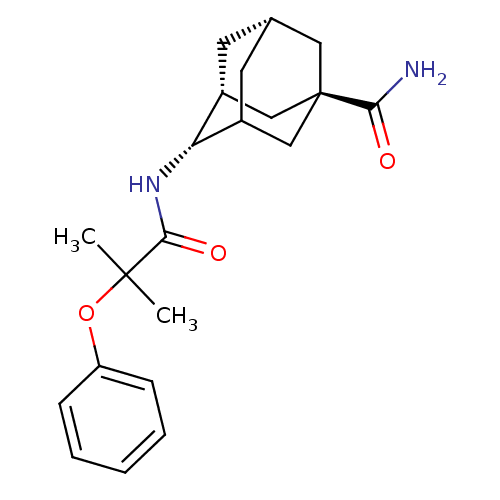 ((1R,3R,4S,7S)-4-(2-methyl-2-phenoxypropanamido)ada...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM13761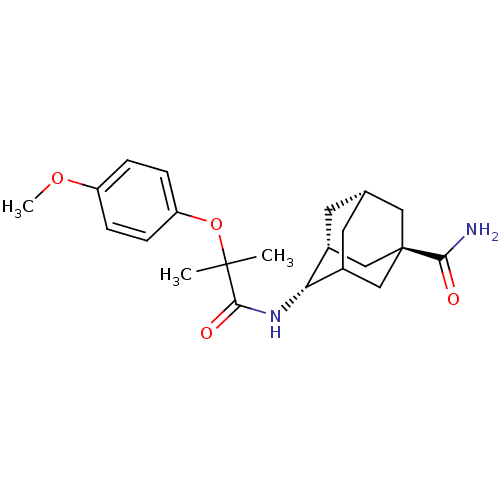 ((1R,3R,4S,7S)-4-[2-(4-methoxyphenoxy)-2-methylprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM20722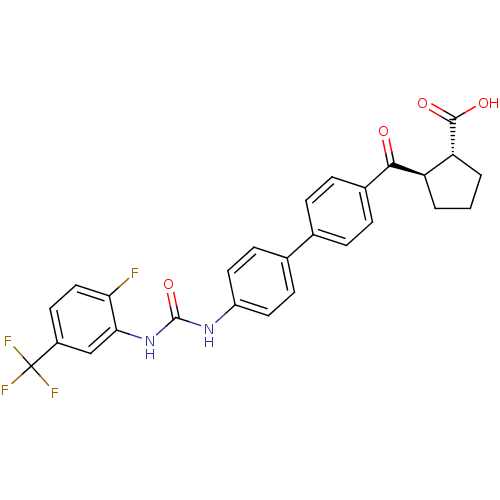 ((1R,2R)-2-({4-[4-({[2-fluoro-5-(trifluoromethyl)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description DGAT-1 activity was characterized by catalyzing the transfer of the radiolabeled decanoyl group onto the syn-3 position of didecanoyl glycerol. The r... | J Med Chem 51: 380-3 (2008) Article DOI: 10.1021/jm7013887 BindingDB Entry DOI: 10.7270/Q2KW5DB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM13756 ((1R,3R,4S,7S)-4-(2-methyl-2-phenoxypropanamido)ada...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50174908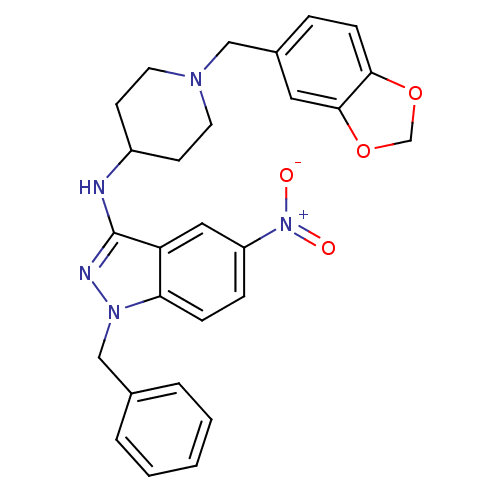 (CHEMBL427367 | N-(1-(benzo[d][1,3]dioxol-5-ylmethy...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to human MCHr1 from neuronal IMR32 cells | Bioorg Med Chem Lett 15: 5293-7 (2005) Article DOI: 10.1016/j.bmcl.2005.08.049 BindingDB Entry DOI: 10.7270/Q2GQ6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM13760 ((1R,3R,4S,7S)-4-[2-(4-chlorophenoxy)-2-methylpropa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM20721 ((1R,2R)-2-{[4-(4-{[(3-chlorophenyl)carbamoyl]amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description DGAT-1 activity was characterized by catalyzing the transfer of the radiolabeled decanoyl group onto the syn-3 position of didecanoyl glycerol. The r... | J Med Chem 51: 380-3 (2008) Article DOI: 10.1021/jm7013887 BindingDB Entry DOI: 10.7270/Q2KW5DB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM13770 (2-(4-chlorophenoxy)-N-[(1R,2S,5R,7S)-5-(hydroxymet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50174921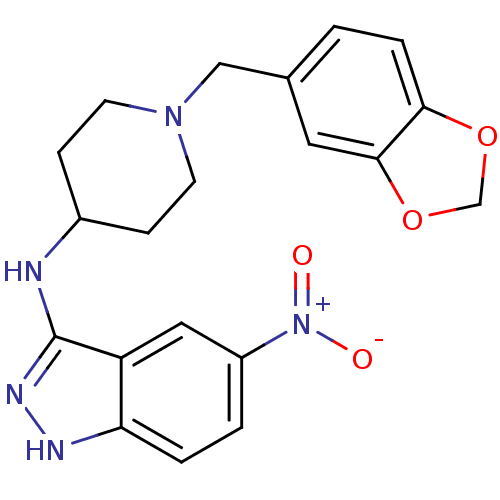 (CHEMBL196973 | N-(1-(benzo[d][1,3]dioxol-5-ylmethy...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to human MCHr1 from neuronal IMR32 cells | Bioorg Med Chem Lett 15: 5293-7 (2005) Article DOI: 10.1016/j.bmcl.2005.08.049 BindingDB Entry DOI: 10.7270/Q2GQ6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM13761 ((1R,3R,4S,7S)-4-[2-(4-methoxyphenoxy)-2-methylprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM20716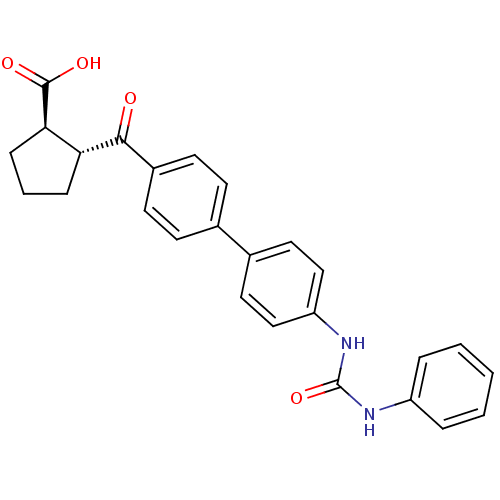 ((1R,2R)-2-[(4-{4-[(phenylcarbamoyl)amino]phenyl}ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description DGAT-1 activity was characterized by catalyzing the transfer of the radiolabeled decanoyl group onto the syn-3 position of didecanoyl glycerol. The r... | J Med Chem 51: 380-3 (2008) Article DOI: 10.1021/jm7013887 BindingDB Entry DOI: 10.7270/Q2KW5DB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM20727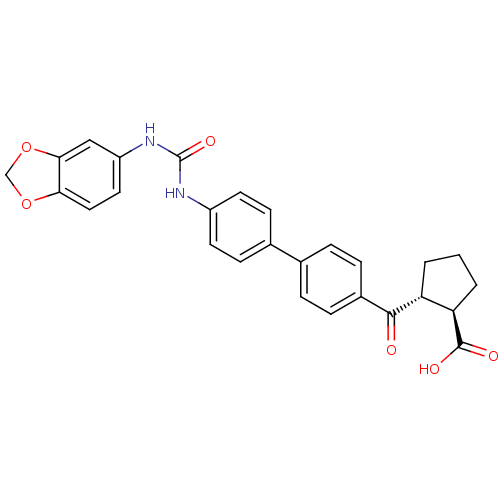 ((1R,2R)-2-[(4-{4-[(2H-1,3-benzodioxol-5-ylcarbamoy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description DGAT-1 activity was characterized by catalyzing the transfer of the radiolabeled decanoyl group onto the syn-3 position of didecanoyl glycerol. The r... | J Med Chem 51: 380-3 (2008) Article DOI: 10.1021/jm7013887 BindingDB Entry DOI: 10.7270/Q2KW5DB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50174920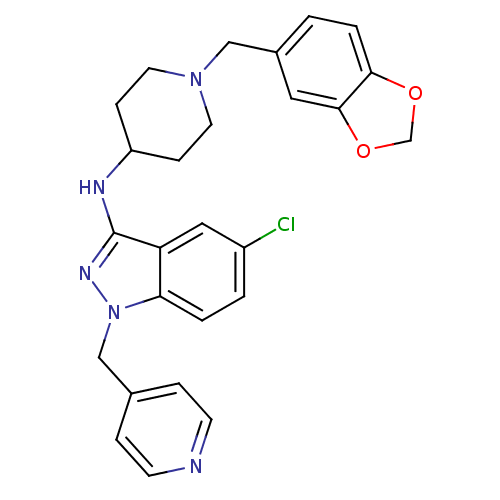 (CHEMBL199025 | N-(1-(benzo[d][1,3]dioxol-5-ylmethy...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to human MCHr1 from neuronal IMR32 cells | Bioorg Med Chem Lett 15: 5293-7 (2005) Article DOI: 10.1016/j.bmcl.2005.08.049 BindingDB Entry DOI: 10.7270/Q2GQ6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM13757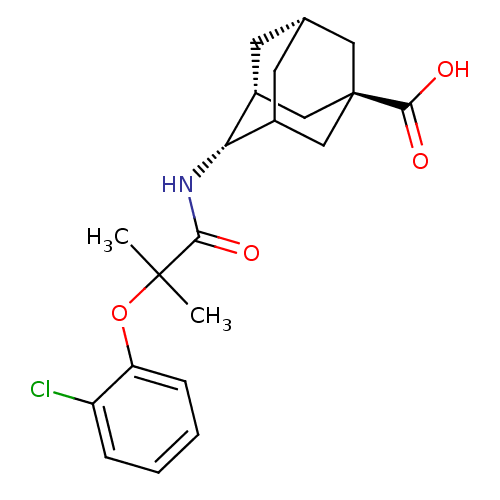 ((1R,3R,4S,7S)-4-[2-(2-chlorophenoxy)-2-methylpropa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM13757 ((1R,3R,4S,7S)-4-[2-(2-chlorophenoxy)-2-methylpropa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50174919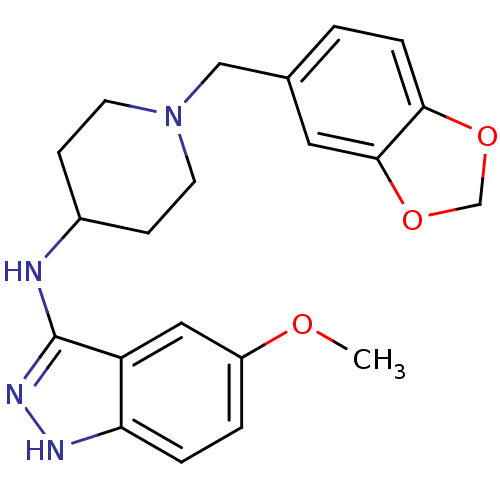 (CHEMBL371222 | N-(1-(benzo[d][1,3]dioxol-5-ylmethy...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to human MCHr1 from neuronal IMR32 cells | Bioorg Med Chem Lett 15: 5293-7 (2005) Article DOI: 10.1016/j.bmcl.2005.08.049 BindingDB Entry DOI: 10.7270/Q2GQ6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM13769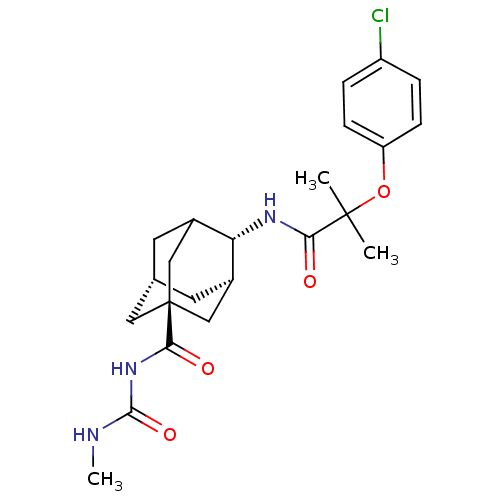 (2-(4-chlorophenoxy)-2-methyl-N-[(1R,2S,5R,7S)-5-{[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM20724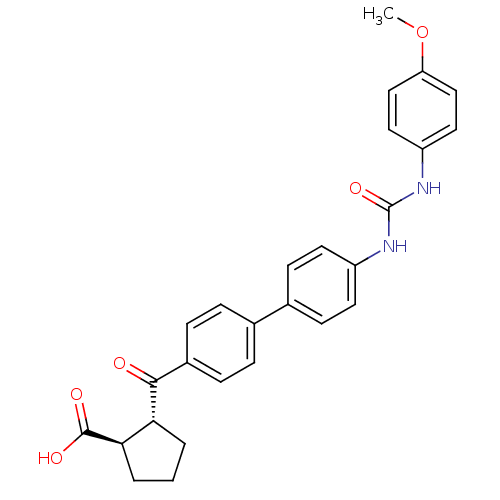 ((1R,2R)-2-{[4-(4-{[(4-methoxyphenyl)carbamoyl]amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description DGAT-1 activity was characterized by catalyzing the transfer of the radiolabeled decanoyl group onto the syn-3 position of didecanoyl glycerol. The r... | J Med Chem 51: 380-3 (2008) Article DOI: 10.1021/jm7013887 BindingDB Entry DOI: 10.7270/Q2KW5DB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM20726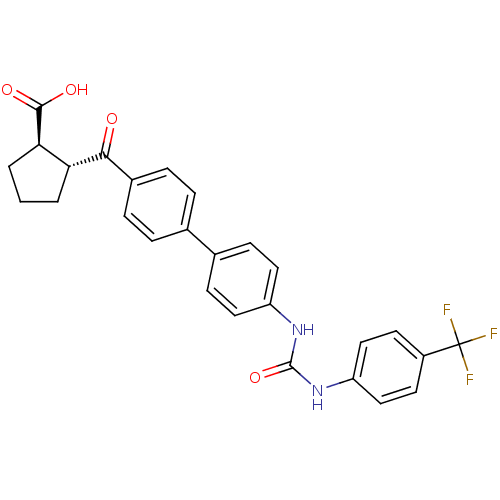 ((1R,2R)-2-({4-[4-({[4-(trifluoromethyl)phenyl]carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description DGAT-1 activity was characterized by catalyzing the transfer of the radiolabeled decanoyl group onto the syn-3 position of didecanoyl glycerol. The r... | J Med Chem 51: 380-3 (2008) Article DOI: 10.1021/jm7013887 BindingDB Entry DOI: 10.7270/Q2KW5DB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50174913 (CHEMBL197232 | N-(1-(benzo[d][1,3]dioxol-5-ylmethy...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to human MCHr1 from neuronal IMR32 cells | Bioorg Med Chem Lett 15: 5293-7 (2005) Article DOI: 10.1016/j.bmcl.2005.08.049 BindingDB Entry DOI: 10.7270/Q2GQ6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM13770 (2-(4-chlorophenoxy)-N-[(1R,2S,5R,7S)-5-(hydroxymet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM20718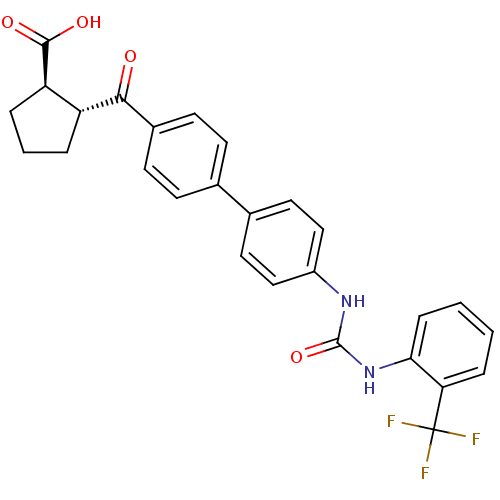 ((1R,2R)-2-({4-[4-({[2-(trifluoromethyl)phenyl]carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description DGAT-1 activity was characterized by catalyzing the transfer of the radiolabeled decanoyl group onto the syn-3 position of didecanoyl glycerol. The r... | J Med Chem 51: 380-3 (2008) Article DOI: 10.1021/jm7013887 BindingDB Entry DOI: 10.7270/Q2KW5DB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM13772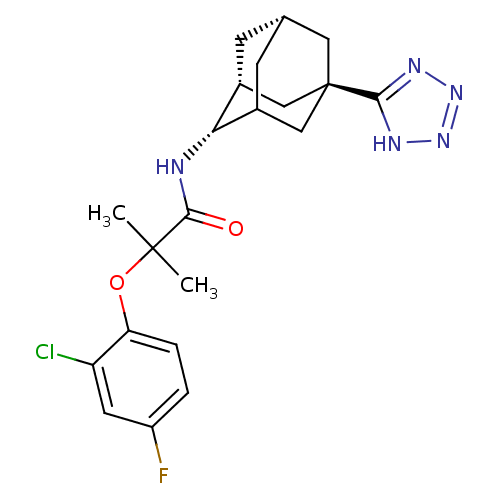 (2-(2-chloro-4-fluorophenoxy)-2-methyl-N-[(1R,2S,5R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM20719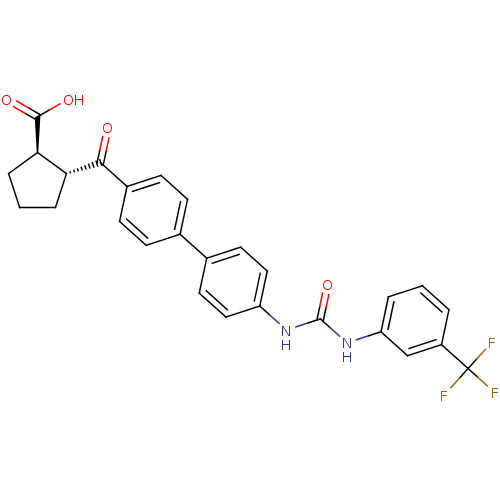 ((1R,2R)-2-({4-[4-({[3-(trifluoromethyl)phenyl]carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description DGAT-1 activity was characterized by catalyzing the transfer of the radiolabeled decanoyl group onto the syn-3 position of didecanoyl glycerol. The r... | J Med Chem 51: 380-3 (2008) Article DOI: 10.1021/jm7013887 BindingDB Entry DOI: 10.7270/Q2KW5DB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM20720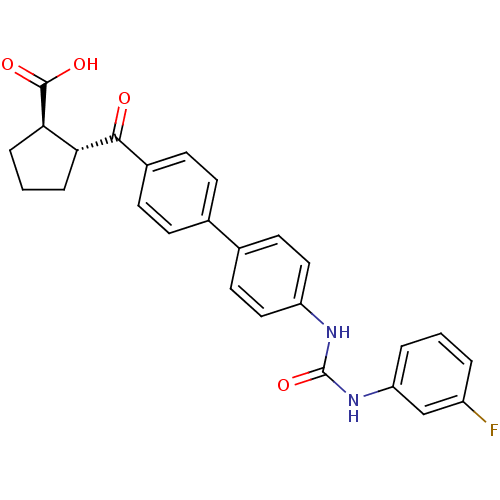 ((1R,2R)-2-{[4-(4-{[(3-fluorophenyl)carbamoyl]amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description DGAT-1 activity was characterized by catalyzing the transfer of the radiolabeled decanoyl group onto the syn-3 position of didecanoyl glycerol. The r... | J Med Chem 51: 380-3 (2008) Article DOI: 10.1021/jm7013887 BindingDB Entry DOI: 10.7270/Q2KW5DB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM13772 (2-(2-chloro-4-fluorophenoxy)-2-methyl-N-[(1R,2S,5R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM13766 ((1R,3R,4S,7S)-4-[2-(4-chlorophenoxy)-2-methylpropa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM13768 (N-[(1R,2S,5R,7S)-5-[(carbamoylmethyl)carbamoyl]ada...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM13762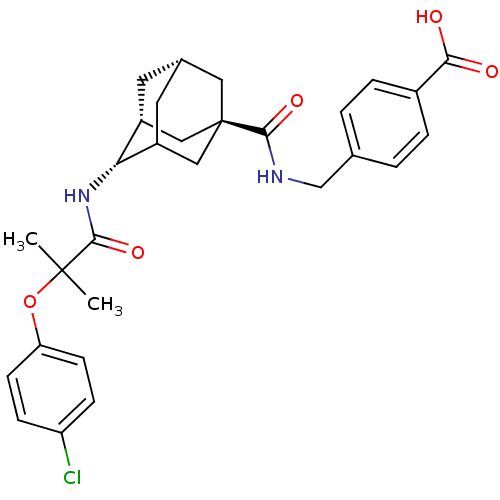 (4-({[(1R,3R,4S,7S)-4-[2-(4-chlorophenoxy)-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM13769 (2-(4-chlorophenoxy)-2-methyl-N-[(1R,2S,5R,7S)-5-{[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Mus musculus (mouse)) | BDBM20726 ((1R,2R)-2-({4-[4-({[4-(trifluoromethyl)phenyl]carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description DGAT-1 activity was characterized by catalyzing the transfer of the radiolabeled decanoyl group onto the syn-3 position of didecanoyl glycerol. The r... | J Med Chem 51: 380-3 (2008) Article DOI: 10.1021/jm7013887 BindingDB Entry DOI: 10.7270/Q2KW5DB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM13767 ((1R,3R,4S,7S)-4-[2-(4-chlorophenoxy)-2-methylpropa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM13762 (4-({[(1R,3R,4S,7S)-4-[2-(4-chlorophenoxy)-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM13758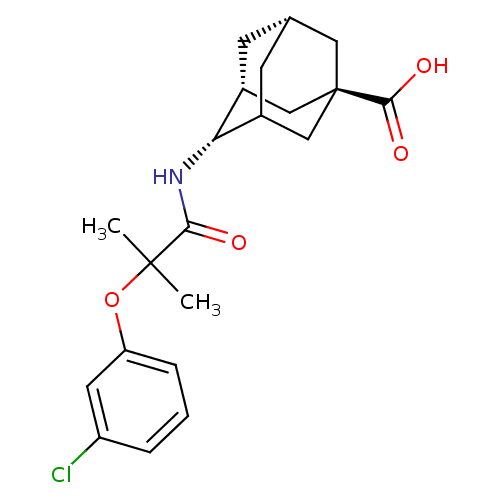 ((1R,3R,4S,7S)-4-[2-(3-chlorophenoxy)-2-methylpropa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM13764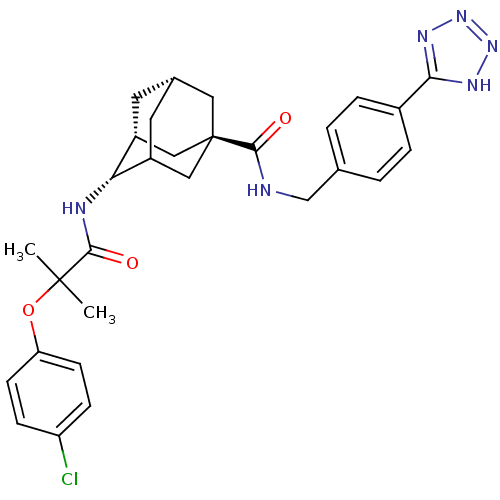 ((1R,3R,4S,7S)-4-[2-(4-chlorophenoxy)-2-methylpropa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Mus musculus (mouse)) | BDBM20718 ((1R,2R)-2-({4-[4-({[2-(trifluoromethyl)phenyl]carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description DGAT-1 activity was characterized by catalyzing the transfer of the radiolabeled decanoyl group onto the syn-3 position of didecanoyl glycerol. The r... | J Med Chem 51: 380-3 (2008) Article DOI: 10.1021/jm7013887 BindingDB Entry DOI: 10.7270/Q2KW5DB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Mus musculus (mouse)) | BDBM20719 ((1R,2R)-2-({4-[4-({[3-(trifluoromethyl)phenyl]carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description DGAT-1 activity was characterized by catalyzing the transfer of the radiolabeled decanoyl group onto the syn-3 position of didecanoyl glycerol. The r... | J Med Chem 51: 380-3 (2008) Article DOI: 10.1021/jm7013887 BindingDB Entry DOI: 10.7270/Q2KW5DB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM13764 ((1R,3R,4S,7S)-4-[2-(4-chlorophenoxy)-2-methylpropa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Mus musculus (mouse)) | BDBM20721 ((1R,2R)-2-{[4-(4-{[(3-chlorophenyl)carbamoyl]amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description DGAT-1 activity was characterized by catalyzing the transfer of the radiolabeled decanoyl group onto the syn-3 position of didecanoyl glycerol. The r... | J Med Chem 51: 380-3 (2008) Article DOI: 10.1021/jm7013887 BindingDB Entry DOI: 10.7270/Q2KW5DB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50174893 (CHEMBL197497 | N-(1-(benzo[d][1,3]dioxol-5-ylmethy...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to human MCHr1 from neuronal IMR32 cells | Bioorg Med Chem Lett 15: 5293-7 (2005) Article DOI: 10.1016/j.bmcl.2005.08.049 BindingDB Entry DOI: 10.7270/Q2GQ6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50174895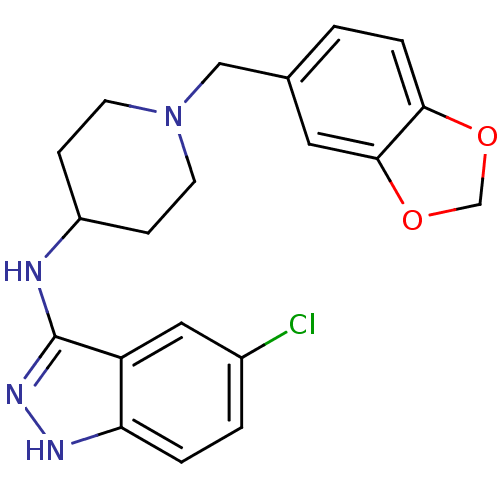 ((1-benzo{1,3}dioxol-5-ylmethyl-piperidin-4-yl)(5-c...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to human MCHr1 from neuronal IMR32 cells | Bioorg Med Chem Lett 15: 5293-7 (2005) Article DOI: 10.1016/j.bmcl.2005.08.049 BindingDB Entry DOI: 10.7270/Q2GQ6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50174899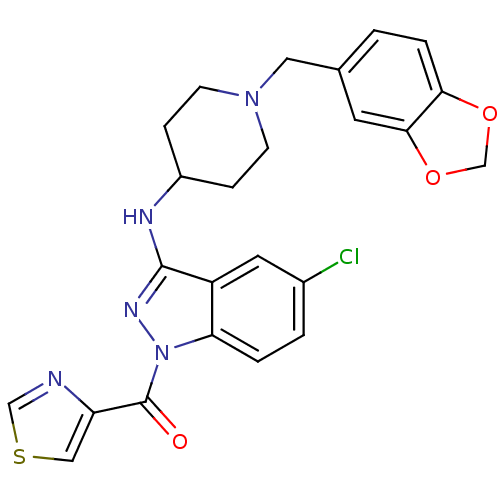 ((3-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to human MCHr1 from neuronal IMR32 cells | Bioorg Med Chem Lett 15: 5293-7 (2005) Article DOI: 10.1016/j.bmcl.2005.08.049 BindingDB Entry DOI: 10.7270/Q2GQ6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50174906 ((3-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to human MCHr1 from neuronal IMR32 cells | Bioorg Med Chem Lett 15: 5293-7 (2005) Article DOI: 10.1016/j.bmcl.2005.08.049 BindingDB Entry DOI: 10.7270/Q2GQ6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50174911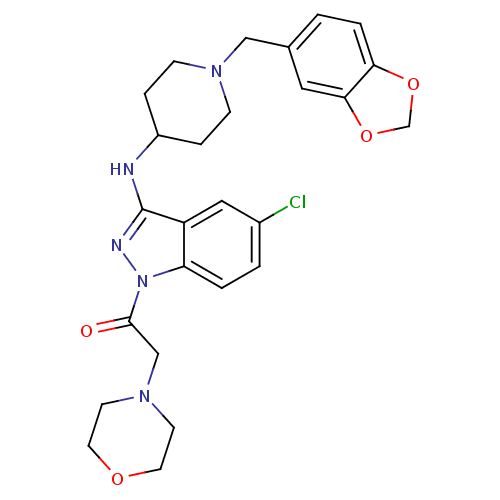 (1-(3-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to human MCHr1 from neuronal IMR32 cells | Bioorg Med Chem Lett 15: 5293-7 (2005) Article DOI: 10.1016/j.bmcl.2005.08.049 BindingDB Entry DOI: 10.7270/Q2GQ6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50174916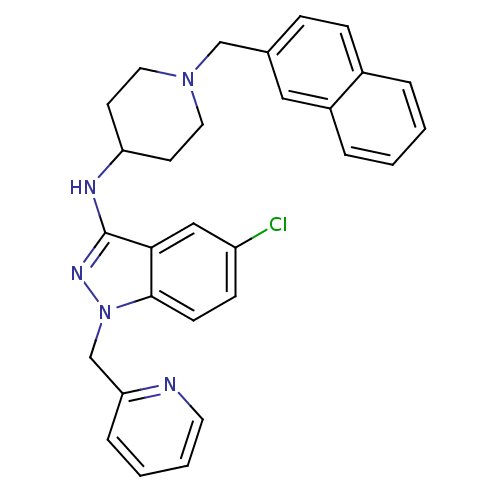 (5-chloro-N-(1-(naphthalen-2-ylmethyl)piperidin-4-y...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to human MCHr1 from neuronal IMR32 cells | Bioorg Med Chem Lett 15: 5293-7 (2005) Article DOI: 10.1016/j.bmcl.2005.08.049 BindingDB Entry DOI: 10.7270/Q2GQ6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Mus musculus (mouse)) | BDBM20722 ((1R,2R)-2-({4-[4-({[2-fluoro-5-(trifluoromethyl)ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description DGAT-1 activity was characterized by catalyzing the transfer of the radiolabeled decanoyl group onto the syn-3 position of didecanoyl glycerol. The r... | J Med Chem 51: 380-3 (2008) Article DOI: 10.1021/jm7013887 BindingDB Entry DOI: 10.7270/Q2KW5DB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM13759 ((1R,3R,4S,7S)-4-[2-(4-chlorophenoxy)-2-methylpropa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Abbott Laboratories | Assay Description Enzyme assays were performed using purified recombinant human or mouse11beta-HSD1. The fractional conversion of cortisone to cortisol was used to det... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Mus musculus (mouse)) | BDBM20716 ((1R,2R)-2-[(4-{4-[(phenylcarbamoyl)amino]phenyl}ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description DGAT-1 activity was characterized by catalyzing the transfer of the radiolabeled decanoyl group onto the syn-3 position of didecanoyl glycerol. The r... | J Med Chem 51: 380-3 (2008) Article DOI: 10.1021/jm7013887 BindingDB Entry DOI: 10.7270/Q2KW5DB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 165 total ) | Next | Last >> |