

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442489 (CHEMBL2440417) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of wild-type human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli using geraniol as substrate by dou... | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50241817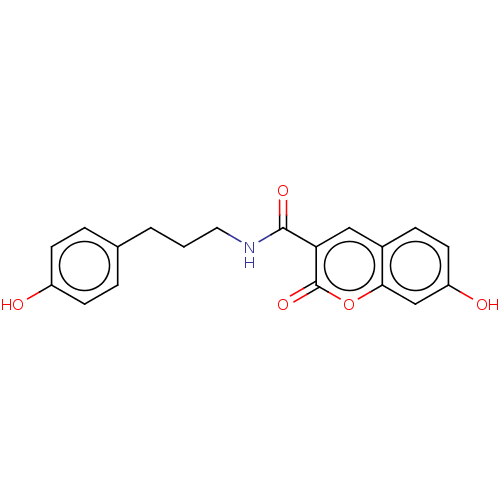 (CHEMBL4081954) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human AKR1B10 in presence of geraniol as substrate by Lineweaver-Burk plot method | J Med Chem 60: 8441-8455 (2017) Article DOI: 10.1021/acs.jmedchem.7b00830 BindingDB Entry DOI: 10.7270/Q2NZ89S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321717 (7-hydroxy-2-(4-methoxyphenylimino)-N-(pyridin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of dehydrogenase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of geraniol deh... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321718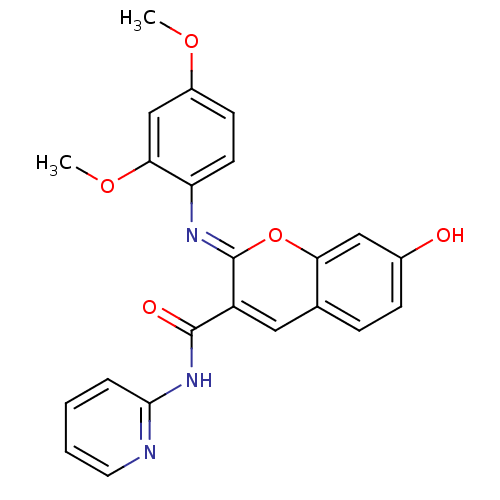 (2-(2,4-dimethoxyphenylimino)-7-hydroxy-N-(pyridin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of dehydrogenase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of geraniol deh... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321715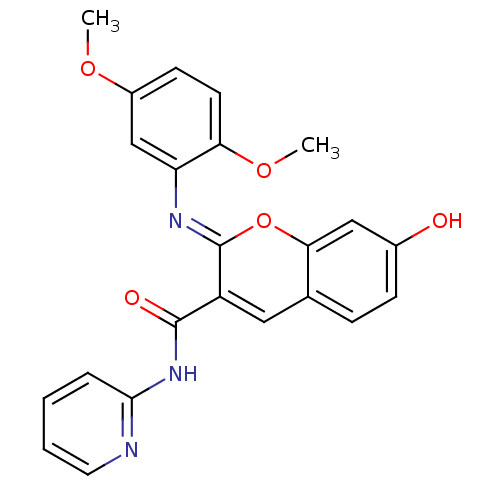 (2-(2,5-dimethoxyphenylimino)-7-hydroxy-N-(pyridin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of dehydrogenase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of geraniol deh... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321716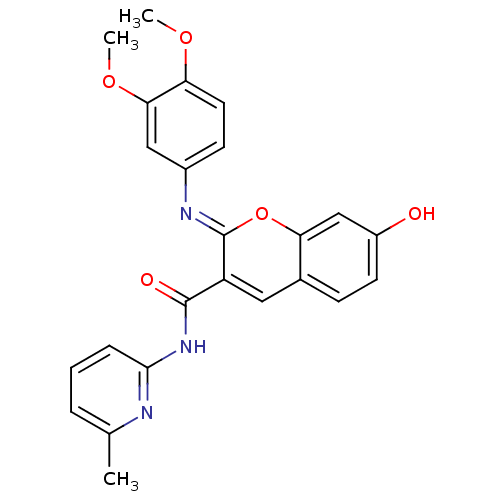 (2-(3,4-dimethoxyphenylimino)-7-hydroxy-N-(6-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of dehydrogenase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of geraniol deh... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/28/2005(H6N2)) neuraminidase N2 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/67/2005(H1N1)) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/394/2005(H5N3)) neuraminidase N3 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins foll... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/20/2007(H8N4)) neuraminidase N4 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/700/2007(H7N7)) neuraminidase N7 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins foll... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Shiga/8/2004(H4N6)) neuraminidase N6 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/Yamaguchi/20/06(H1N1) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed by ... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/duck/Tsukuba/441/05(H11N9) neuraminidase N9 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followe... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/04/2009(H1N1)) neuraminidase N1 V149I mutant activity using 4MU-Neu5Ac as substrate preincubated for 15... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/mallard/Hokkaido/24/2009(H5N1)) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins f... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Aichi/102/2008(H3N2)) neuraminidase N2 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed by... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Narita/1/2009(H1N1)) neuraminidase N1 V149I mutant activity using 4MU-Neu5Ac as substrate preincubated for 15 mins... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/20/2007(H8N4)) neuraminidase N4 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/duck/Chiba/13/06(H12N5) neuraminidase N5 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed b... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/394/2005(H5N3)) neuraminidase N3 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins foll... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/28/2005(H6N2)) neuraminidase N2 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182248 (CHEMBL3818654) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/28/2005(H6N2)) neuraminidase N2 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/Kitakyushu/10/06(H1N1) neuraminidase N1 H274Y mutant activity using 4MU-Neu5Ac as substrate preincubated for 15 min... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/duck/Chiba/13/06(H12N5) neuraminidase N5 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed b... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/Yamaguchi/20/06(H1N1) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed by ... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/67/2005(H1N1)) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50241828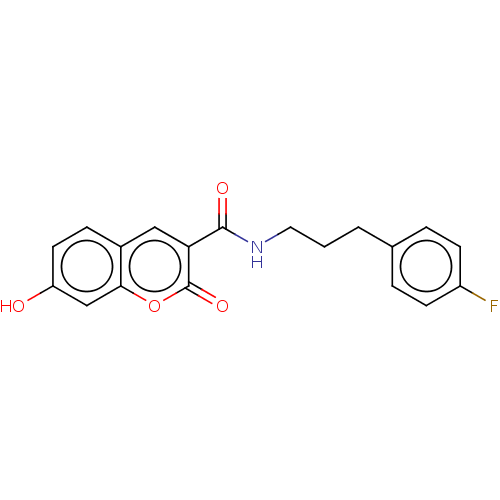 (CHEMBL4089817) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate | J Med Chem 60: 8441-8455 (2017) Article DOI: 10.1021/acs.jmedchem.7b00830 BindingDB Entry DOI: 10.7270/Q2NZ89S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/700/2007(H7N7)) neuraminidase N7 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins foll... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50241817 (CHEMBL4081954) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate | J Med Chem 60: 8441-8455 (2017) Article DOI: 10.1021/acs.jmedchem.7b00830 BindingDB Entry DOI: 10.7270/Q2NZ89S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182248 (CHEMBL3818654) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/duck/Chiba/13/06(H12N5) neuraminidase N5 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed b... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442489 (CHEMBL2440417) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of AKR1B10 (unknown origin) | J Med Chem 60: 8441-8455 (2017) Article DOI: 10.1021/acs.jmedchem.7b00830 BindingDB Entry DOI: 10.7270/Q2NZ89S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442489 (CHEMBL2440417) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/04/2009(H1N1)) neuraminidase N1 V149I mutant activity using 4MU-Neu5Ac as substrate preincubated for 15... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/duck/Tsukuba/441/05(H11N9) neuraminidase N9 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followe... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/28/2006(H3N8)) neuraminidase N8 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182248 (CHEMBL3818654) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/20/2007(H8N4)) neuraminidase N4 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182248 (CHEMBL3818654) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/700/2007(H7N7)) neuraminidase N7 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins foll... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Shiga/8/2004(H4N6)) neuraminidase N6 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321717 (7-hydroxy-2-(4-methoxyphenylimino)-N-(pyridin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of reductase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of NADPH linked pyr... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50241818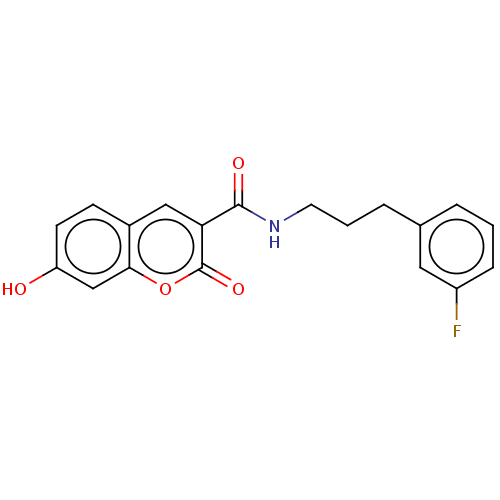 (CHEMBL4060049) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate | J Med Chem 60: 8441-8455 (2017) Article DOI: 10.1021/acs.jmedchem.7b00830 BindingDB Entry DOI: 10.7270/Q2NZ89S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321717 (7-hydroxy-2-(4-methoxyphenylimino)-N-(pyridin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50241816 (CHEMBL4098452) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate | J Med Chem 60: 8441-8455 (2017) Article DOI: 10.1021/acs.jmedchem.7b00830 BindingDB Entry DOI: 10.7270/Q2NZ89S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182248 (CHEMBL3818654) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Aichi/102/2008(H3N2)) neuraminidase N2 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed by... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/mallard/Hokkaido/24/2009(H5N1)) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins f... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM50038843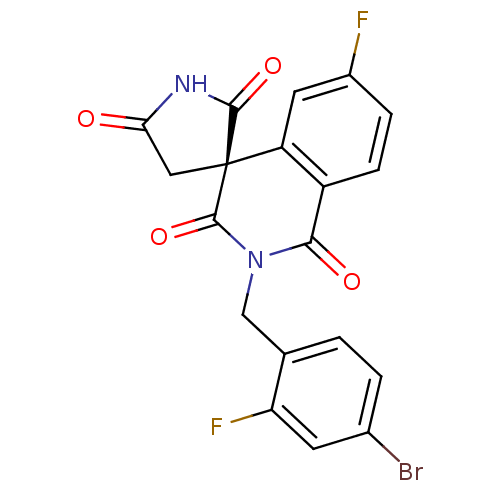 ((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human aldehyde reductase expressed in Escherichia coli BL21(DE3) mediated D-glucuronate reduction | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50442506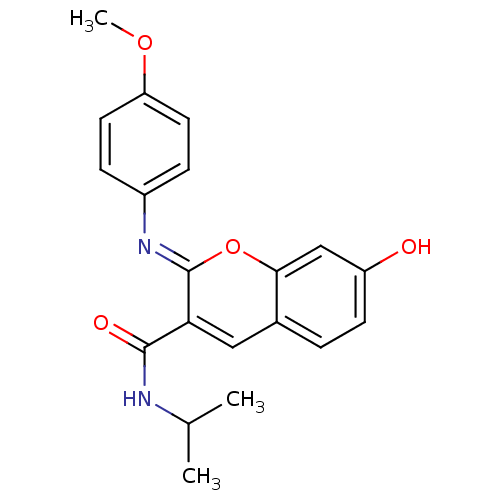 (CHEMBL2440411) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B1 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442501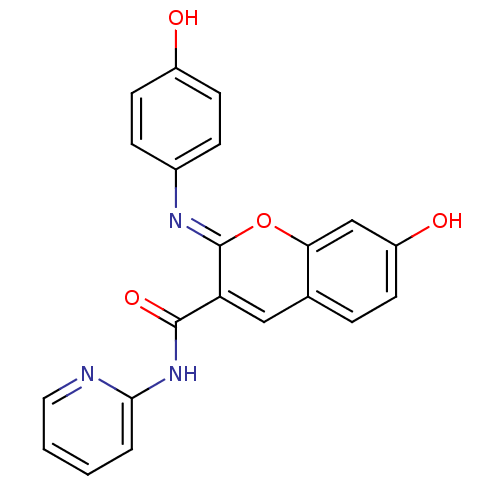 (CHEMBL2440422) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli by fluorescence assay in presence of NADPH | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Aichi/75/2008(H3N2)) neuraminidase N2 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed by ... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182248 (CHEMBL3818654) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/394/2005(H5N3)) neuraminidase N3 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins foll... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 214 total ) | Next | Last >> |