 Found 1460 hits with Last Name = 'stefan' and Initial = 'k'
Found 1460 hits with Last Name = 'stefan' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166849
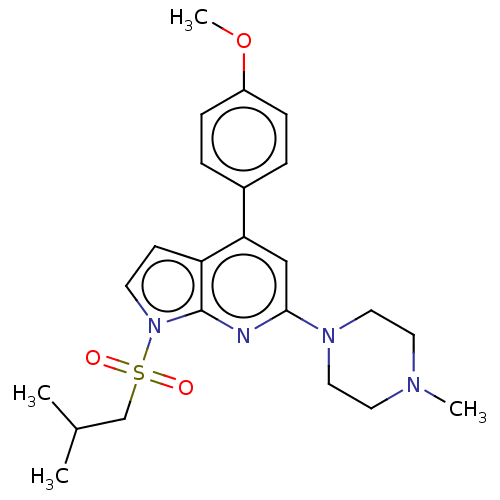
(CHEMBL3797651)Show SMILES COc1ccc(cc1)-c1cc(nc2n(ccc12)S(=O)(=O)CC(C)C)N1CCN(C)CC1 Show InChI InChI=1S/C23H30N4O3S/c1-17(2)16-31(28,29)27-10-9-20-21(18-5-7-19(30-4)8-6-18)15-22(24-23(20)27)26-13-11-25(3)12-14-26/h5-10,15,17H,11-14,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166863

(CHEMBL3799529)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCNCC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H19NO5/c1-13-4-5-17(10-14(13)2)19(24)12-28-22(27)16-6-8-18(9-7-16)23-20(25)11-15(3)21(23)26/h4-11H,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166852
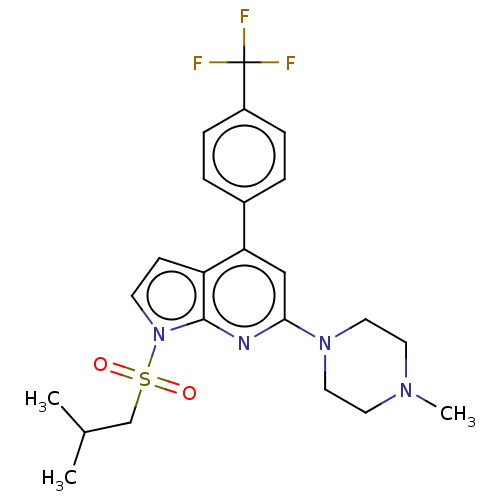
(CHEMBL3797717)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C23H27F3N4O2S/c1-16(2)15-33(31,32)30-9-8-19-20(17-4-6-18(7-5-17)23(24,25)26)14-21(27-22(19)30)29-12-10-28(3)11-13-29/h4-9,14,16H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166844
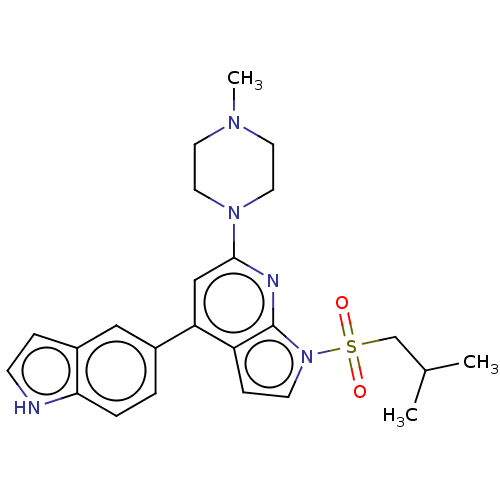
(CHEMBL3798478)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc2[nH]ccc2c1 Show InChI InChI=1S/C24H29N5O2S/c1-17(2)16-32(30,31)29-9-7-20-21(18-4-5-22-19(14-18)6-8-25-22)15-23(26-24(20)29)28-12-10-27(3)11-13-28/h4-9,14-15,17,25H,10-13,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166859
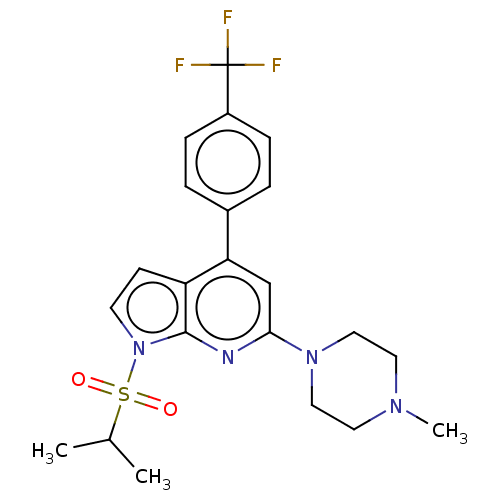
(CHEMBL3799120)Show SMILES CC(C)S(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H25F3N4O2S/c1-15(2)32(30,31)29-9-8-18-19(16-4-6-17(7-5-16)22(23,24)25)14-20(26-21(18)29)28-12-10-27(3)11-13-28/h4-9,14-15H,10-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166861
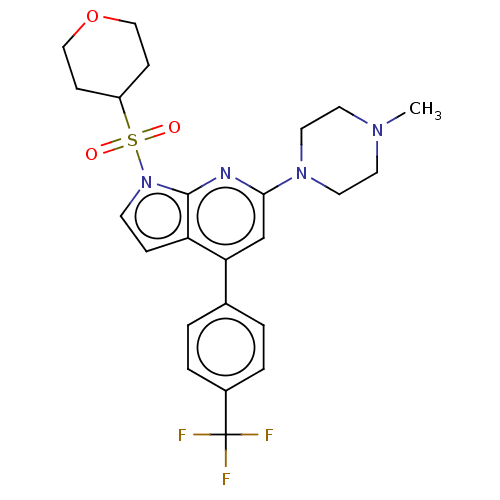
(CHEMBL3799448)Show SMILES CN1CCN(CC1)c1cc(-c2ccc(cc2)C(F)(F)F)c2ccn(c2n1)S(=O)(=O)C1CCOCC1 Show InChI InChI=1S/C26H23NO5/c1-14-3-4-17(11-15(14)2)21(28)13-32-26(31)16-7-9-20(10-8-16)27-24(29)22-18-5-6-19(12-18)23(22)25(27)30/h3-11,18-19,29-30H,12-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166846
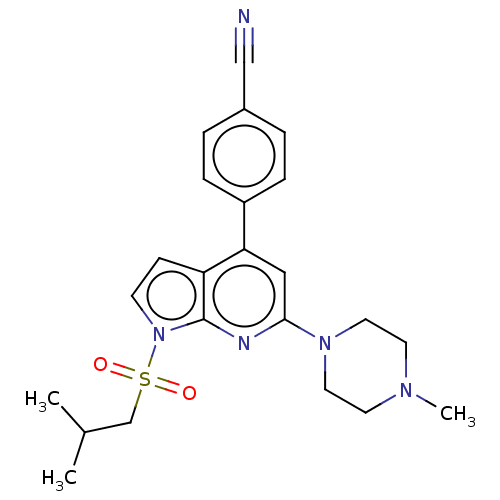
(CHEMBL3797435)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C#N Show InChI InChI=1S/C23H27N5O2S/c1-17(2)16-31(29,30)28-9-8-20-21(19-6-4-18(15-24)5-7-19)14-22(25-23(20)28)27-12-10-26(3)11-13-27/h4-9,14,17H,10-13,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166862
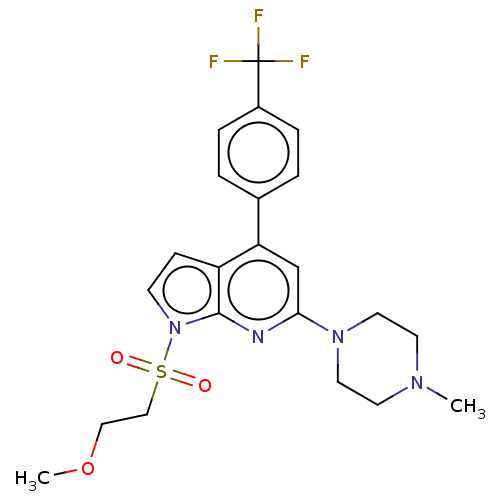
(CHEMBL3798490)Show SMILES COCCS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H21NO5/c1-13-4-5-17(10-14(13)2)19(24)12-28-22(27)16-6-8-18(9-7-16)23-20(25)11-15(3)21(23)26/h4-11,25-26H,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166853

(CHEMBL3798953)Show SMILES CCc1ccc(cc1)-c1cc(nc2n(ccc12)S(=O)(=O)CC(C)C)N1CCN(C)CC1 Show InChI InChI=1S/C24H32N4O2S/c1-5-19-6-8-20(9-7-19)22-16-23(27-14-12-26(4)13-15-27)25-24-21(22)10-11-28(24)31(29,30)17-18(2)3/h6-11,16,18H,5,12-15,17H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166856
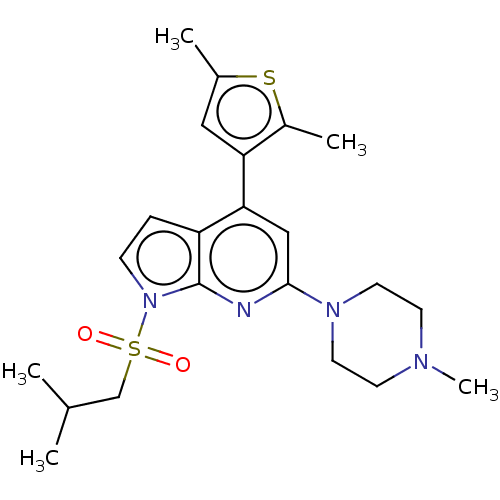
(CHEMBL3800251)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1cc(C)sc1C Show InChI InChI=1S/C22H30N4O2S2/c1-15(2)14-30(27,28)26-7-6-18-20(19-12-16(3)29-17(19)4)13-21(23-22(18)26)25-10-8-24(5)9-11-25/h6-7,12-13,15H,8-11,14H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166860
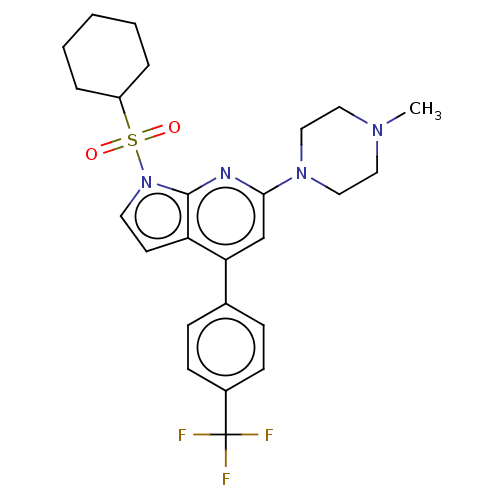
(CHEMBL3799330)Show SMILES CN1CCN(CC1)c1cc(-c2ccc(cc2)C(F)(F)F)c2ccn(c2n1)S(=O)(=O)C1CCCCC1 Show InChI InChI=1S/C25H29F3N4O2S/c1-30-13-15-31(16-14-30)23-17-22(18-7-9-19(10-8-18)25(26,27)28)21-11-12-32(24(21)29-23)35(33,34)20-5-3-2-4-6-20/h7-12,17,20H,2-6,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166854

(CHEMBL3799638)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccccc1 Show InChI InChI=1S/C22H28N4O2S/c1-17(2)16-29(27,28)26-10-9-19-20(18-7-5-4-6-8-18)15-21(23-22(19)26)25-13-11-24(3)12-14-25/h4-10,15,17H,11-14,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166847
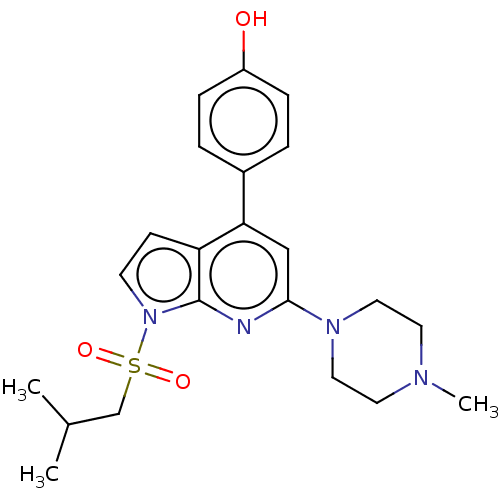
(CHEMBL3800539)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(O)cc1 Show InChI InChI=1S/C22H28N4O3S/c1-16(2)15-30(28,29)26-9-8-19-20(17-4-6-18(27)7-5-17)14-21(23-22(19)26)25-12-10-24(3)11-13-25/h4-9,14,16,27H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166849

(CHEMBL3797651)Show SMILES COc1ccc(cc1)-c1cc(nc2n(ccc12)S(=O)(=O)CC(C)C)N1CCN(C)CC1 Show InChI InChI=1S/C23H30N4O3S/c1-17(2)16-31(28,29)27-10-9-20-21(18-5-7-19(30-4)8-6-18)15-22(24-23(20)27)26-13-11-25(3)12-14-26/h5-10,15,17H,11-14,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166850
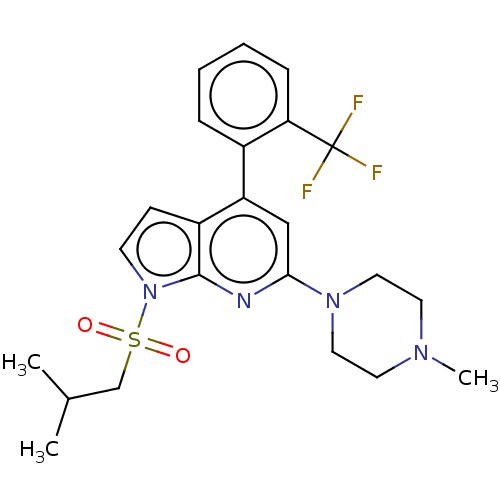
(CHEMBL3799547)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C23H27F3N4O2S/c1-16(2)15-33(31,32)30-9-8-18-19(17-6-4-5-7-20(17)23(24,25)26)14-21(27-22(18)30)29-12-10-28(3)11-13-29/h4-9,14,16H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166859

(CHEMBL3799120)Show SMILES CC(C)S(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H25F3N4O2S/c1-15(2)32(30,31)29-9-8-18-19(16-4-6-17(7-5-16)22(23,24)25)14-20(26-21(18)29)28-12-10-27(3)11-13-28/h4-9,14-15H,10-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166843
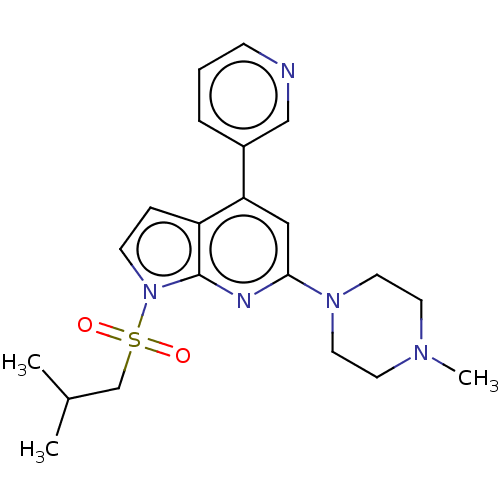
(CHEMBL3797293)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1cccnc1 Show InChI InChI=1S/C21H27N5O2S/c1-16(2)15-29(27,28)26-8-6-18-19(17-5-4-7-22-14-17)13-20(23-21(18)26)25-11-9-24(3)10-12-25/h4-8,13-14,16H,9-12,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166851
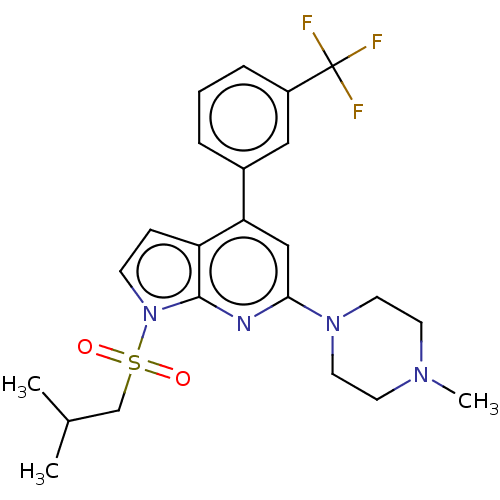
(CHEMBL3798840)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H27F3N4O2S/c1-16(2)15-33(31,32)30-8-7-19-20(17-5-4-6-18(13-17)23(24,25)26)14-21(27-22(19)30)29-11-9-28(3)10-12-29/h4-8,13-14,16H,9-12,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166862

(CHEMBL3798490)Show SMILES COCCS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H21NO5/c1-13-4-5-17(10-14(13)2)19(24)12-28-22(27)16-6-8-18(9-7-16)23-20(25)11-15(3)21(23)26/h4-11,25-26H,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166858
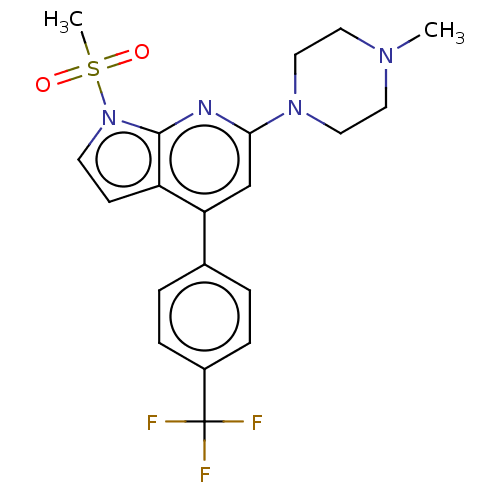
(CHEMBL3800153)Show SMILES CN1CCN(CC1)c1cc(-c2ccc(cc2)C(F)(F)F)c2ccn(c2n1)S(C)(=O)=O Show InChI InChI=1S/C20H21F3N4O2S/c1-25-9-11-26(12-10-25)18-13-17(14-3-5-15(6-4-14)20(21,22)23)16-7-8-27(19(16)24-18)30(2,28)29/h3-8,13H,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166848
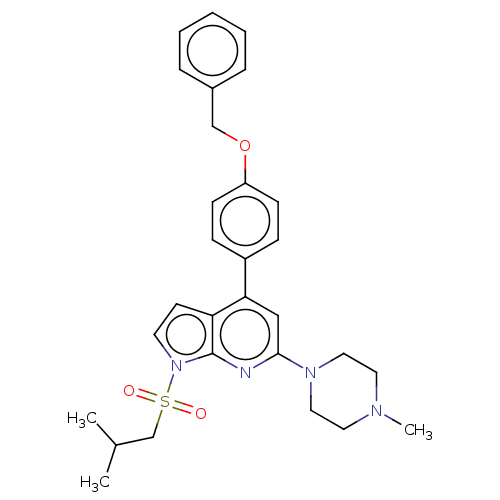
(CHEMBL3800150)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C29H34N4O3S/c1-22(2)21-37(34,35)33-14-13-26-27(19-28(30-29(26)33)32-17-15-31(3)16-18-32)24-9-11-25(12-10-24)36-20-23-7-5-4-6-8-23/h4-14,19,22H,15-18,20-21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166855

(CHEMBL3800576)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1cnn(C)c1 Show InChI InChI=1S/C20H28N6O2S/c1-15(2)14-29(27,28)26-6-5-17-18(16-12-21-24(4)13-16)11-19(22-20(17)26)25-9-7-23(3)8-10-25/h5-6,11-13,15H,7-10,14H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166847

(CHEMBL3800539)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(O)cc1 Show InChI InChI=1S/C22H28N4O3S/c1-16(2)15-30(28,29)26-9-8-19-20(17-4-6-18(27)7-5-17)14-21(23-22(19)26)25-12-10-24(3)11-13-25/h4-9,14,16,27H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166850

(CHEMBL3799547)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C23H27F3N4O2S/c1-16(2)15-33(31,32)30-9-8-18-19(17-6-4-5-7-20(17)23(24,25)26)14-21(27-22(18)30)29-12-10-28(3)11-13-29/h4-9,14,16H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166857

(CHEMBL3800055)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1c(C)noc1C |(.88,-6.25,;1.7,-5.33,;2.91,-5.58,;1.22,-3.86,;2.25,-2.71,;3.46,-2.96,;2.64,-3.88,;1.77,-1.24,;2.67,.02,;1.77,1.24,;.3,.77,;-1.03,1.56,;-2.39,.77,;-2.39,-.77,;-1.03,-1.56,;.3,-.77,;-3.73,-1.54,;-3.73,-3.09,;-5.07,-3.85,;-6.41,-3.08,;-7.48,-3.69,;-6.4,-1.53,;-5.06,-.76,;-1.03,3.1,;.22,4,;1.39,3.61,;-.27,5.46,;-1.81,5.46,;-2.28,3.99,;-3.45,3.59,)| Show InChI InChI=1S/C21H29N5O3S/c1-14(2)13-30(27,28)26-7-6-17-18(20-15(3)23-29-16(20)4)12-19(22-21(17)26)25-10-8-24(5)9-11-25/h6-7,12,14H,8-11,13H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166846

(CHEMBL3797435)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C#N Show InChI InChI=1S/C23H27N5O2S/c1-17(2)16-31(29,30)28-9-8-20-21(19-6-4-18(15-24)5-7-19)14-22(25-23(20)28)27-12-10-26(3)11-13-27/h4-9,14,17H,10-13,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166842
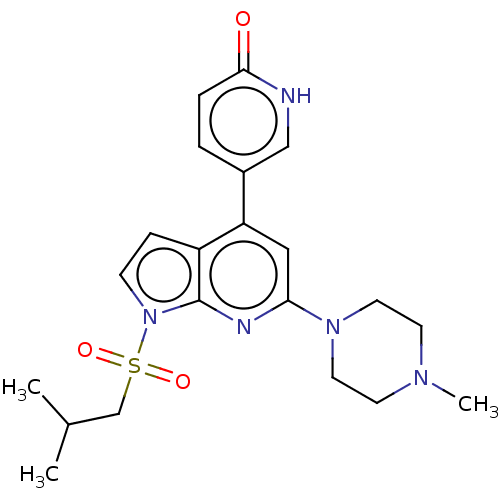
(CHEMBL3800442)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(=O)[nH]c1 Show InChI InChI=1S/C21H27N5O3S/c1-15(2)14-30(28,29)26-7-6-17-18(16-4-5-20(27)22-13-16)12-19(23-21(17)26)25-10-8-24(3)9-11-25/h4-7,12-13,15H,8-11,14H2,1-3H3,(H,22,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166848

(CHEMBL3800150)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C29H34N4O3S/c1-22(2)21-37(34,35)33-14-13-26-27(19-28(30-29(26)33)32-17-15-31(3)16-18-32)24-9-11-25(12-10-24)36-20-23-7-5-4-6-8-23/h4-14,19,22H,15-18,20-21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166852

(CHEMBL3797717)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C23H27F3N4O2S/c1-16(2)15-33(31,32)30-9-8-19-20(17-4-6-18(7-5-17)23(24,25)26)14-21(27-22(19)30)29-12-10-28(3)11-13-29/h4-9,14,16H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166844

(CHEMBL3798478)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc2[nH]ccc2c1 Show InChI InChI=1S/C24H29N5O2S/c1-17(2)16-32(30,31)29-9-7-20-21(18-4-5-22-19(14-18)6-8-25-22)15-23(26-24(20)29)28-12-10-27(3)11-13-28/h4-9,14-15,17,25H,10-13,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 382 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166854

(CHEMBL3799638)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccccc1 Show InChI InChI=1S/C22H28N4O2S/c1-17(2)16-29(27,28)26-10-9-19-20(18-7-5-4-6-8-18)15-21(23-22(19)26)25-13-11-24(3)12-14-25/h4-10,15,17H,11-14,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 547 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166853

(CHEMBL3798953)Show SMILES CCc1ccc(cc1)-c1cc(nc2n(ccc12)S(=O)(=O)CC(C)C)N1CCN(C)CC1 Show InChI InChI=1S/C24H32N4O2S/c1-5-19-6-8-20(9-7-19)22-16-23(27-14-12-26(4)13-15-27)25-24-21(22)10-11-28(24)31(29,30)17-18(2)3/h6-11,16,18H,5,12-15,17H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 614 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166851

(CHEMBL3798840)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H27F3N4O2S/c1-16(2)15-33(31,32)30-8-7-19-20(17-5-4-6-18(13-17)23(24,25)26)14-21(27-22(19)30)29-11-9-28(3)10-12-29/h4-8,13-14,16H,9-12,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166856

(CHEMBL3800251)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1cc(C)sc1C Show InChI InChI=1S/C22H30N4O2S2/c1-15(2)14-30(27,28)26-7-6-18-20(19-12-16(3)29-17(19)4)13-21(23-22(18)26)25-10-8-24(5)9-11-25/h6-7,12-13,15H,8-11,14H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166858

(CHEMBL3800153)Show SMILES CN1CCN(CC1)c1cc(-c2ccc(cc2)C(F)(F)F)c2ccn(c2n1)S(C)(=O)=O Show InChI InChI=1S/C20H21F3N4O2S/c1-25-9-11-26(12-10-25)18-13-17(14-3-5-15(6-4-14)20(21,22)23)16-7-8-27(19(16)24-18)30(2,28)29/h3-8,13H,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166863

(CHEMBL3799529)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCNCC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H19NO5/c1-13-4-5-17(10-14(13)2)19(24)12-28-22(27)16-6-8-18(9-7-16)23-20(25)11-15(3)21(23)26/h4-11H,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166857

(CHEMBL3800055)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1c(C)noc1C |(.88,-6.25,;1.7,-5.33,;2.91,-5.58,;1.22,-3.86,;2.25,-2.71,;3.46,-2.96,;2.64,-3.88,;1.77,-1.24,;2.67,.02,;1.77,1.24,;.3,.77,;-1.03,1.56,;-2.39,.77,;-2.39,-.77,;-1.03,-1.56,;.3,-.77,;-3.73,-1.54,;-3.73,-3.09,;-5.07,-3.85,;-6.41,-3.08,;-7.48,-3.69,;-6.4,-1.53,;-5.06,-.76,;-1.03,3.1,;.22,4,;1.39,3.61,;-.27,5.46,;-1.81,5.46,;-2.28,3.99,;-3.45,3.59,)| Show InChI InChI=1S/C21H29N5O3S/c1-14(2)13-30(27,28)26-7-6-17-18(20-15(3)23-29-16(20)4)12-19(22-21(17)26)25-10-8-24(5)9-11-25/h6-7,12,14H,8-11,13H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166843

(CHEMBL3797293)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1cccnc1 Show InChI InChI=1S/C21H27N5O2S/c1-16(2)15-29(27,28)26-8-6-18-19(17-5-4-7-22-14-17)13-20(23-21(18)26)25-11-9-24(3)10-12-25/h4-8,13-14,16H,9-12,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166842

(CHEMBL3800442)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(=O)[nH]c1 Show InChI InChI=1S/C21H27N5O3S/c1-15(2)14-30(28,29)26-7-6-17-18(16-4-5-20(27)22-13-16)12-19(23-21(17)26)25-10-8-24(3)9-11-25/h4-7,12-13,15H,8-11,14H2,1-3H3,(H,22,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166860

(CHEMBL3799330)Show SMILES CN1CCN(CC1)c1cc(-c2ccc(cc2)C(F)(F)F)c2ccn(c2n1)S(=O)(=O)C1CCCCC1 Show InChI InChI=1S/C25H29F3N4O2S/c1-30-13-15-31(16-14-30)23-17-22(18-7-9-19(10-8-18)25(26,27)28)21-11-12-32(24(21)29-23)35(33,34)20-5-3-2-4-6-20/h7-12,17,20H,2-6,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166861

(CHEMBL3799448)Show SMILES CN1CCN(CC1)c1cc(-c2ccc(cc2)C(F)(F)F)c2ccn(c2n1)S(=O)(=O)C1CCOCC1 Show InChI InChI=1S/C26H23NO5/c1-14-3-4-17(11-15(14)2)21(28)13-32-26(31)16-7-9-20(10-8-16)27-24(29)22-18-5-6-19(12-18)23(22)25(27)30/h3-11,18-19,29-30H,12-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166845
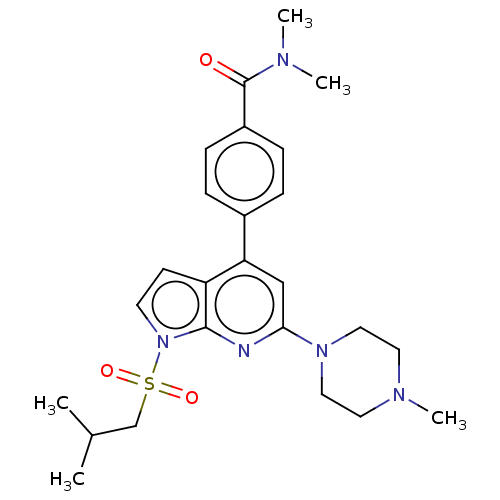
(CHEMBL3799430)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(=O)N(C)C Show InChI InChI=1S/C25H33N5O3S/c1-18(2)17-34(32,33)30-11-10-21-22(19-6-8-20(9-7-19)25(31)27(3)4)16-23(26-24(21)30)29-14-12-28(5)13-15-29/h6-11,16,18H,12-15,17H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50166855

(CHEMBL3800576)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1cnn(C)c1 Show InChI InChI=1S/C20H28N6O2S/c1-15(2)14-29(27,28)26-6-5-17-18(16-12-21-24(4)13-16)11-19(22-20(17)26)25-9-7-23(3)8-10-25/h5-6,11-13,15H,7-10,14H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMSP from human dopamine D2S receptor expressed in CHO cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166845

(CHEMBL3799430)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(=O)N(C)C Show InChI InChI=1S/C25H33N5O3S/c1-18(2)17-34(32,33)30-11-10-21-22(19-6-8-20(9-7-19)25(31)27(3)4)16-23(26-24(21)30)29-14-12-28(5)13-15-29/h6-11,16,18H,12-15,17H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239718
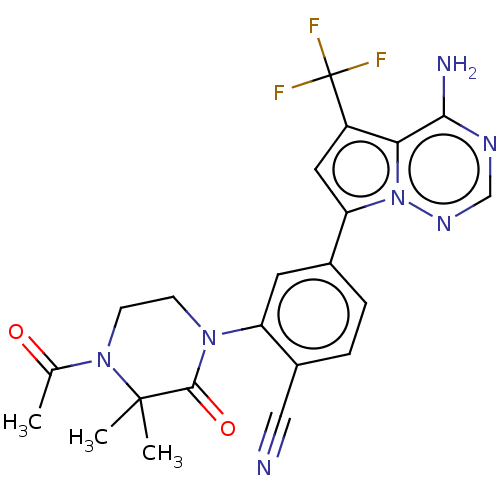
(CHEMBL4064666 | US10214537, Example 639)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F Show InChI InChI=1S/C22H20F3N7O2/c1-12(33)31-7-6-30(20(34)21(31,2)3)16-8-13(4-5-14(16)10-26)17-9-15(22(23,24)25)18-19(27)28-11-29-32(17)18/h4-5,8-9,11H,6-7H2,1-3H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50232433
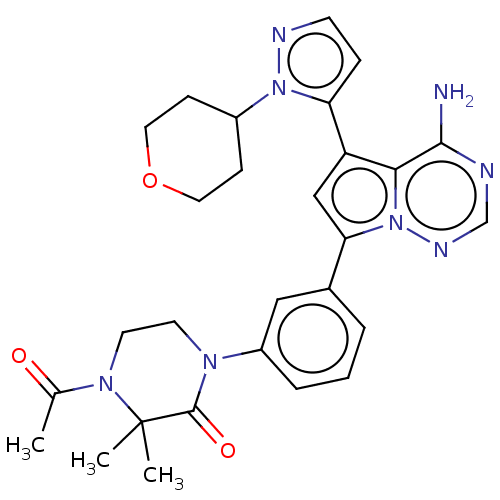
(CHEMBL4068514 | US10214537, Example 619)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cccc(c1)-c1cc(-c2ccnn2C2CCOCC2)c2c(N)ncnn12 Show InChI InChI=1S/C28H32N8O3/c1-18(37)34-12-11-33(27(38)28(34,2)3)21-6-4-5-19(15-21)24-16-22(25-26(29)30-17-32-36(24)25)23-7-10-31-35(23)20-8-13-39-14-9-20/h4-7,10,15-17,20H,8-9,11-14H2,1-3H3,(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2:PS as substrate incubated for 3 hrs by ADP-Glo assay |
Bioorg Med Chem Lett 27: 855-861 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.016
BindingDB Entry DOI: 10.7270/Q2BR8VFH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50232433

(CHEMBL4068514 | US10214537, Example 619)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cccc(c1)-c1cc(-c2ccnn2C2CCOCC2)c2c(N)ncnn12 Show InChI InChI=1S/C28H32N8O3/c1-18(37)34-12-11-33(27(38)28(34,2)3)21-6-4-5-19(15-21)24-16-22(25-26(29)30-17-32-36(24)25)23-7-10-31-35(23)20-8-13-39-14-9-20/h4-7,10,15-17,20H,8-9,11-14H2,1-3H3,(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50232438
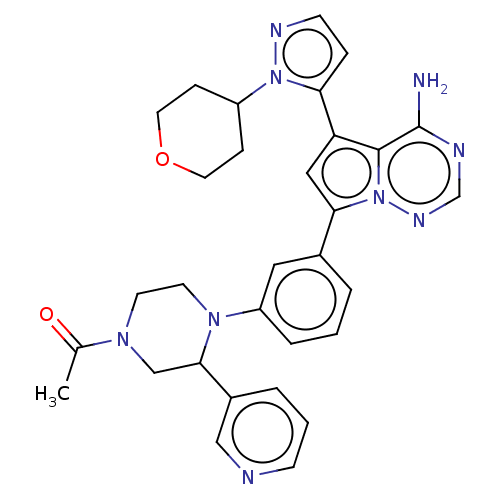
(CHEMBL4100063 | US10214537, Example 256)Show SMILES CC(=O)N1CCN(C(C1)c1cccnc1)c1cccc(c1)-c1cc(-c2ccnn2C2CCOCC2)c2c(N)ncnn12 Show InChI InChI=1S/C31H33N9O2/c1-21(41)37-12-13-38(29(19-37)23-5-3-10-33-18-23)25-6-2-4-22(16-25)28-17-26(30-31(32)34-20-36-40(28)30)27-7-11-35-39(27)24-8-14-42-15-9-24/h2-7,10-11,16-18,20,24,29H,8-9,12-15,19H2,1H3,(H2,32,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2:PS as substrate incubated for 3 hrs by ADP-Glo assay |
Bioorg Med Chem Lett 27: 855-861 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.016
BindingDB Entry DOI: 10.7270/Q2BR8VFH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM284821
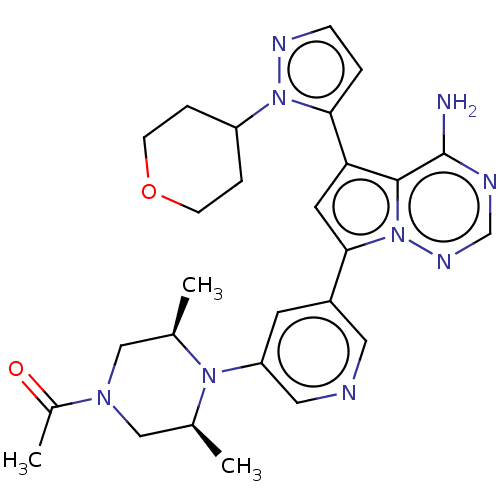
(1-((cis)-4-(5-(4-amino-5-(1-(tetrahydro-2H-pyran-4...)Show SMILES C[C@H]1CN(C[C@@H](C)N1c1cncc(c1)-c1cc(-c2ccnn2C2CCOCC2)c2c(N)ncnn12)C(C)=O |r| Show InChI InChI=1S/C27H33N9O2/c1-17-14-33(19(3)37)15-18(2)34(17)22-10-20(12-29-13-22)25-11-23(26-27(28)30-16-32-36(25)26)24-4-7-31-35(24)21-5-8-38-9-6-21/h4,7,10-13,16-18,21H,5-6,8-9,14-15H2,1-3H3,(H2,28,30,32)/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2:PS as substrate incubated for 3 hrs by ADP-Glo assay |
Bioorg Med Chem Lett 27: 855-861 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.016
BindingDB Entry DOI: 10.7270/Q2BR8VFH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50122323
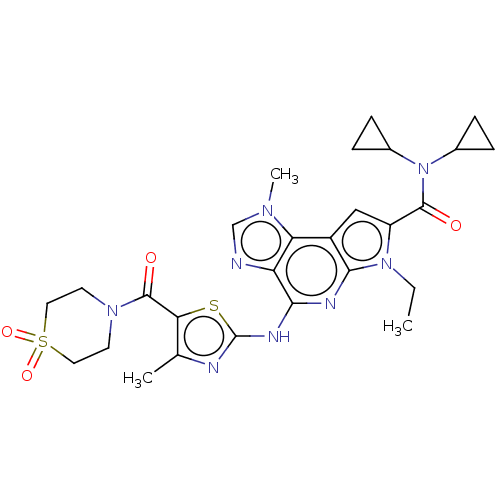
(CHEMBL3622146)Show SMILES CCn1c(cc2c1nc(Nc1nc(C)c(s1)C(=O)N1CCS(=O)(=O)CC1)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C27H32N8O4S2/c1-4-34-19(25(36)35(16-5-6-16)17-7-8-17)13-18-21-20(28-14-32(21)3)23(30-24(18)34)31-27-29-15(2)22(40-27)26(37)33-9-11-41(38,39)12-10-33/h13-14,16-17H,4-12H2,1-3H3,(H,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 (unknown origin) using 5-FAM-KKKKEEIYFFFG-OH substrate and ATP incubated for 180 mins by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data