

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522827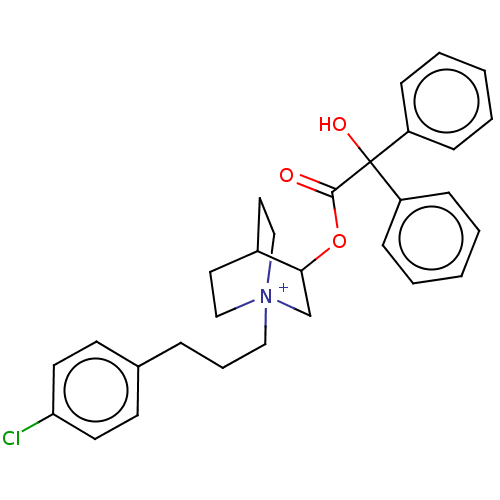 (CHEMBL4519930) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50327943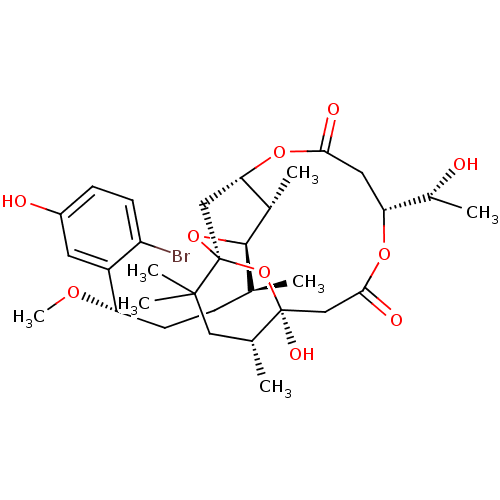 (Aplysiatoxin | CHEMBL1256416) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCtheta C1B peptide | Bioorg Med Chem Lett 20: 6064-6 (2010) Article DOI: 10.1016/j.bmcl.2010.08.051 BindingDB Entry DOI: 10.7270/Q2FJ2H04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522833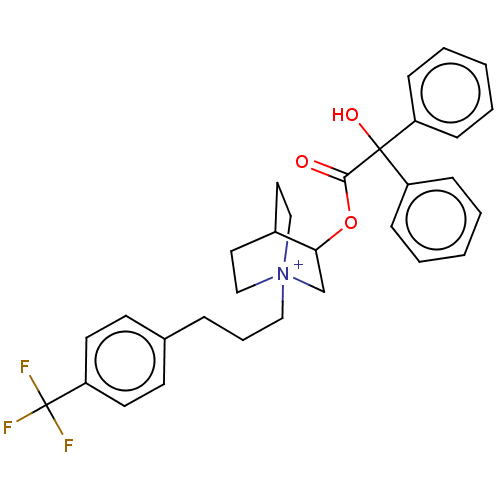 (CHEMBL4516342) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50375451 (CHEMBL408579) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells | Bioorg Med Chem 16: 1966-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.100 BindingDB Entry DOI: 10.7270/Q2TH8NKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50375457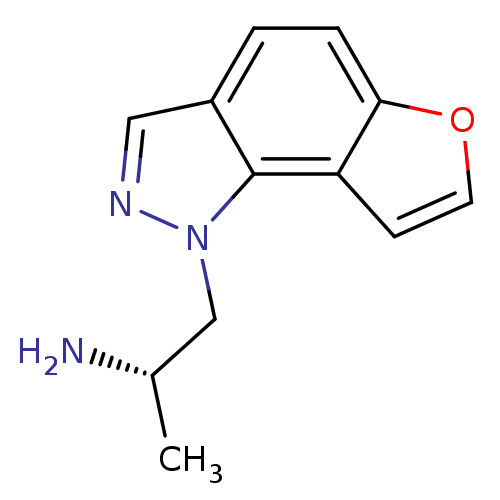 (CHEMBL261476) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells | Bioorg Med Chem 16: 1966-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.100 BindingDB Entry DOI: 10.7270/Q2TH8NKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50239981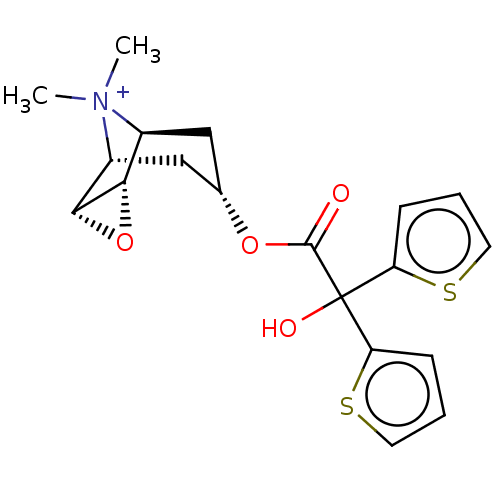 (Braltus | Spiriva | Spiriva Respimat | Tiotropium) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Homo sapiens (Human)) | BDBM50327943 (Aplysiatoxin | CHEMBL1256416) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCeta C1B peptide | Bioorg Med Chem Lett 20: 6064-6 (2010) Article DOI: 10.1016/j.bmcl.2010.08.051 BindingDB Entry DOI: 10.7270/Q2FJ2H04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522829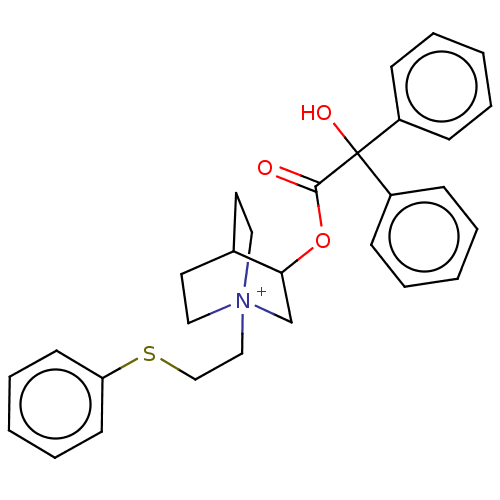 (CHEMBL4457734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50327943 (Aplysiatoxin | CHEMBL1256416) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKC alpha C1A peptide | Bioorg Med Chem Lett 20: 6064-6 (2010) Article DOI: 10.1016/j.bmcl.2010.08.051 BindingDB Entry DOI: 10.7270/Q2FJ2H04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50522832 (CHEMBL4483083) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50327943 (Aplysiatoxin | CHEMBL1256416) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKC delta C1B peptide | Bioorg Med Chem Lett 20: 6064-6 (2010) Article DOI: 10.1016/j.bmcl.2010.08.051 BindingDB Entry DOI: 10.7270/Q2FJ2H04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296331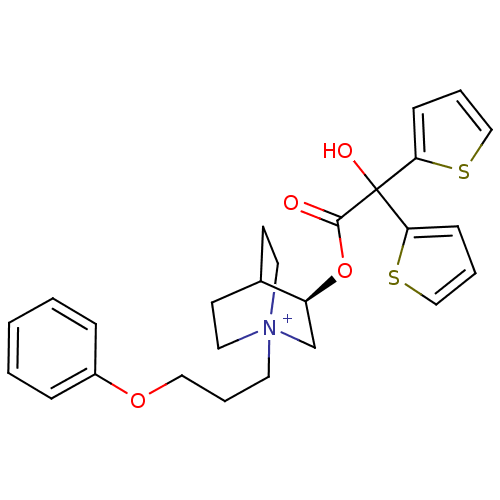 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50522817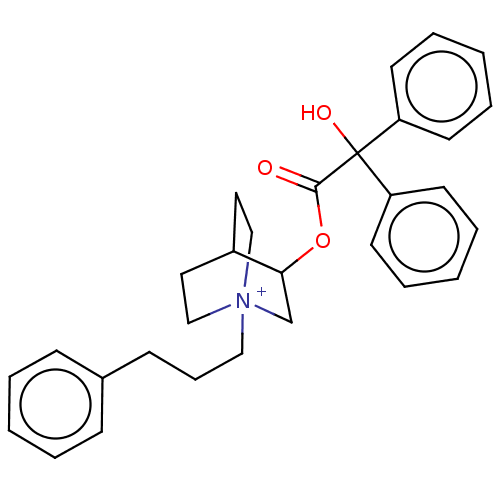 (CHEMBL4448944) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM50327943 (Aplysiatoxin | CHEMBL1256416) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKC beta C1A peptide | Bioorg Med Chem Lett 20: 6064-6 (2010) Article DOI: 10.1016/j.bmcl.2010.08.051 BindingDB Entry DOI: 10.7270/Q2FJ2H04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50019290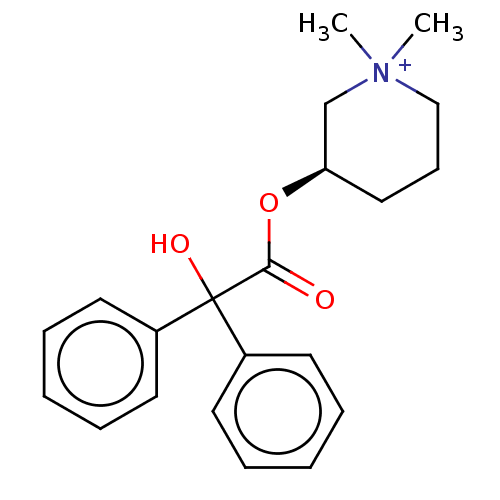 (CHEMBL3289390) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysis | Bioorg Med Chem 22: 3488-97 (2014) Article DOI: 10.1016/j.bmc.2014.04.029 BindingDB Entry DOI: 10.7270/Q2VD7111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50239981 (Braltus | Spiriva | Spiriva Respimat | Tiotropium) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522821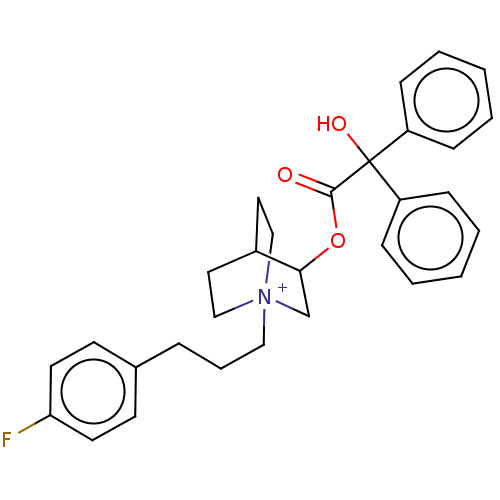 (CHEMBL4450188) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50375452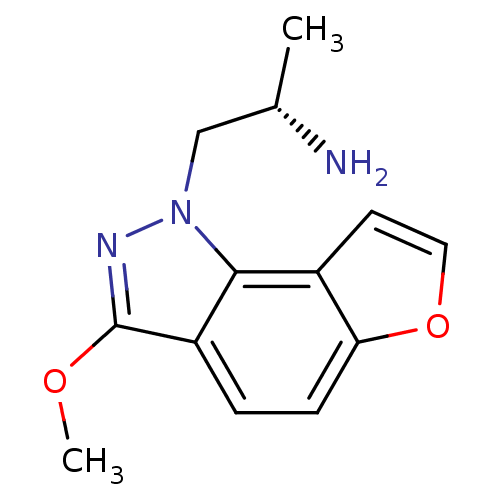 (CHEMBL262092) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells | Bioorg Med Chem 16: 1966-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.100 BindingDB Entry DOI: 10.7270/Q2TH8NKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50522827 (CHEMBL4519930) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296331 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C gamma type (Homo sapiens (Human)) | BDBM50327943 (Aplysiatoxin | CHEMBL1256416) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKC gamma C1A peptide | Bioorg Med Chem Lett 20: 6064-6 (2010) Article DOI: 10.1016/j.bmcl.2010.08.051 BindingDB Entry DOI: 10.7270/Q2FJ2H04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50522829 (CHEMBL4457734) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522814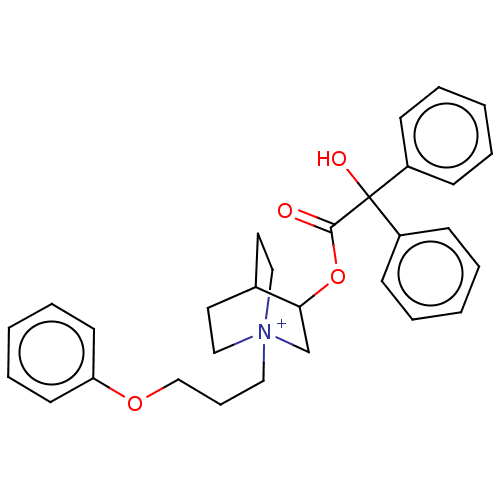 (CHEMBL4461243) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50377964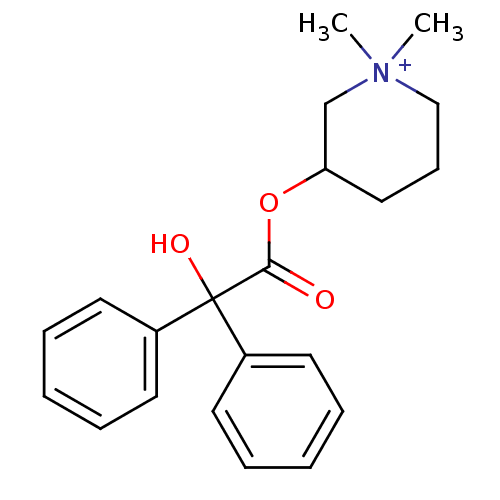 (Cantil | Glycophenylate | MEPENZOLATE BROMIDE | Me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysis | Bioorg Med Chem 22: 3488-97 (2014) Article DOI: 10.1016/j.bmc.2014.04.029 BindingDB Entry DOI: 10.7270/Q2VD7111 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522818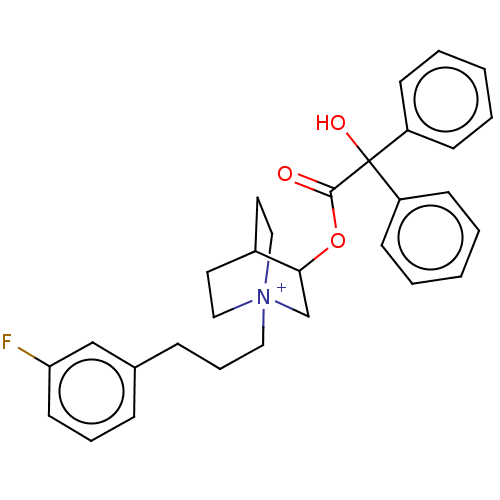 (CHEMBL4436930) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522817 (CHEMBL4448944) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50375457 (CHEMBL261476) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5HT2A receptor expressed in CHO cells | Bioorg Med Chem 16: 1966-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.100 BindingDB Entry DOI: 10.7270/Q2TH8NKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50375451 (CHEMBL408579) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5HT2A receptor expressed in CHO cells | Bioorg Med Chem 16: 1966-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.100 BindingDB Entry DOI: 10.7270/Q2TH8NKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50417445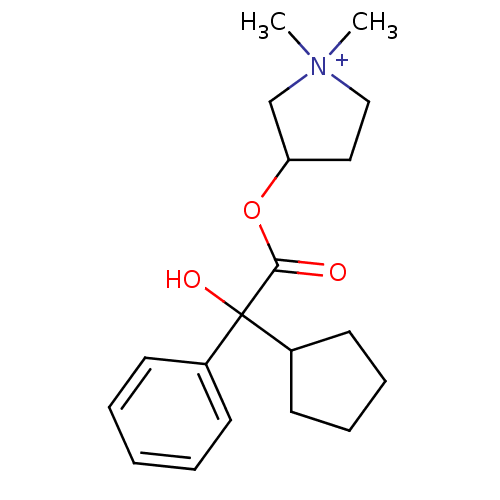 (AHR-504 | CUVPOSA | GLYCOPYRROLATE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50522809 (CHEMBL4540604) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50522822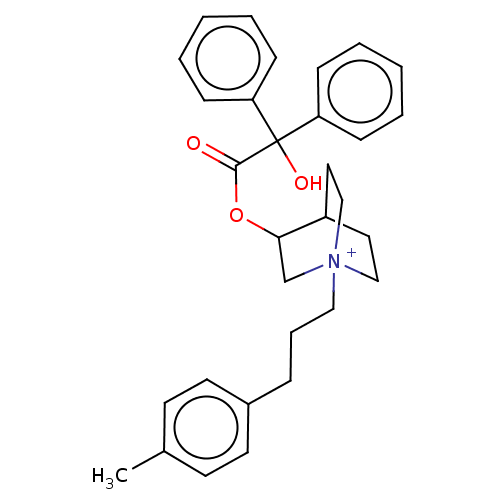 (CHEMBL4590868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50522814 (CHEMBL4461243) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50375450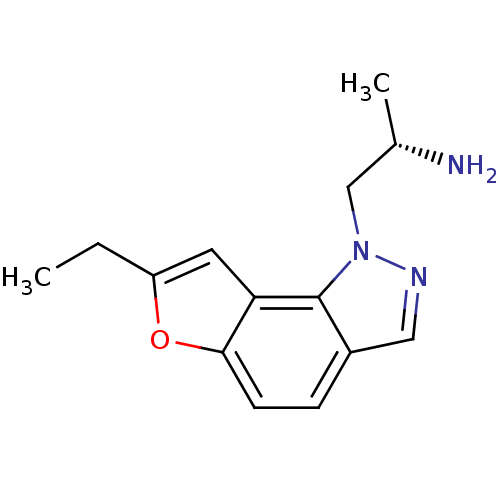 (CHEMBL407909 | YM-348) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells | Bioorg Med Chem 16: 1966-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.100 BindingDB Entry DOI: 10.7270/Q2TH8NKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM86441 (CAS_3045225 | NSC_3045225 | YM348) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | Eur J Pharmacol 483: 37-43 (2004) Article DOI: 10.1016/j.ejphar.2003.10.004 BindingDB Entry DOI: 10.7270/Q2ZG6QTV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50375450 (CHEMBL407909 | YM-348) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells | Bioorg Med Chem 16: 3309-20 (2008) Article DOI: 10.1016/j.bmc.2007.12.009 BindingDB Entry DOI: 10.7270/Q2PN96H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522823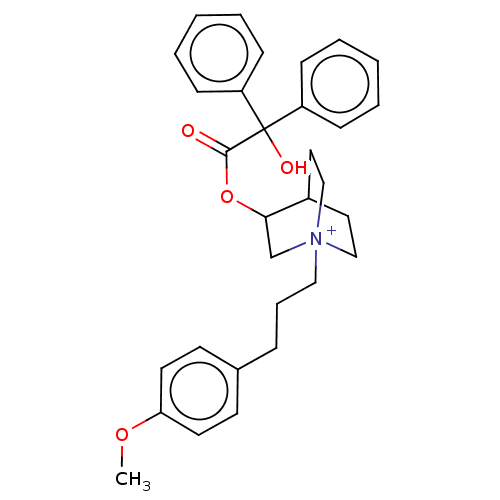 (CHEMBL4545504) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522832 (CHEMBL4483083) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50522818 (CHEMBL4436930) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522822 (CHEMBL4590868) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50327943 (Aplysiatoxin | CHEMBL1256416) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCepsilon C1B peptide | Bioorg Med Chem Lett 20: 6064-6 (2010) Article DOI: 10.1016/j.bmcl.2010.08.051 BindingDB Entry DOI: 10.7270/Q2FJ2H04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491400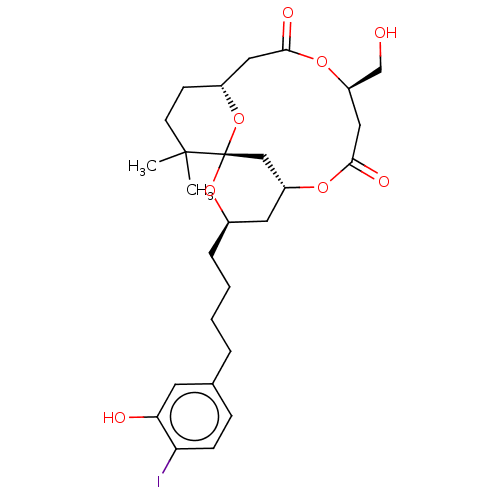 (CHEMBL2381165) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1B domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50375458 (CHEMBL406851) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells | Bioorg Med Chem 16: 1966-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.100 BindingDB Entry DOI: 10.7270/Q2TH8NKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522820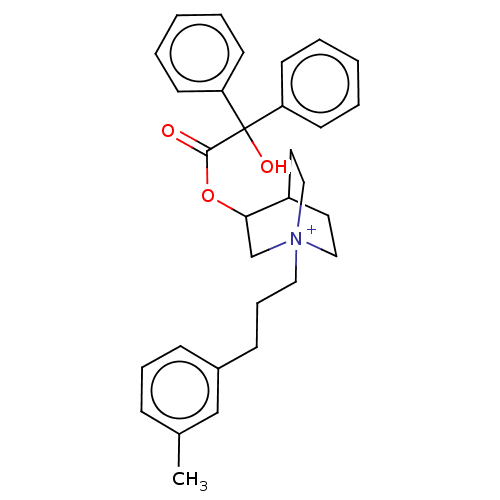 (CHEMBL4463636) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50522824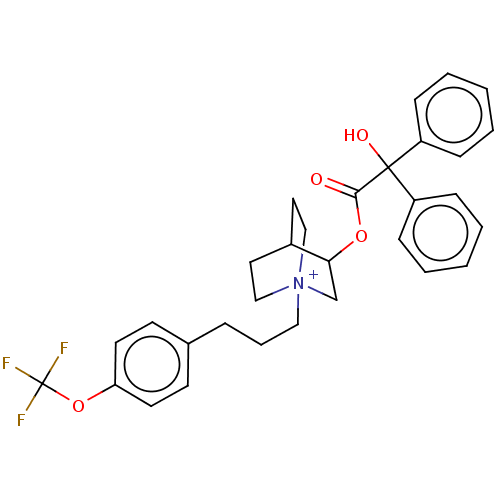 (CHEMBL4521993) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50417445 (AHR-504 | CUVPOSA | GLYCOPYRROLATE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522813 (CHEMBL4539799) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50522821 (CHEMBL4450188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50522820 (CHEMBL4463636) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50377964 (Cantil | Glycophenylate | MEPENZOLATE BROMIDE | Me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50522819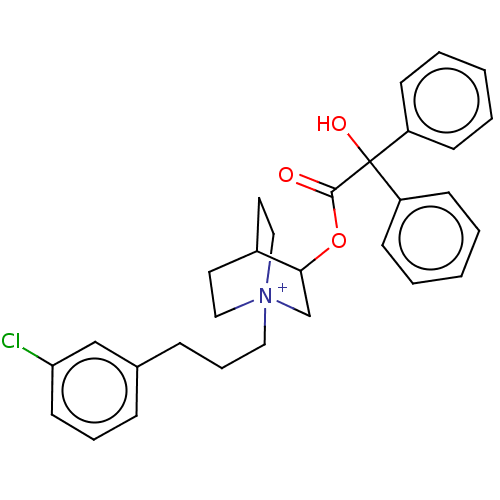 (CHEMBL4452706) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method | Bioorg Med Chem 27: 3339-3346 (2019) Article DOI: 10.1016/j.bmc.2019.06.016 BindingDB Entry DOI: 10.7270/Q2DR2ZXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1726 total ) | Next | Last >> |