 Found 986 hits with Last Name = 'ho' and Initial = 'kh'
Found 986 hits with Last Name = 'ho' and Initial = 'kh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053067
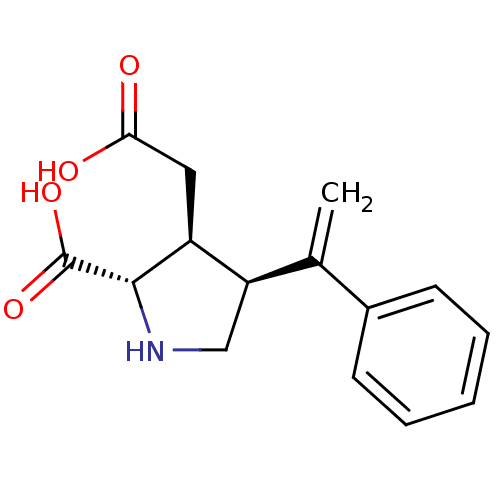
((2S,3S,4S)-3-Carboxymethyl-4-(1-phenyl-vinyl)-pyrr...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccccc1 Show InChI InChI=1S/C15H17NO4/c1-9(10-5-3-2-4-6-10)12-8-16-14(15(19)20)11(12)7-13(17)18/h2-6,11-12,14,16H,1,7-8H2,(H,17,18)(H,19,20)/t11-,12+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053071
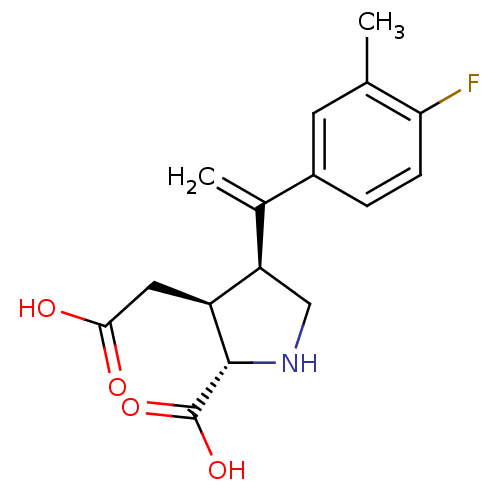
((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-fluoro-3-methyl...)Show SMILES Cc1cc(ccc1F)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H18FNO4/c1-8-5-10(3-4-13(8)17)9(2)12-7-18-15(16(21)22)11(12)6-14(19)20/h3-5,11-12,15,18H,2,6-7H2,1H3,(H,19,20)(H,21,22)/t11-,12+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053075
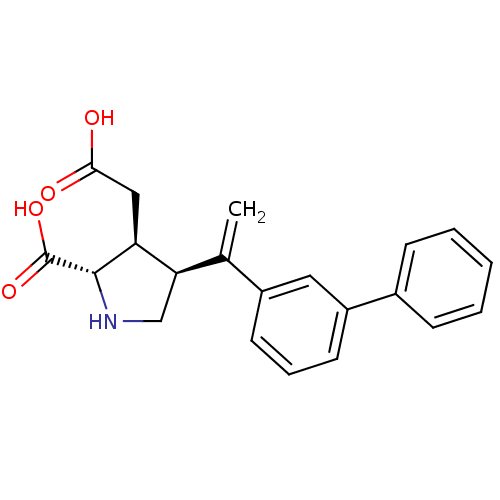
((2S,3S,4S)-4-(1-Biphenyl-3-yl-vinyl)-3-carboxymeth...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C21H21NO4/c1-13(18-12-22-20(21(25)26)17(18)11-19(23)24)15-8-5-9-16(10-15)14-6-3-2-4-7-14/h2-10,17-18,20,22H,1,11-12H2,(H,23,24)(H,25,26)/t17-,18+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053083
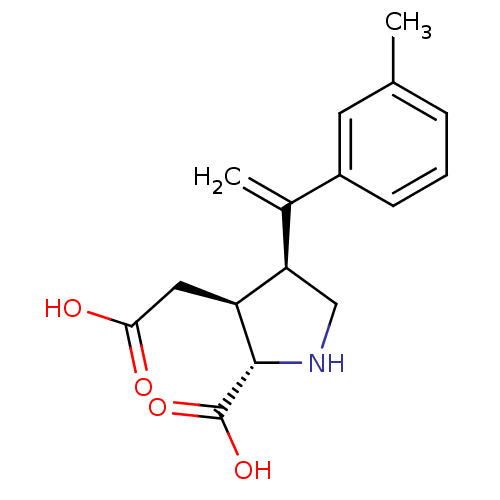
((2S,3S,4S)-3-Carboxymethyl-4-(1-m-tolyl-vinyl)-pyr...)Show SMILES Cc1cccc(c1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H19NO4/c1-9-4-3-5-11(6-9)10(2)13-8-17-15(16(20)21)12(13)7-14(18)19/h3-6,12-13,15,17H,2,7-8H2,1H3,(H,18,19)(H,20,21)/t12-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053080
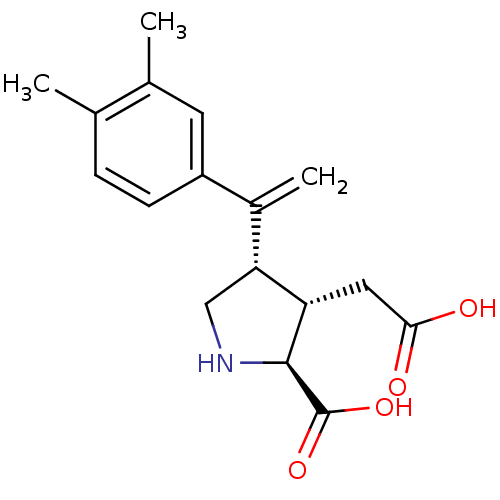
((2S,3S,4S)-3-Carboxymethyl-4-[1-(3,4-dimethyl-phen...)Show SMILES Cc1ccc(cc1C)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C17H21NO4/c1-9-4-5-12(6-10(9)2)11(3)14-8-18-16(17(21)22)13(14)7-15(19)20/h4-6,13-14,16,18H,3,7-8H2,1-2H3,(H,19,20)(H,21,22)/t13-,14+,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053088
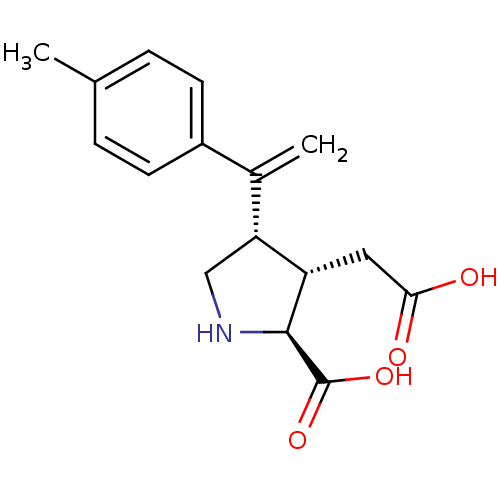
((2S,3S,4S)-3-Carboxymethyl-4-(1-p-tolyl-vinyl)-pyr...)Show SMILES Cc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H19NO4/c1-9-3-5-11(6-4-9)10(2)13-8-17-15(16(20)21)12(13)7-14(18)19/h3-6,12-13,15,17H,2,7-8H2,1H3,(H,18,19)(H,20,21)/t12-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053087
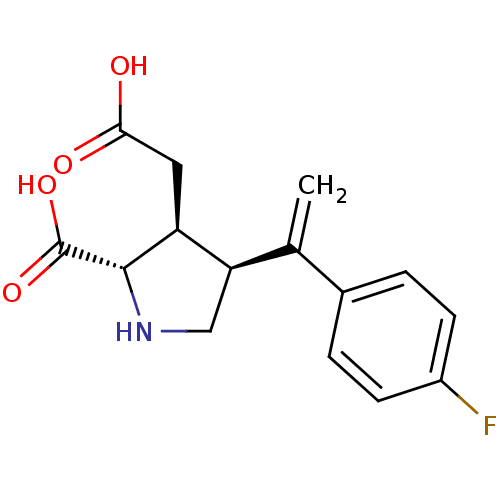
((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-fluoro-phenyl)-...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(F)cc1 Show InChI InChI=1S/C15H16FNO4/c1-8(9-2-4-10(16)5-3-9)12-7-17-14(15(20)21)11(12)6-13(18)19/h2-5,11-12,14,17H,1,6-7H2,(H,18,19)(H,20,21)/t11-,12+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053064
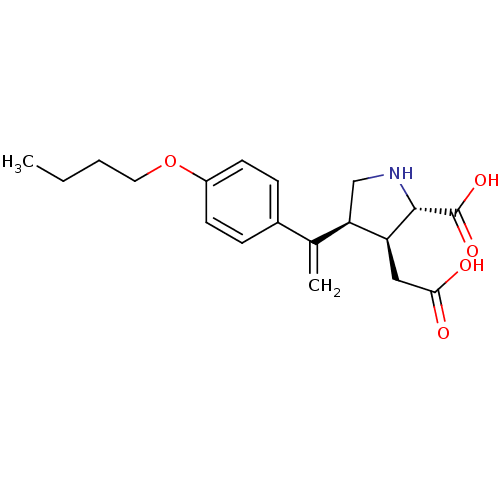
((2S,3S,4S)-4-[1-(4-Butoxy-phenyl)-vinyl]-3-carboxy...)Show SMILES CCCCOc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C19H25NO5/c1-3-4-9-25-14-7-5-13(6-8-14)12(2)16-11-20-18(19(23)24)15(16)10-17(21)22/h5-8,15-16,18,20H,2-4,9-11H2,1H3,(H,21,22)(H,23,24)/t15-,16+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50494356
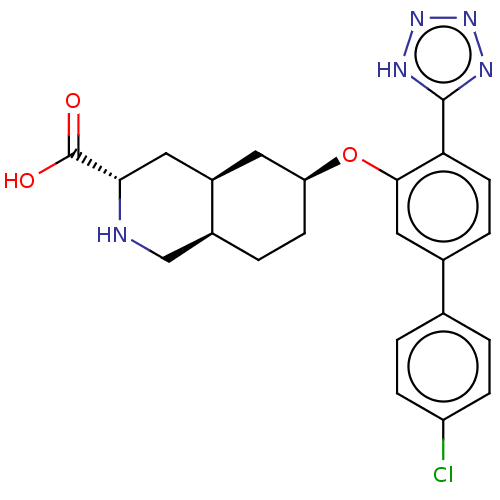
(CHEMBL3088070)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(ccc1-c1nnn[nH]1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H24ClN5O3.ClH/c24-17-5-1-13(2-6-17)14-4-8-19(22-26-28-29-27-22)21(11-14)32-18-7-3-15-12-25-20(23(30)31)10-16(15)9-18;/h1-2,4-6,8,11,15-16,18,20,25H,3,7,9-10,12H2,(H,30,31)(H,26,27,28,29);1H/t15-,16+,18-,20-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from homomeric recombinant GluA2 receptor (unknown origin) |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053063

((2S,3S,4S)-3-Carboxymethyl-4-[1-(3-methoxy-phenyl)...)Show SMILES COc1cccc(c1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H19NO5/c1-9(10-4-3-5-11(6-10)22-2)13-8-17-15(16(20)21)12(13)7-14(18)19/h3-6,12-13,15,17H,1,7-8H2,2H3,(H,18,19)(H,20,21)/t12-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053059

((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-propyl-phenyl)-...)Show SMILES CCCc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C18H23NO4/c1-3-4-12-5-7-13(8-6-12)11(2)15-10-19-17(18(22)23)14(15)9-16(20)21/h5-8,14-15,17,19H,2-4,9-10H2,1H3,(H,20,21)(H,22,23)/t14-,15+,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053068
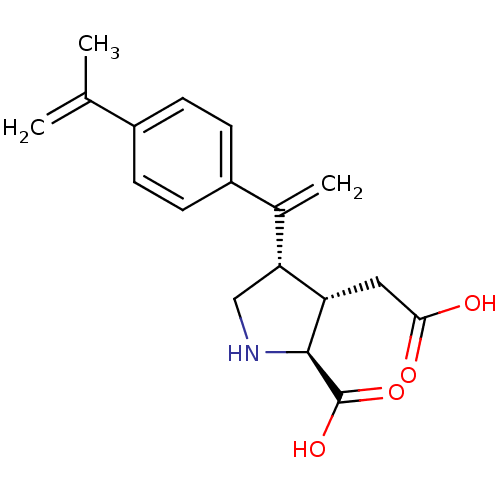
((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-isopropenyl-phe...)Show SMILES CC(=C)c1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C18H21NO4/c1-10(2)12-4-6-13(7-5-12)11(3)15-9-19-17(18(22)23)14(15)8-16(20)21/h4-7,14-15,17,19H,1,3,8-9H2,2H3,(H,20,21)(H,22,23)/t14-,15+,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50002369
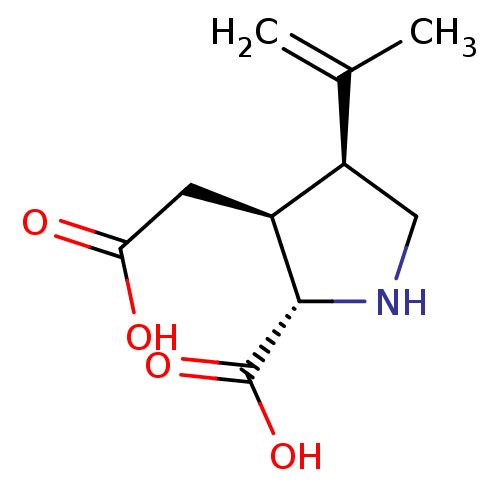
((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053073

((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-methoxy-phenyl)...)Show SMILES COc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H19NO5/c1-9(10-3-5-11(22-2)6-4-10)13-8-17-15(16(20)21)12(13)7-14(18)19/h3-6,12-13,15,17H,1,7-8H2,2H3,(H,18,19)(H,20,21)/t12-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053061
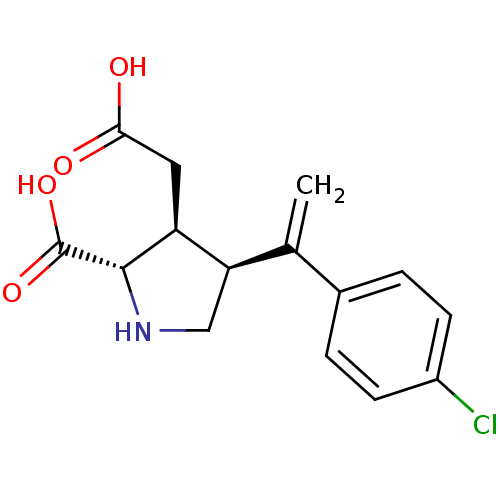
((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-chloro-phenyl)-...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H16ClNO4/c1-8(9-2-4-10(16)5-3-9)12-7-17-14(15(20)21)11(12)6-13(18)19/h2-5,11-12,14,17H,1,6-7H2,(H,18,19)(H,20,21)/t11-,12+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053082
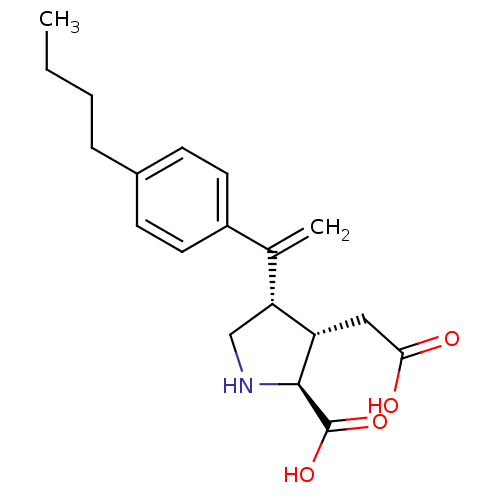
((2S,3S,4S)-4-[1-(4-Butyl-phenyl)-vinyl]-3-carboxym...)Show SMILES CCCCc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C19H25NO4/c1-3-4-5-13-6-8-14(9-7-13)12(2)16-11-20-18(19(23)24)15(16)10-17(21)22/h6-9,15-16,18,20H,2-5,10-11H2,1H3,(H,21,22)(H,23,24)/t15-,16+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053065
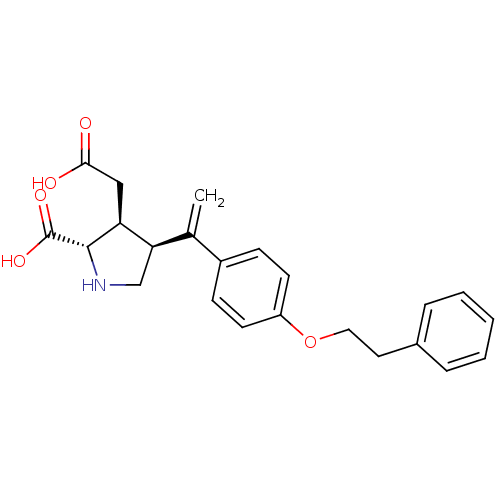
((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-phenethyloxy-ph...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(OCCc2ccccc2)cc1 Show InChI InChI=1S/C23H25NO5/c1-15(20-14-24-22(23(27)28)19(20)13-21(25)26)17-7-9-18(10-8-17)29-12-11-16-5-3-2-4-6-16/h2-10,19-20,22,24H,1,11-14H2,(H,25,26)(H,27,28)/t19-,20+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053089
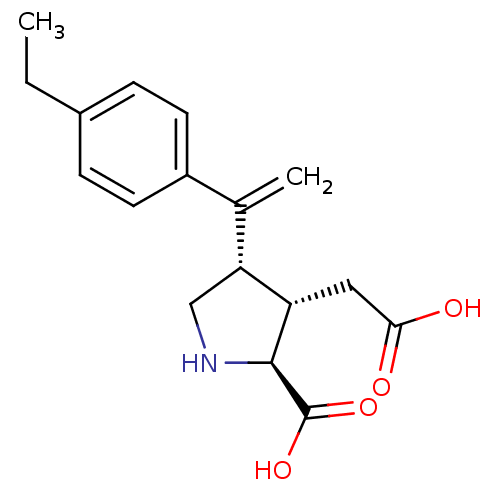
((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-ethyl-phenyl)-v...)Show SMILES CCc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C17H21NO4/c1-3-11-4-6-12(7-5-11)10(2)14-9-18-16(17(21)22)13(14)8-15(19)20/h4-7,13-14,16,18H,2-3,8-9H2,1H3,(H,19,20)(H,21,22)/t13-,14+,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053070
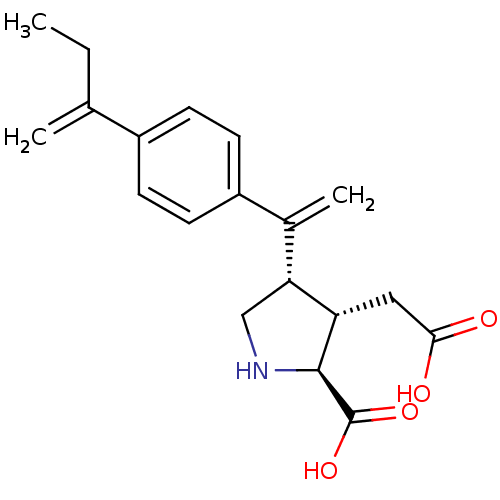
((2S,3S,4S)-3-Carboxymethyl-4-{1-[4-(1-methylene-pr...)Show SMILES CCC(=C)c1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C19H23NO4/c1-4-11(2)13-5-7-14(8-6-13)12(3)16-10-20-18(19(23)24)15(16)9-17(21)22/h5-8,15-16,18,20H,2-4,9-10H2,1H3,(H,21,22)(H,23,24)/t15-,16+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053076
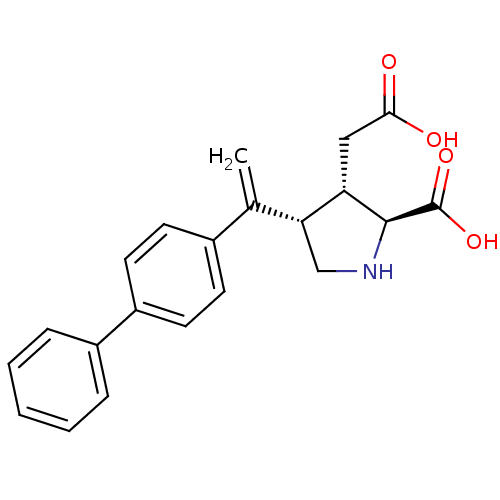
((2S,3S,4S)-4-(1-Biphenyl-4-yl-vinyl)-3-carboxymeth...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C21H21NO4/c1-13(18-12-22-20(21(25)26)17(18)11-19(23)24)14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-10,17-18,20,22H,1,11-12H2,(H,23,24)(H,25,26)/t17-,18+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053062

((2S,3S,4S)-3-Carboxymethyl-4-[1-(3,5-dimethyl-phen...)Show SMILES Cc1cc(C)cc(c1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C17H21NO4/c1-9-4-10(2)6-12(5-9)11(3)14-8-18-16(17(21)22)13(14)7-15(19)20/h4-6,13-14,16,18H,3,7-8H2,1-2H3,(H,19,20)(H,21,22)/t13-,14+,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053074
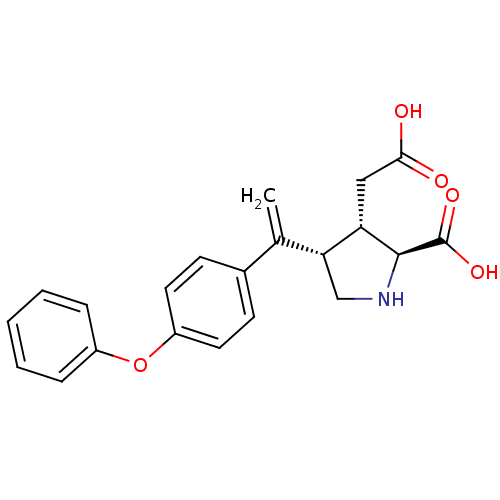
((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-phenoxy-phenyl)...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C21H21NO5/c1-13(18-12-22-20(21(25)26)17(18)11-19(23)24)14-7-9-16(10-8-14)27-15-5-3-2-4-6-15/h2-10,17-18,20,22H,1,11-12H2,(H,23,24)(H,25,26)/t17-,18+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053077
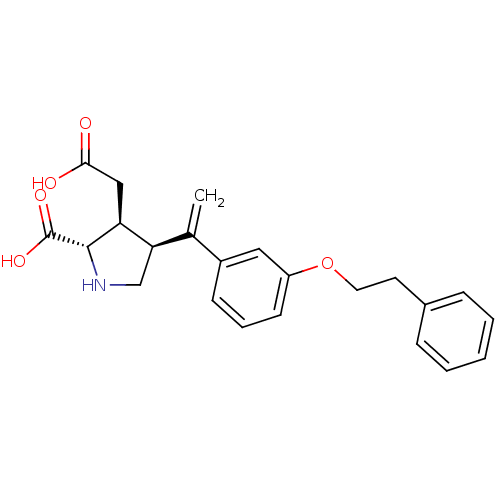
((2S,3S,4S)-3-Carboxymethyl-4-[1-(3-phenethyloxy-ph...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1cccc(OCCc2ccccc2)c1 Show InChI InChI=1S/C23H25NO5/c1-15(20-14-24-22(23(27)28)19(20)13-21(25)26)17-8-5-9-18(12-17)29-11-10-16-6-3-2-4-7-16/h2-9,12,19-20,22,24H,1,10-11,13-14H2,(H,25,26)(H,27,28)/t19-,20+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053058
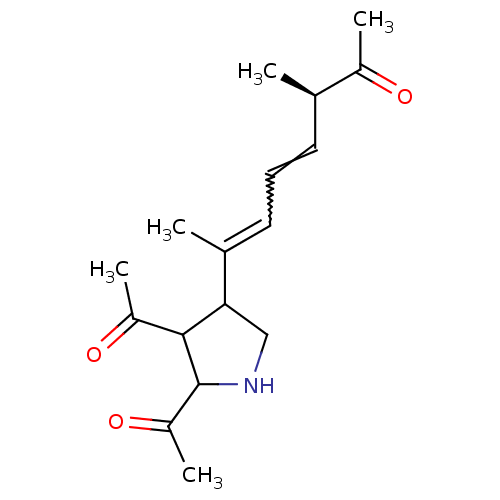
((4E,6E)-(R)-7-[4,5-Bis-(1-hydroxy-vinyl)-pyrrolidi...)Show SMILES C[C@H](C=CC=C(C)C1CNC(C1C(C)=O)C(C)=O)C(C)=O |w:4.3| Show InChI InChI=1S/C17H25NO3/c1-10(12(3)19)7-6-8-11(2)15-9-18-17(14(5)21)16(15)13(4)20/h6-8,10,15-18H,9H2,1-5H3/t10-,15?,16?,17?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053060
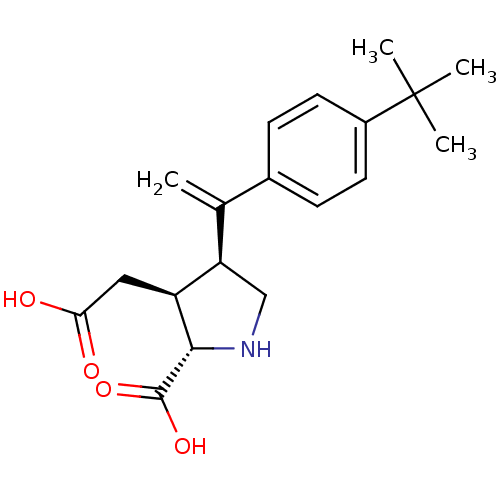
((2S,3S,4S)-4-[1-(4-tert-Butyl-phenyl)-vinyl]-3-car...)Show SMILES CC(C)(C)c1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C19H25NO4/c1-11(12-5-7-13(8-6-12)19(2,3)4)15-10-20-17(18(23)24)14(15)9-16(21)22/h5-8,14-15,17,20H,1,9-10H2,2-4H3,(H,21,22)(H,23,24)/t14-,15+,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494356

(CHEMBL3088070)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(ccc1-c1nnn[nH]1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H24ClN5O3.ClH/c24-17-5-1-13(2-6-17)14-4-8-19(22-26-28-29-27-22)21(11-14)32-18-7-3-15-12-25-20(23(30)31)10-16(15)9-18;/h1-2,4-6,8,11,15-16,18,20,25H,3,7,9-10,12H2,(H,30,31)(H,26,27,28,29);1H/t15-,16+,18-,20-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053079
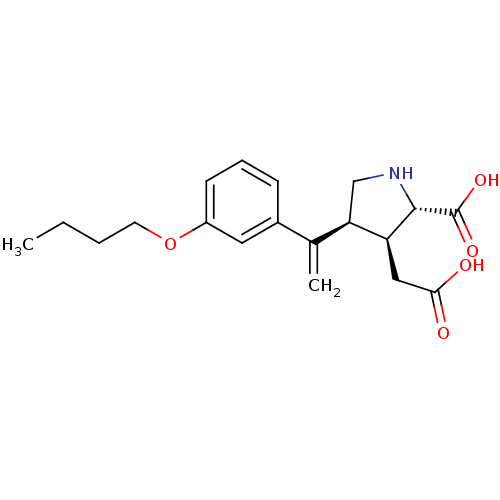
((2S,3S,4S)-4-[1-(3-Butoxy-phenyl)-vinyl]-3-carboxy...)Show SMILES CCCCOc1cccc(c1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C19H25NO5/c1-3-4-8-25-14-7-5-6-13(9-14)12(2)16-11-20-18(19(23)24)15(16)10-17(21)22/h5-7,9,15-16,18,20H,2-4,8,10-11H2,1H3,(H,21,22)(H,23,24)/t15-,16+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053067

((2S,3S,4S)-3-Carboxymethyl-4-(1-phenyl-vinyl)-pyrr...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccccc1 Show InChI InChI=1S/C15H17NO4/c1-9(10-5-3-2-4-6-10)12-8-16-14(15(19)20)11(12)7-13(17)18/h2-6,11-12,14,16H,1,7-8H2,(H,17,18)(H,19,20)/t11-,12+,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053061

((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-chloro-phenyl)-...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H16ClNO4/c1-8(9-2-4-10(16)5-3-9)12-7-17-14(15(20)21)11(12)6-13(18)19/h2-5,11-12,14,17H,1,6-7H2,(H,18,19)(H,20,21)/t11-,12+,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053087

((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-fluoro-phenyl)-...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(F)cc1 Show InChI InChI=1S/C15H16FNO4/c1-8(9-2-4-10(16)5-3-9)12-7-17-14(15(20)21)11(12)6-13(18)19/h2-5,11-12,14,17H,1,6-7H2,(H,18,19)(H,20,21)/t11-,12+,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053088

((2S,3S,4S)-3-Carboxymethyl-4-(1-p-tolyl-vinyl)-pyr...)Show SMILES Cc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H19NO4/c1-9-3-5-11(6-4-9)10(2)13-8-17-15(16(20)21)12(13)7-14(18)19/h3-6,12-13,15,17H,2,7-8H2,1H3,(H,18,19)(H,20,21)/t12-,13+,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 5
(Homo sapiens (Human)) | BDBM86754
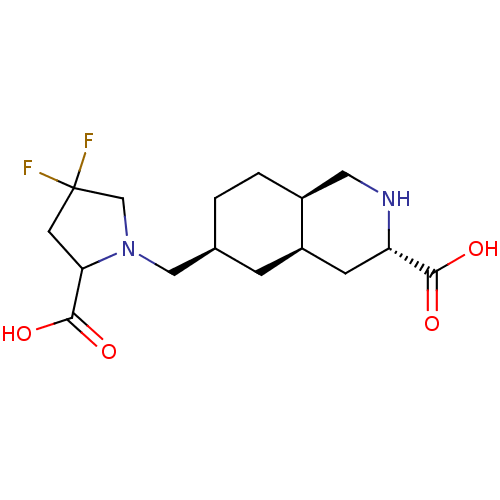
(LY 466195 | LY-466195)Show SMILES OC(=O)C1CC(F)(F)CN1C[C@H]1CC[C@H]2CN[C@@H](C[C@H]2C1)C(O)=O |r| Show InChI InChI=1S/C16H24F2N2O4/c17-16(18)5-13(15(23)24)20(8-16)7-9-1-2-10-6-19-12(14(21)22)4-11(10)3-9/h9-13,19H,1-8H2,(H,21,22)(H,23,24)/t9-,10-,11+,12-,13?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50002369

((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053071

((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-fluoro-3-methyl...)Show SMILES Cc1cc(ccc1F)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H18FNO4/c1-8-5-10(3-4-13(8)17)9(2)12-7-18-15(16(21)22)11(12)6-14(19)20/h3-5,11-12,15,18H,2,6-7H2,1H3,(H,19,20)(H,21,22)/t11-,12+,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053073

((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-methoxy-phenyl)...)Show SMILES COc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H19NO5/c1-9(10-3-5-11(22-2)6-4-10)13-8-17-15(16(20)21)12(13)7-14(18)19/h3-6,12-13,15,17H,1,7-8H2,2H3,(H,18,19)(H,20,21)/t12-,13+,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit alpha
(Homo sapiens (Human)) | BDBM50257823
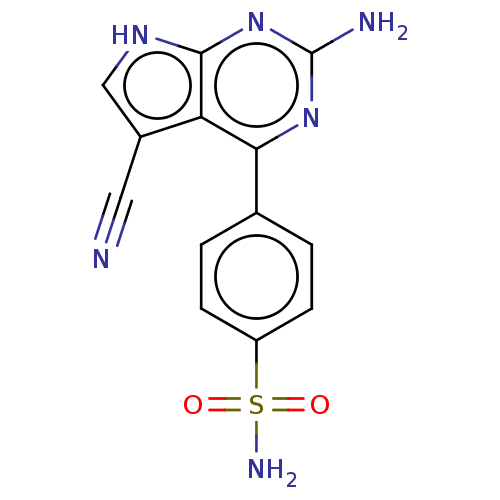
(CHEMBL4089551)Show SMILES Nc1nc(-c2ccc(cc2)S(N)(=O)=O)c2c(c[nH]c2n1)C#N Show InChI InChI=1S/C13H10N6O2S/c14-5-8-6-17-12-10(8)11(18-13(15)19-12)7-1-3-9(4-2-7)22(16,20)21/h1-4,6H,(H2,16,20,21)(H3,15,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde , 161 Cathedral Street, Glasgow G4 0NR, Scotland, United Kingdom.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged IKKalpha (1 to 745 residues) expressed in baculovirus expression system using biotinylated IkBa... |
J Med Chem 60: 7043-7066 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00484
BindingDB Entry DOI: 10.7270/Q2M90C4N |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053089

((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-ethyl-phenyl)-v...)Show SMILES CCc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C17H21NO4/c1-3-11-4-6-12(7-5-11)10(2)14-9-18-16(17(21)22)13(14)8-15(19)20/h4-7,13-14,16,18H,2-3,8-9H2,1H3,(H,19,20)(H,21,22)/t13-,14+,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494342

(CHEMBL3088072)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(ccc1-c1nnn[nH]1)-c1cccs1 |r| Show InChI InChI=1S/C21H23N5O3S.ClH/c27-21(28)17-9-14-8-15(5-3-13(14)11-22-17)29-18-10-12(19-2-1-7-30-19)4-6-16(18)20-23-25-26-24-20;/h1-2,4,6-7,10,13-15,17,22H,3,5,8-9,11H2,(H,27,28)(H,23,24,25,26);1H/t13-,14+,15-,17-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053075

((2S,3S,4S)-4-(1-Biphenyl-3-yl-vinyl)-3-carboxymeth...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C21H21NO4/c1-13(18-12-22-20(21(25)26)17(18)11-19(23)24)15-8-5-9-16(10-15)14-6-3-2-4-7-14/h2-10,17-18,20,22H,1,11-12H2,(H,23,24)(H,25,26)/t17-,18+,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053063

((2S,3S,4S)-3-Carboxymethyl-4-[1-(3-methoxy-phenyl)...)Show SMILES COc1cccc(c1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H19NO5/c1-9(10-4-3-5-11(6-10)22-2)13-8-17-15(16(20)21)12(13)7-14(18)19/h3-6,12-13,15,17H,1,7-8H2,2H3,(H,18,19)(H,20,21)/t12-,13+,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 93.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50257884
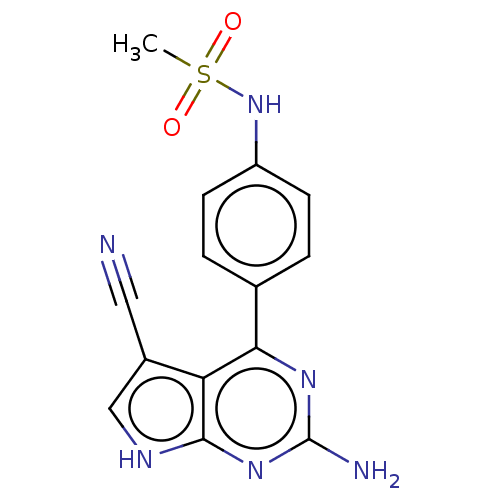
(CHEMBL4065551)Show SMILES CS(=O)(=O)Nc1ccc(cc1)-c1nc(N)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C14H12N6O2S/c1-23(21,22)20-10-4-2-8(3-5-10)12-11-9(6-15)7-17-13(11)19-14(16)18-12/h2-5,7,20H,1H3,(H3,16,17,18,19) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde , 161 Cathedral Street, Glasgow G4 0NR, Scotland, United Kingdom.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human IKKbeta expressed in baculovirus infected sf9 insect cells using biotinylated IkBalpha as substrate after... |
J Med Chem 60: 7043-7066 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00484
BindingDB Entry DOI: 10.7270/Q2M90C4N |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494350
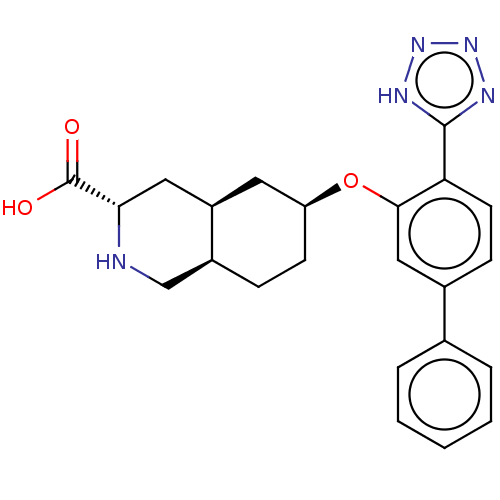
(CHEMBL3088064)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(ccc1-c1nnn[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C23H25N5O3.ClH/c29-23(30)20-11-17-10-18(8-6-16(17)13-24-20)31-21-12-15(14-4-2-1-3-5-14)7-9-19(21)22-25-27-28-26-22;/h1-5,7,9,12,16-18,20,24H,6,8,10-11,13H2,(H,29,30)(H,25,26,27,28);1H/t16-,17+,18-,20-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053080

((2S,3S,4S)-3-Carboxymethyl-4-[1-(3,4-dimethyl-phen...)Show SMILES Cc1ccc(cc1C)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C17H21NO4/c1-9-4-5-12(6-10(9)2)11(3)14-8-18-16(17(21)22)13(14)7-15(19)20/h4-6,13-14,16,18H,3,7-8H2,1-2H3,(H,19,20)(H,21,22)/t13-,14+,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50439332
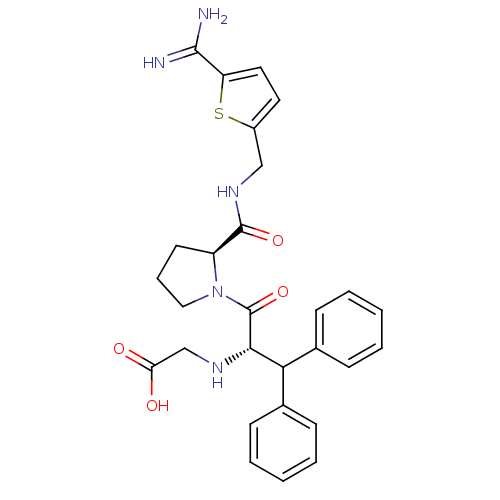
(CHEMBL2419745)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCCN2C(=O)[C@@H](NCC(O)=O)C(c2ccccc2)c2ccccc2)s1 |r| Show InChI InChI=1S/C28H31N5O4S/c29-26(30)22-14-13-20(38-22)16-32-27(36)21-12-7-15-33(21)28(37)25(31-17-23(34)35)24(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-6,8-11,13-14,21,24-25,31H,7,12,15-17H2,(H3,29,30)(H,32,36)(H,34,35)/t21-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human t-PA |
Bioorg Med Chem Lett 23: 4779-84 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.008
BindingDB Entry DOI: 10.7270/Q2X92CR8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053083

((2S,3S,4S)-3-Carboxymethyl-4-(1-m-tolyl-vinyl)-pyr...)Show SMILES Cc1cccc(c1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H19NO4/c1-9-4-3-5-11(6-9)10(2)13-8-17-15(16(20)21)12(13)7-14(18)19/h3-6,12-13,15,17H,2,7-8H2,1H3,(H,18,19)(H,20,21)/t12-,13+,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053066
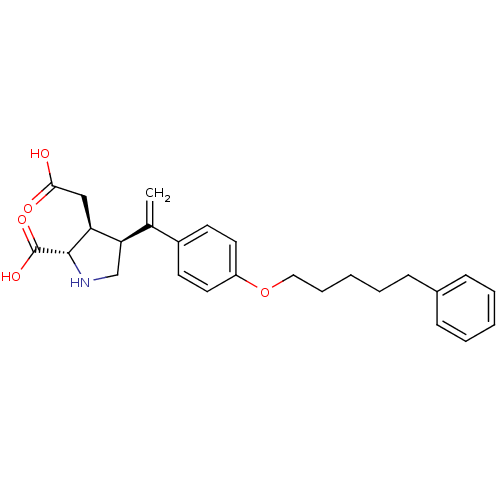
((2S,3S,4S)-3-Carboxymethyl-4-{1-[4-(5-phenyl-penty...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(OCCCCCc2ccccc2)cc1 Show InChI InChI=1S/C26H31NO5/c1-18(23-17-27-25(26(30)31)22(23)16-24(28)29)20-11-13-21(14-12-20)32-15-7-3-6-10-19-8-4-2-5-9-19/h2,4-5,8-9,11-14,22-23,25,27H,1,3,6-7,10,15-17H2,(H,28,29)(H,30,31)/t22-,23+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053065

((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-phenethyloxy-ph...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(OCCc2ccccc2)cc1 Show InChI InChI=1S/C23H25NO5/c1-15(20-14-24-22(23(27)28)19(20)13-21(25)26)17-7-9-18(10-8-17)29-12-11-16-5-3-2-4-6-16/h2-10,19-20,22,24H,1,11-14H2,(H,25,26)(H,27,28)/t19-,20+,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053084
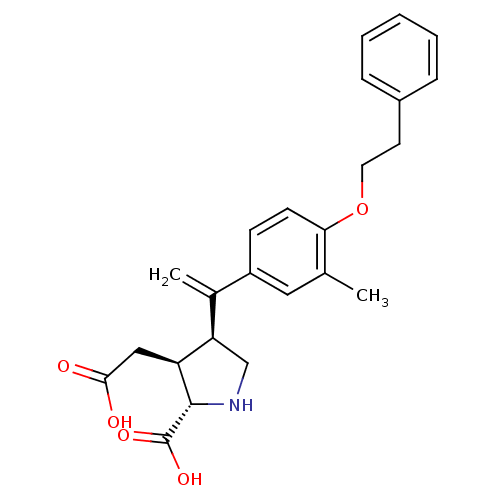
((2S,3S,4S)-3-Carboxymethyl-4-[1-(3-methyl-4-phenet...)Show SMILES Cc1cc(ccc1OCCc1ccccc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C24H27NO5/c1-15-12-18(8-9-21(15)30-11-10-17-6-4-3-5-7-17)16(2)20-14-25-23(24(28)29)19(20)13-22(26)27/h3-9,12,19-20,23,25H,2,10-11,13-14H2,1H3,(H,26,27)(H,28,29)/t19-,20+,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053076

((2S,3S,4S)-4-(1-Biphenyl-4-yl-vinyl)-3-carboxymeth...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C21H21NO4/c1-13(18-12-22-20(21(25)26)17(18)11-19(23)24)14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-10,17-18,20,22H,1,11-12H2,(H,23,24)(H,25,26)/t17-,18+,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053068

((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-isopropenyl-phe...)Show SMILES CC(=C)c1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C18H21NO4/c1-10(2)12-4-6-13(7-5-12)11(3)15-9-19-17(18(22)23)14(15)8-16(20)21/h4-7,14-15,17,19H,1,3,8-9H2,2H3,(H,20,21)(H,22,23)/t14-,15+,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data