

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50243396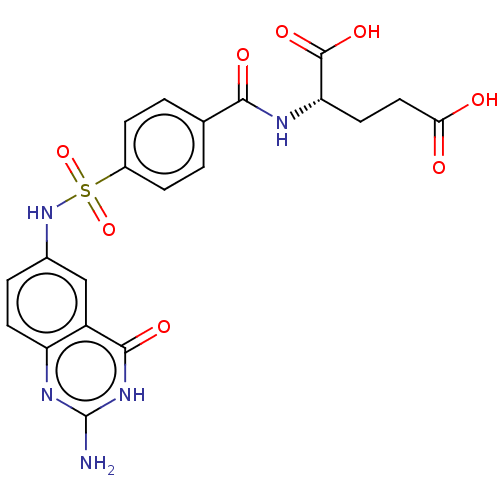 (CHEMBL1231520) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human AICARFT | J Med Chem 60: 9599-9616 (2017) Article DOI: 10.1021/acs.jmedchem.7b01046 BindingDB Entry DOI: 10.7270/Q2222X6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50006878 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled gastrin binding to gastrin receptor in guinea pig brain membranes | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50243461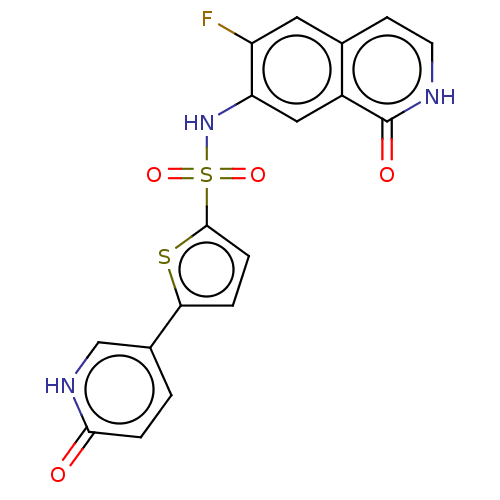 (CHEMBL4075503) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... | J Med Chem 60: 9599-9616 (2017) Article DOI: 10.1021/acs.jmedchem.7b01046 BindingDB Entry DOI: 10.7270/Q2222X6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50243462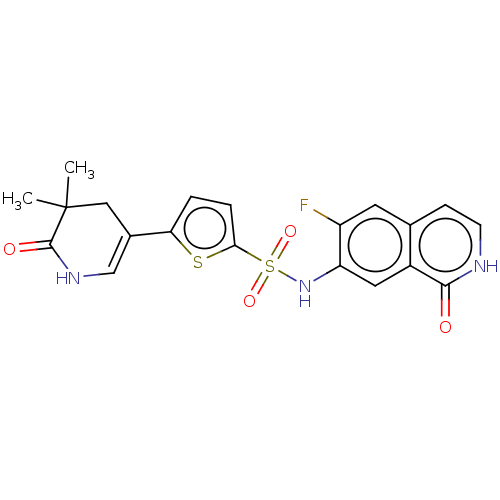 (CHEMBL4083899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... | J Med Chem 60: 9599-9616 (2017) Article DOI: 10.1021/acs.jmedchem.7b01046 BindingDB Entry DOI: 10.7270/Q2222X6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50243463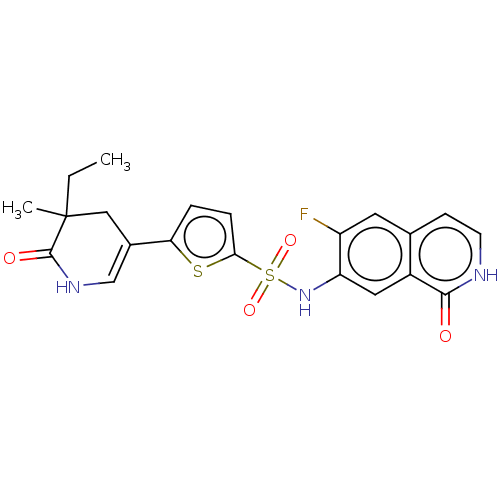 (CHEMBL4100363) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... | J Med Chem 60: 9599-9616 (2017) Article DOI: 10.1021/acs.jmedchem.7b01046 BindingDB Entry DOI: 10.7270/Q2222X6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50006878 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50006878 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled CCK-8 sulfate binding to CCK-B receptor in mouse brain membranes | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50243486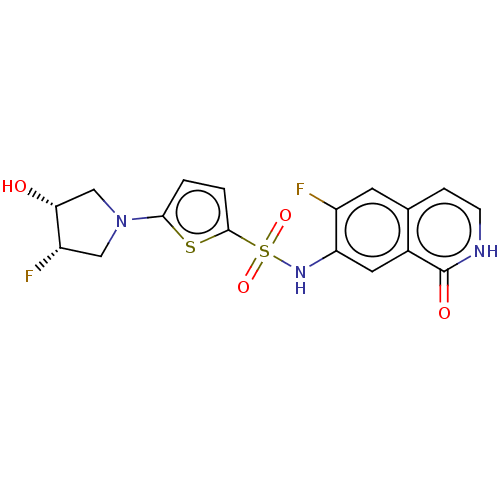 (CHEMBL4081385) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... | J Med Chem 60: 9599-9616 (2017) Article DOI: 10.1021/acs.jmedchem.7b01046 BindingDB Entry DOI: 10.7270/Q2222X6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50006842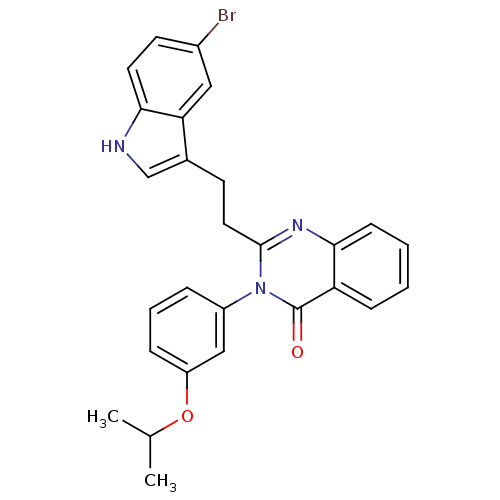 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-isopropox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50243487 (CHEMBL4091668) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... | J Med Chem 60: 9599-9616 (2017) Article DOI: 10.1021/acs.jmedchem.7b01046 BindingDB Entry DOI: 10.7270/Q2222X6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50243441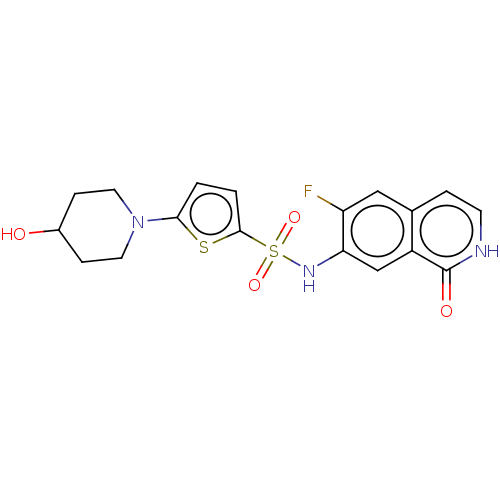 (CHEMBL4076500) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... | J Med Chem 60: 9599-9616 (2017) Article DOI: 10.1021/acs.jmedchem.7b01046 BindingDB Entry DOI: 10.7270/Q2222X6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50243443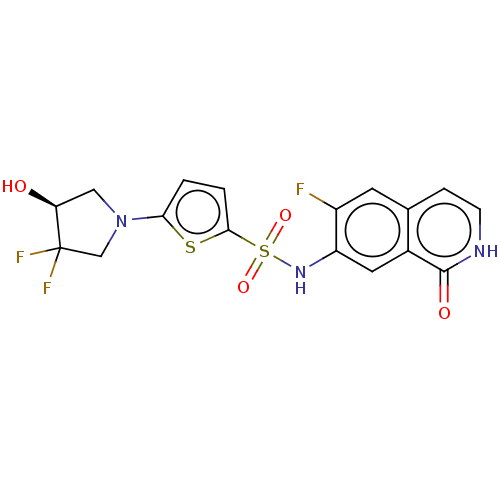 (CHEMBL4070790) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... | J Med Chem 60: 9599-9616 (2017) Article DOI: 10.1021/acs.jmedchem.7b01046 BindingDB Entry DOI: 10.7270/Q2222X6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50243485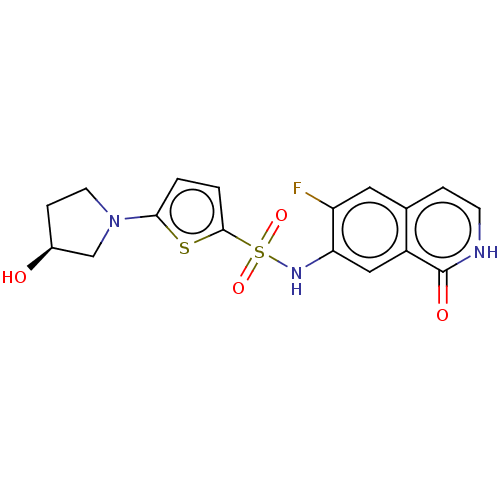 (CHEMBL4074469) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... | J Med Chem 60: 9599-9616 (2017) Article DOI: 10.1021/acs.jmedchem.7b01046 BindingDB Entry DOI: 10.7270/Q2222X6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011943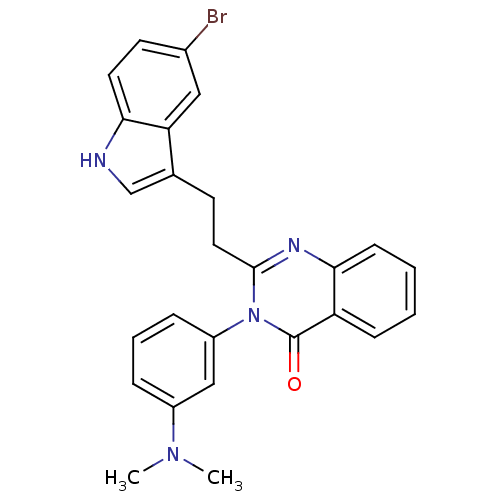 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-dimethyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50243415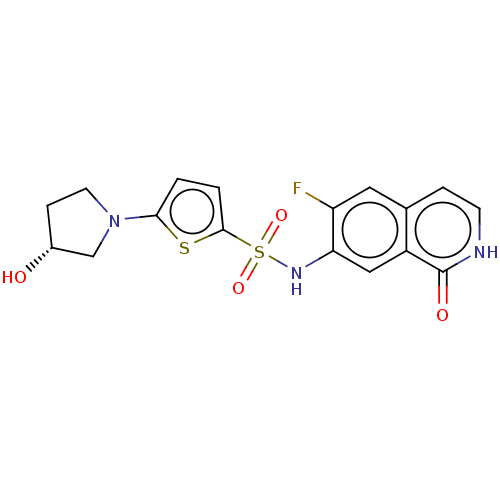 (CHEMBL4063104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... | J Med Chem 60: 9599-9616 (2017) Article DOI: 10.1021/acs.jmedchem.7b01046 BindingDB Entry DOI: 10.7270/Q2222X6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50243434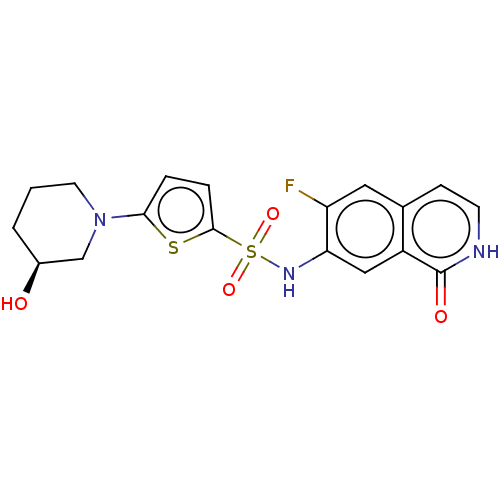 (CHEMBL4079085) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... | J Med Chem 60: 9599-9616 (2017) Article DOI: 10.1021/acs.jmedchem.7b01046 BindingDB Entry DOI: 10.7270/Q2222X6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50243442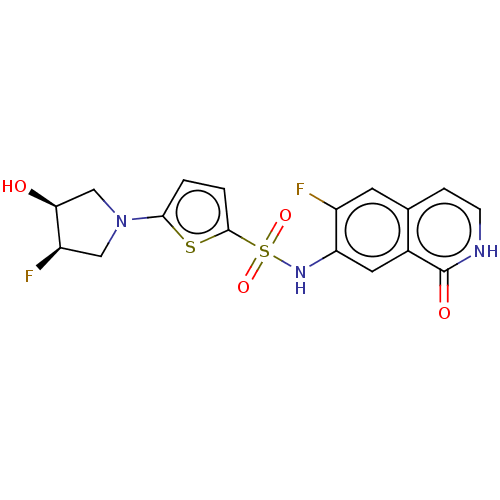 (CHEMBL4099409) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... | J Med Chem 60: 9599-9616 (2017) Article DOI: 10.1021/acs.jmedchem.7b01046 BindingDB Entry DOI: 10.7270/Q2222X6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50006838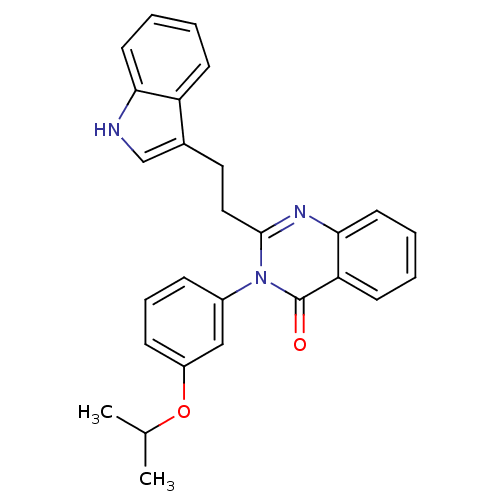 (2-[2-(1H-Indol-3-yl)-ethyl]-3-(3-isopropoxy-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50243433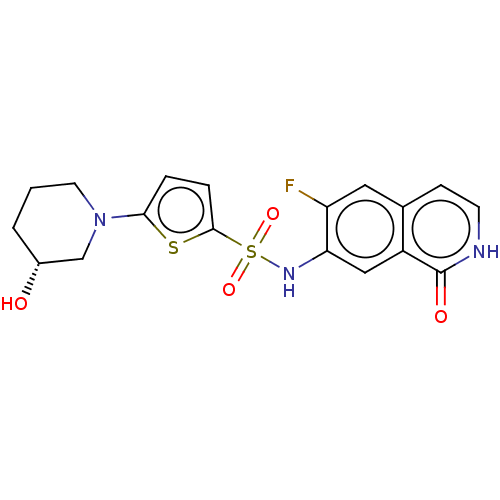 (CHEMBL4101204) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... | J Med Chem 60: 9599-9616 (2017) Article DOI: 10.1021/acs.jmedchem.7b01046 BindingDB Entry DOI: 10.7270/Q2222X6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011957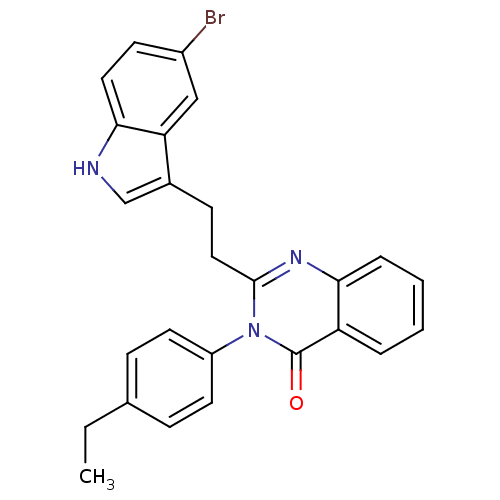 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(4-ethyl-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011939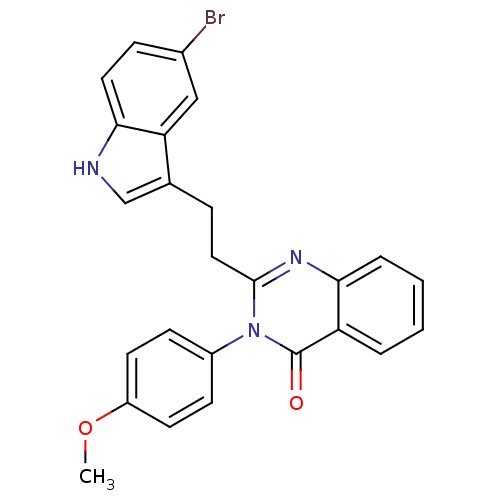 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(4-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011953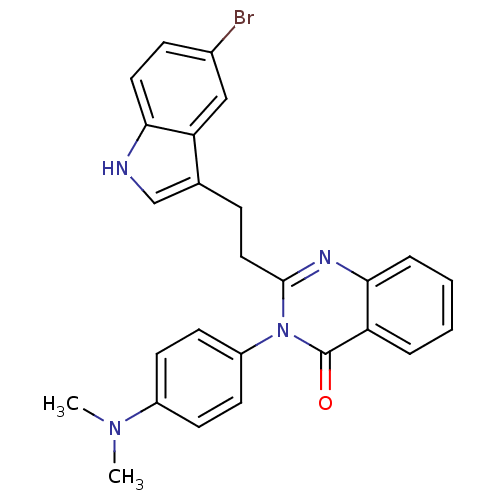 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(4-dimethyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011954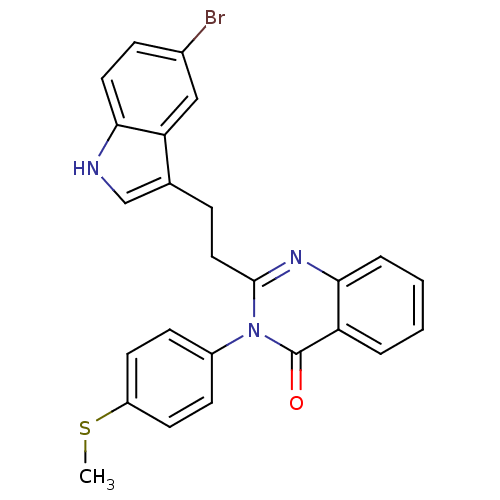 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(4-methylsul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011965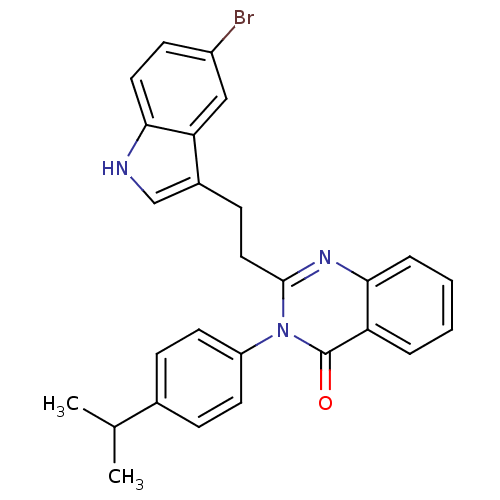 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(4-isopropyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011942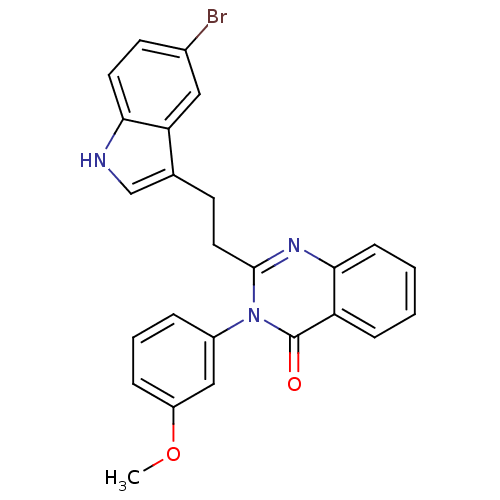 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011942 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled CCK-8 sulfate binding to Cholecystokinin type B receptor in guinea pig brain membranes | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011948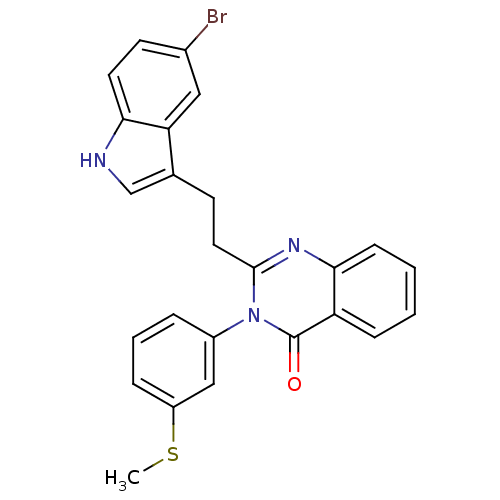 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-methylsul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011940 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-ethyl-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011945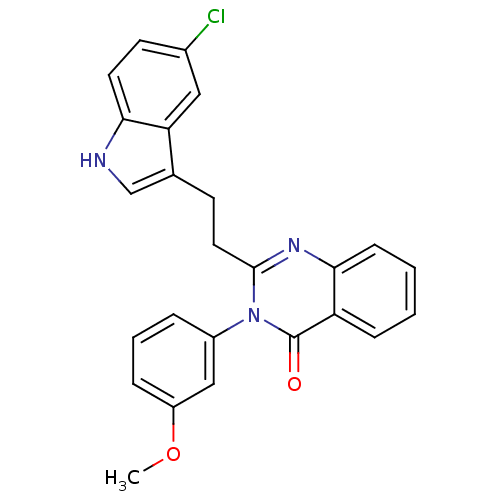 (2-[2-(5-Chloro-1H-indol-3-yl)-ethyl]-3-(3-methoxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011946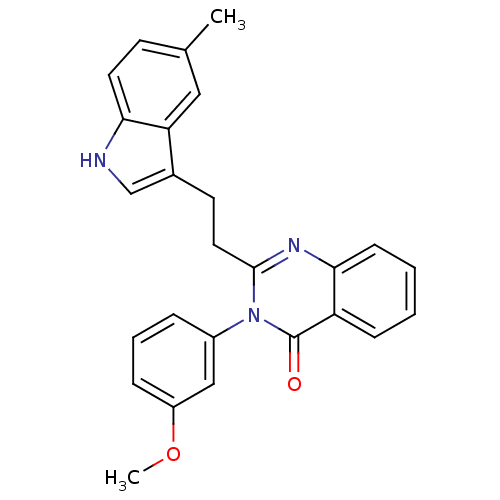 (3-(3-Methoxy-phenyl)-2-[2-(5-methyl-1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011950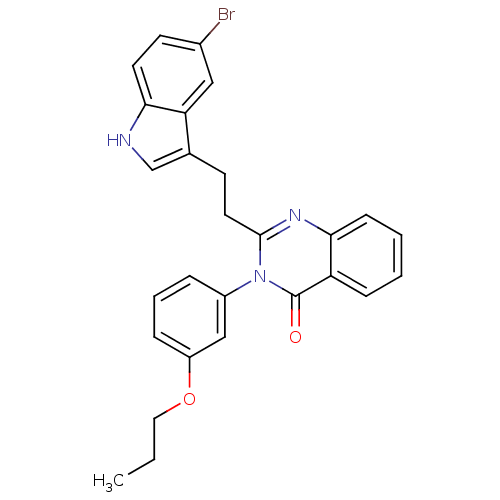 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-propoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011966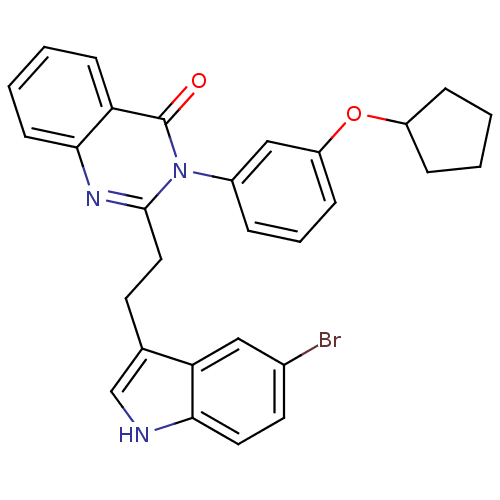 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-cyclopent...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011958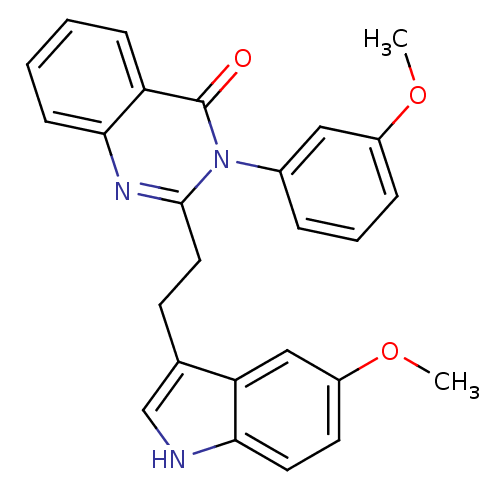 (2-[2-(5-Methoxy-1H-indol-3-yl)-ethyl]-3-(3-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011962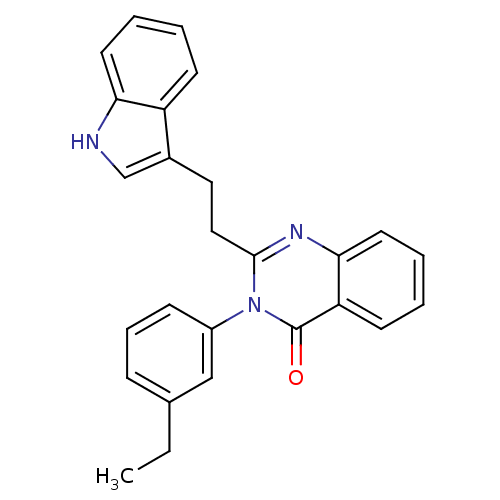 (3-(3-Ethyl-phenyl)-2-[2-(1H-indol-3-yl)-ethyl]-3H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50006842 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-isopropox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled CCK-8 sulfate binding to Cholecystokinin type B receptor in guinea pig brain membranes | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011961 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(4-ethoxy-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011952 (2-[2-(5-Bromo-1H-indol-3-yl)-propyl]-3-(3-ethyl-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011947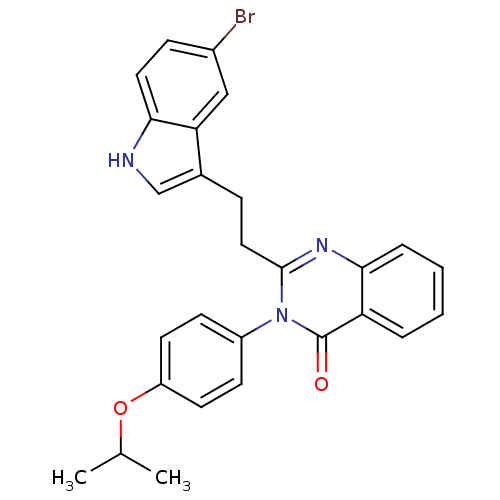 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(4-isopropox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011941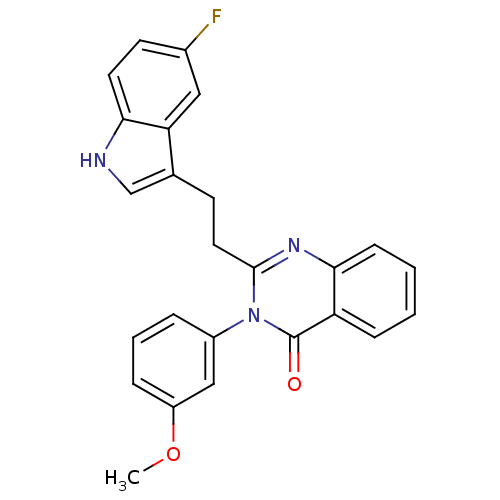 (2-[2-(5-Fluoro-1H-indol-3-yl)-ethyl]-3-(3-methoxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011964 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3,4-dimetho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011949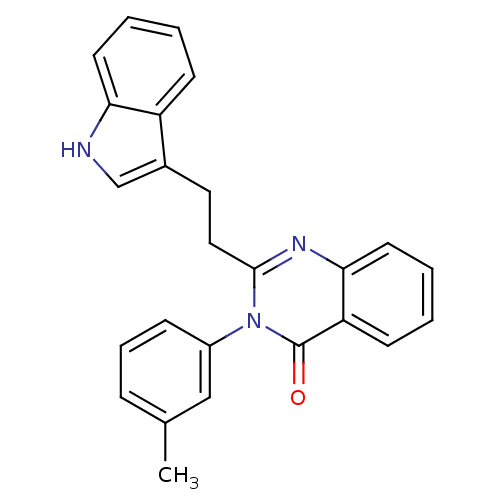 (2-[2-(1H-Indol-3-yl)-ethyl]-3-m-tolyl-3H-quinazoli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50006842 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-isopropox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled gastrin binding to gastrin/cholecystokinin type B receptor | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011956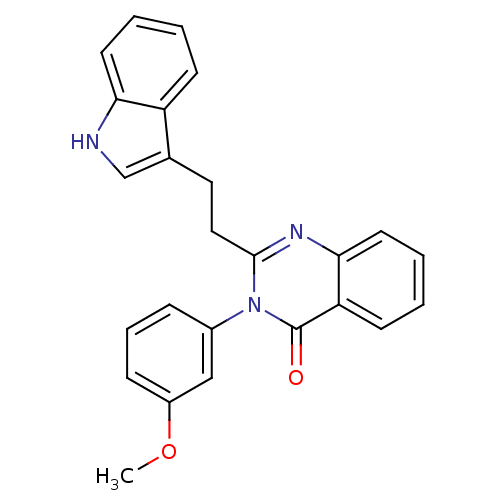 (2-[2-(1H-Indol-3-yl)-ethyl]-3-(3-methoxy-phenyl)-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011963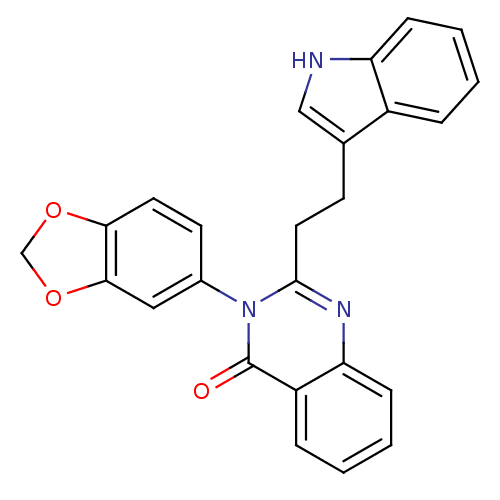 (3-Benzo[1,3]dioxol-5-yl-2-[2-(1H-indol-3-yl)-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011960 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-trifluoro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50011940 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-ethyl-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled CCK-8 sulfate binding to CCK-B receptor in guinea pig brain membranes | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50011942 (2-[2-(5-Bromo-1H-indol-3-yl)-ethyl]-3-(3-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled CCK-8 sulfate binding to Cholecystokinin type B receptor in guinea pig brain membranes | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011959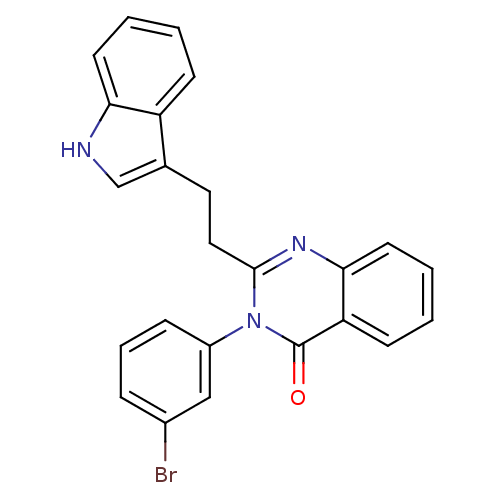 (3-(3-Bromo-phenyl)-2-[2-(1H-indol-3-yl)-ethyl]-3H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50011955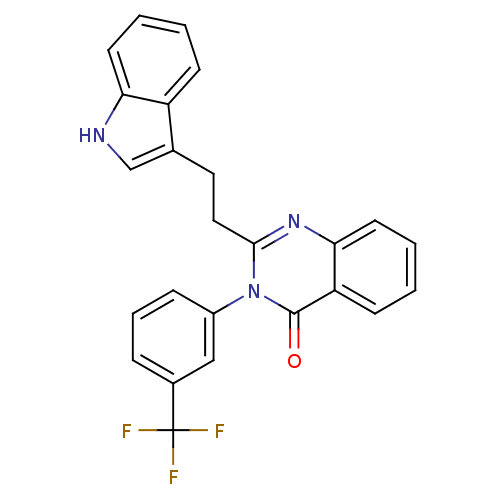 (2-[2-(1H-Indol-3-yl)-ethyl]-3-(3-trifluoromethyl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-CCK-8 sulfate binding to cholecystokinin type B receptor in mouse brain membranes. | J Med Chem 34: 1505-8 (1991) BindingDB Entry DOI: 10.7270/Q2Z60N1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50243477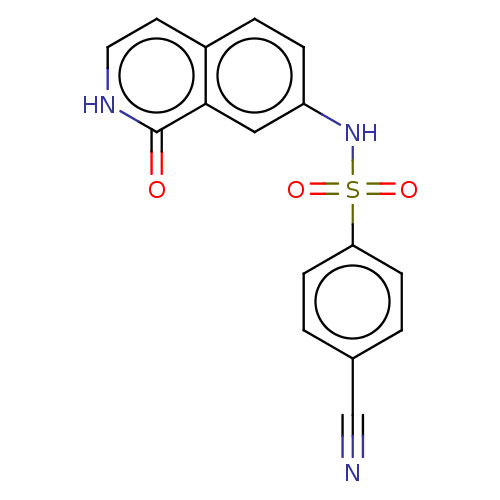 (CHEMBL4092503) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 608 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... | J Med Chem 60: 9599-9616 (2017) Article DOI: 10.1021/acs.jmedchem.7b01046 BindingDB Entry DOI: 10.7270/Q2222X6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 85 total ) | Next | Last >> |