

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ROS1 (unknown origin) by Pfizer mobility shift assay | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2 (Homo sapiens (Human)) | BDBM430025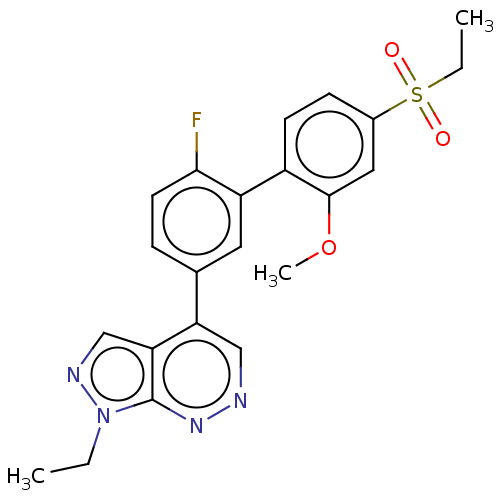 (4-(4′-Ethanesulfonyl-6-fluoro-2′-metho...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PFIZER LIMITED US Patent | Assay Description The affinity of the test compounds was determined by radioligand competition binding assay, using the known compound [3H]Ro-15-1788 (Flumazenil) (Per... | US Patent US10538523 (2020) BindingDB Entry DOI: 10.7270/Q2RB771W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2 (Homo sapiens (Human)) | BDBM430027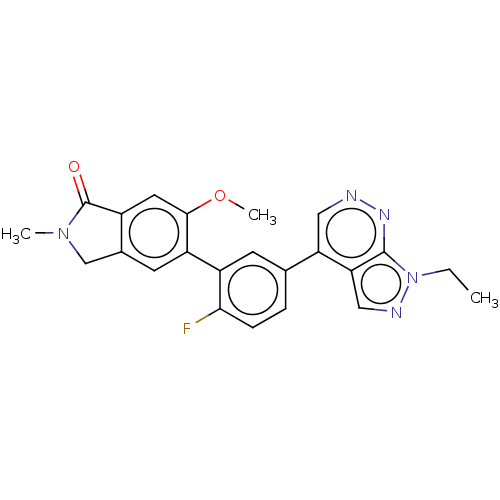 (5-[5-(1-Ethyl-1H-pyrazolo[3,4-c]pyridazin-4-yl)-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PFIZER LIMITED US Patent | Assay Description The affinity of the test compounds was determined by radioligand competition binding assay, using the known compound [3H]Ro-15-1788 (Flumazenil) (Per... | US Patent US10538523 (2020) BindingDB Entry DOI: 10.7270/Q2RB771W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2 (Homo sapiens (Human)) | BDBM430026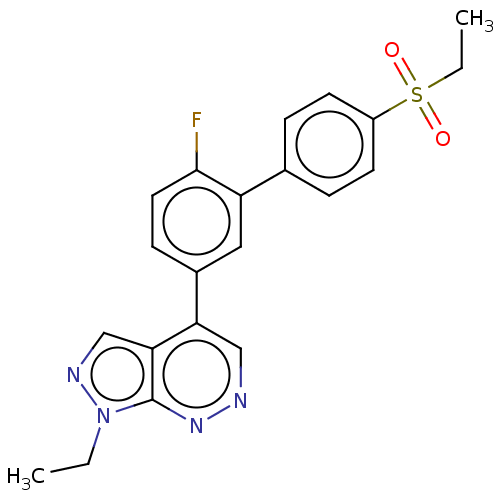 (4-(4′-Ethanesulfonyl-6-fluorobiphenyl-3-yl)-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PFIZER LIMITED US Patent | Assay Description The affinity of the test compounds was determined by radioligand competition binding assay, using the known compound [3H]Ro-15-1788 (Flumazenil) (Per... | US Patent US10538523 (2020) BindingDB Entry DOI: 10.7270/Q2RB771W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50344193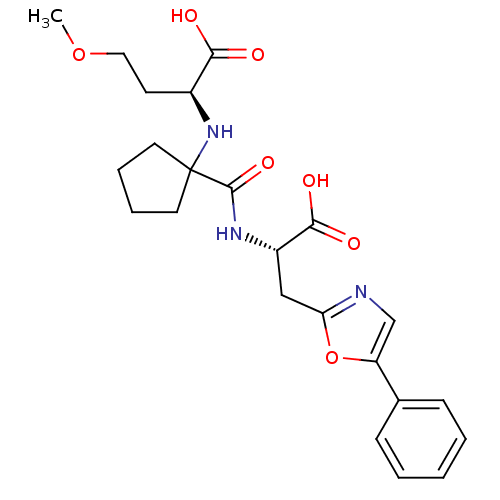 ((S)-2-(1-((S)-1-carboxy-2-(5-phenyloxazol-2-yl)eth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase | Bioorg Med Chem Lett 21: 3404-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.109 BindingDB Entry DOI: 10.7270/Q2PG1S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50344195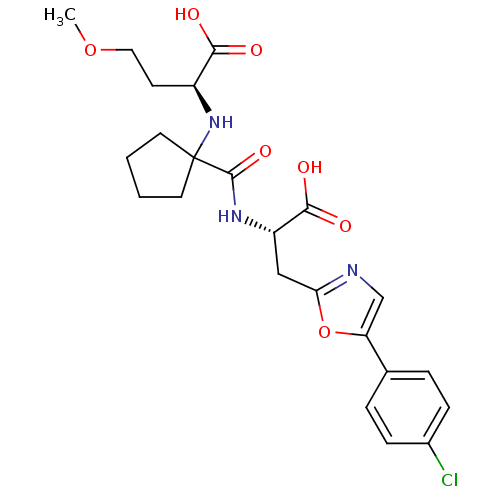 ((S)-2-(1-((S)-1-carboxy-2-(5-(4-chlorophenyl)oxazo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase | Bioorg Med Chem Lett 21: 3404-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.109 BindingDB Entry DOI: 10.7270/Q2PG1S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50227724 (CHEMBL400083 | N-hydroxy-2-methyl-2-{4-[2-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP3 | Bioorg Med Chem Lett 17: 6750-3 (2008) Article DOI: 10.1016/j.bmcl.2007.10.042 BindingDB Entry DOI: 10.7270/Q20K289K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50227723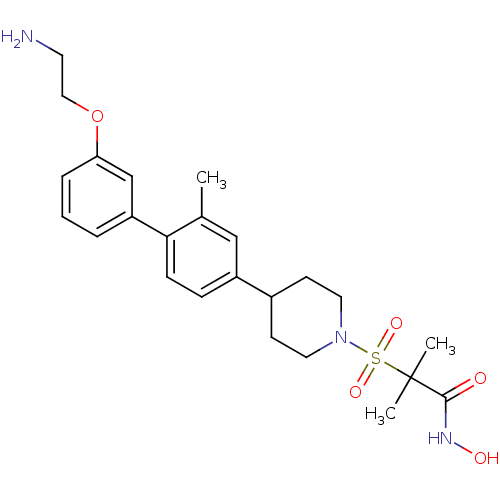 (2-{4-[3'-(2-amino-ethoxy)-2-methyl-biphenyl-4-yl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP3 | Bioorg Med Chem Lett 17: 6750-3 (2008) Article DOI: 10.1016/j.bmcl.2007.10.042 BindingDB Entry DOI: 10.7270/Q20K289K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50354912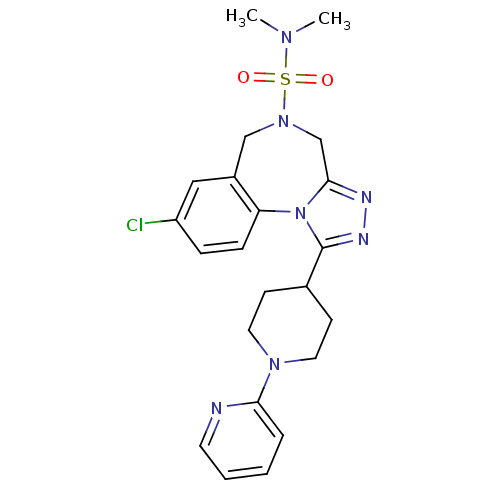 (CHEMBL1837039) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds... | Bioorg Med Chem Lett 21: 5684-7 (2011) Article DOI: 10.1016/j.bmcl.2011.08.038 BindingDB Entry DOI: 10.7270/Q2TB179T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50108204 (3-[1-Benzo[1,3]dioxol-5-yl-2-(2-ethoxy-4-methyl-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Displacement of [125I]-labeled ET-1 from human cloned endothelin A (ETA) receptor | Bioorg Med Chem Lett 12: 125-8 (2001) BindingDB Entry DOI: 10.7270/Q2JH3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50085452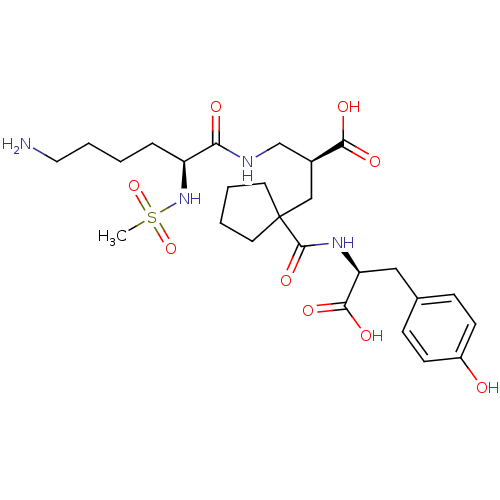 ((S)-2-[((S)-6-Amino-2-methanesulfonylamino-hexanoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase | Bioorg Med Chem Lett 21: 3404-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.109 BindingDB Entry DOI: 10.7270/Q2PG1S2F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50344191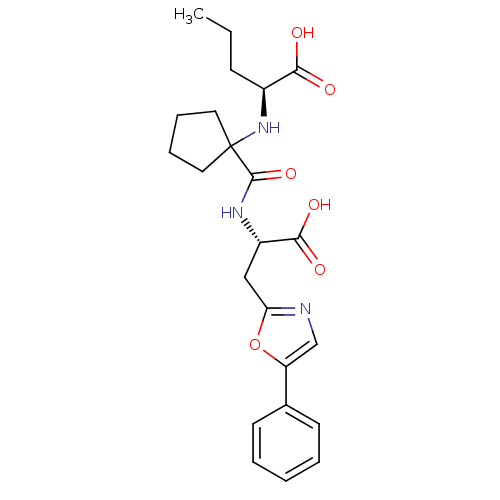 ((S)-2-(1-((S)-1-carboxy-2-(5-phenyloxazol-2-yl)eth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase | Bioorg Med Chem Lett 21: 3404-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.109 BindingDB Entry DOI: 10.7270/Q2PG1S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50108202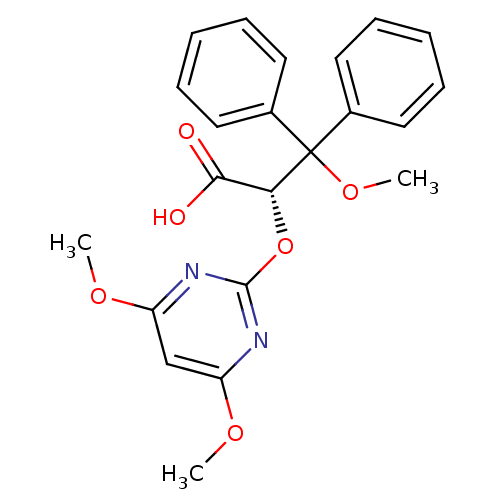 ((S)-2-(4,6-Dimethoxy-pyrimidin-2-yloxy)-3-methoxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Displacement of [125I]-labeled ET-1 from human cloned endothelin A (ETA) receptor | Bioorg Med Chem Lett 12: 125-8 (2001) BindingDB Entry DOI: 10.7270/Q2JH3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human wild type EML4-fused ALK expressed in mouse NIH-3T3 cells assessed as phosphorylated ALK level after 1 hr by sandwich ELISA | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50227722 (CHEMBL398641 | N-hydroxy-2-{4-[3'-(2-hydroxy-ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP3 | Bioorg Med Chem Lett 17: 6750-3 (2008) Article DOI: 10.1016/j.bmcl.2007.10.042 BindingDB Entry DOI: 10.7270/Q20K289K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50560311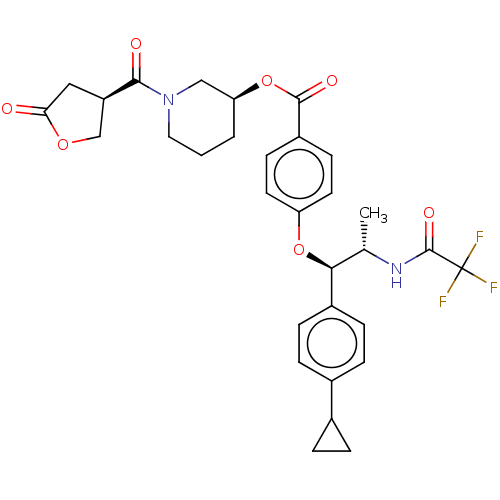 (CHEMBL4756197) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human glucocorticoid receptor in PBMC assessed as inhibition of LPS-induced TNFalpha release | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127402 BindingDB Entry DOI: 10.7270/Q26D5XPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50227725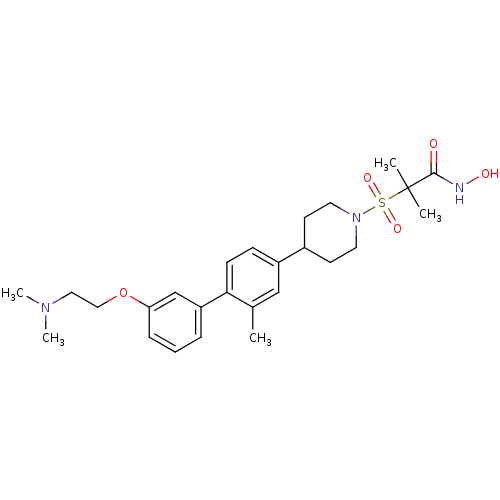 (2-{4-[3'-(2-dimethylamino-ethoxy)-2-methyl-bipheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP3 | Bioorg Med Chem Lett 17: 6750-3 (2008) Article DOI: 10.1016/j.bmcl.2007.10.042 BindingDB Entry DOI: 10.7270/Q20K289K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50227709 (CHEMBL251917 | N-hydroxy-2-(4-(4-(6-(2-hydroxyetho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP3 | Bioorg Med Chem Lett 17: 6750-3 (2008) Article DOI: 10.1016/j.bmcl.2007.10.042 BindingDB Entry DOI: 10.7270/Q20K289K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50108192 (3-[1-Benzo[1,3]dioxol-5-yl-2-(2-methoxy-4-methyl-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Displacement of [125I]-labeled ET-1 from human cloned endothelin A (ETA) receptor | Bioorg Med Chem Lett 12: 125-8 (2001) BindingDB Entry DOI: 10.7270/Q2JH3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50354897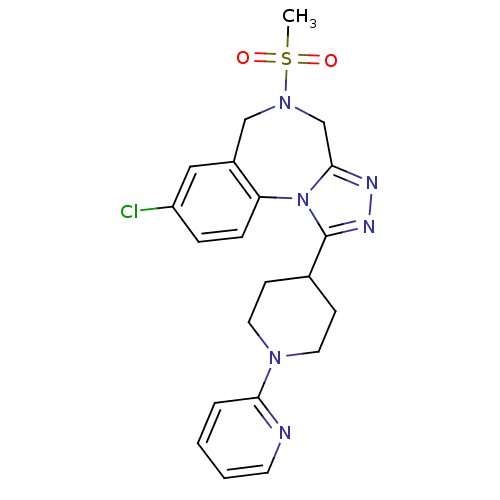 (CHEMBL1837041) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds... | Bioorg Med Chem Lett 21: 5684-7 (2011) Article DOI: 10.1016/j.bmcl.2011.08.038 BindingDB Entry DOI: 10.7270/Q2TB179T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Natriuretic peptides A (Homo sapiens (Human)) | BDBM50344187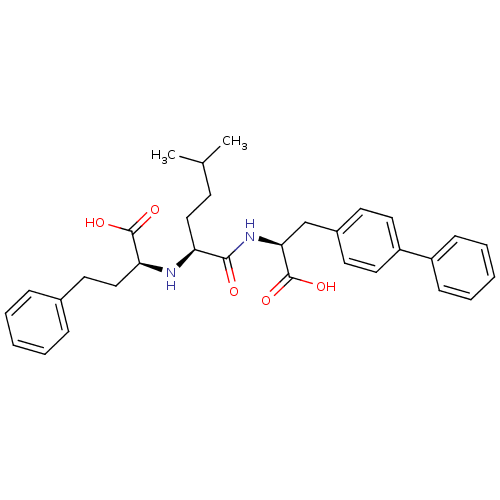 ((S)-2-((S)-1-((S)-2-(biphenyl-4-yl)-1-carboxyethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of atrial natriuretic peptide | Bioorg Med Chem Lett 21: 3404-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.109 BindingDB Entry DOI: 10.7270/Q2PG1S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50108194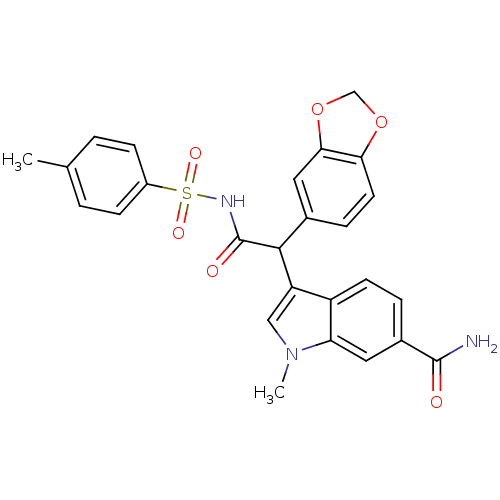 (3-[1-Benzo[1,3]dioxol-5-yl-2-oxo-2-(toluene-4-sulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Displacement of [125I]-labeled ET-1 from human cloned endothelin A (ETA) receptor | Bioorg Med Chem Lett 12: 125-8 (2001) BindingDB Entry DOI: 10.7270/Q2JH3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50344197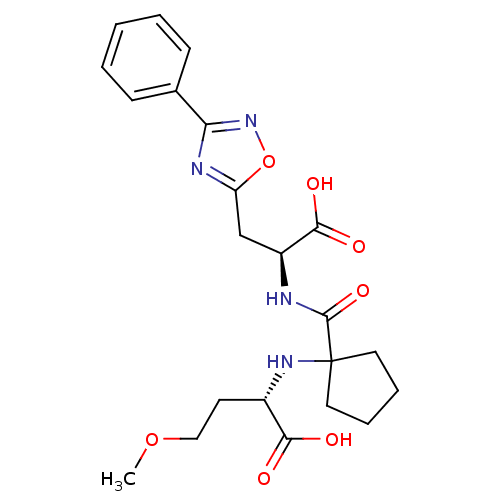 ((S)-2-(1-((S)-1-carboxy-2-(3-phenyl-1,2,4-oxadiazo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase | Bioorg Med Chem Lett 21: 3404-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.109 BindingDB Entry DOI: 10.7270/Q2PG1S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50560315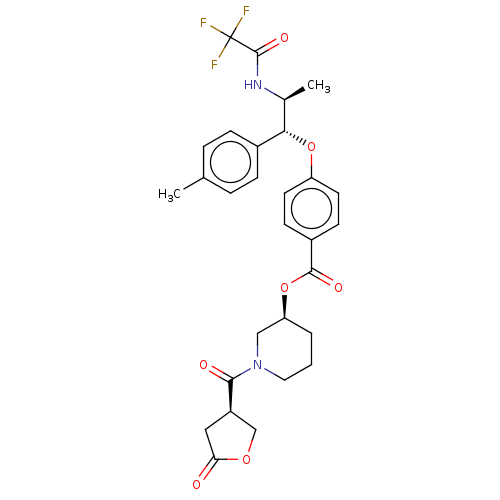 (CHEMBL4763360) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human glucocorticoid receptor in PBMC assessed as inhibition of LPS-induced TNFalpha release | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127402 BindingDB Entry DOI: 10.7270/Q26D5XPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50560313 (CHEMBL4762028) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human glucocorticoid receptor in PBMC assessed as inhibition of LPS-induced TNFalpha release | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127402 BindingDB Entry DOI: 10.7270/Q26D5XPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50227711 (2-[4-(3'-ethoxy-2-methyl-biphenyl-4-yl)-piperidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP3 | Bioorg Med Chem Lett 17: 6750-3 (2008) Article DOI: 10.1016/j.bmcl.2007.10.042 BindingDB Entry DOI: 10.7270/Q20K289K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50560326 (CHEMBL4758563) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human glucocorticoid receptor in PBMC assessed as inhibition of LPS-induced TNFalpha release | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127402 BindingDB Entry DOI: 10.7270/Q26D5XPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of LTK (unknown origin) using Km levels of ATP | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fer (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of FER (unknown origin) using Km levels of ATP | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50536220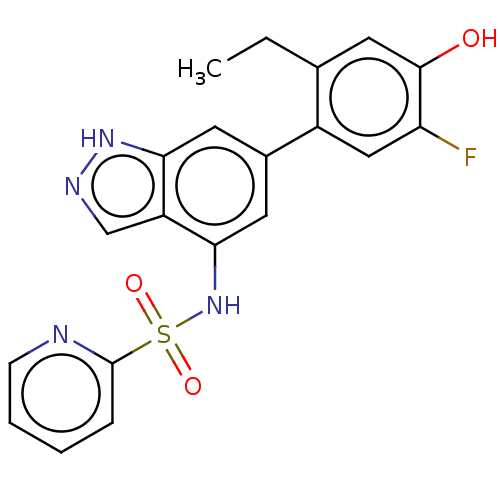 (CHEMBL4528395) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Design, In Vitro Biology, Skin PK and Early Safety, and Preformulation & Early Analytical Development, Global R&D, LEO Pharma A/S , Industriparken 55, DK-2750 Ballerup, Denmark. Curated by ChEMBL | Assay Description Inhibition of human His-tagged JAK3 expressed in baculovirus using biotin-synthetic peptide as substrate after 20 mins by HTRF assay | ACS Med Chem Lett 7: 641-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00087 BindingDB Entry DOI: 10.7270/Q2CC146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50108187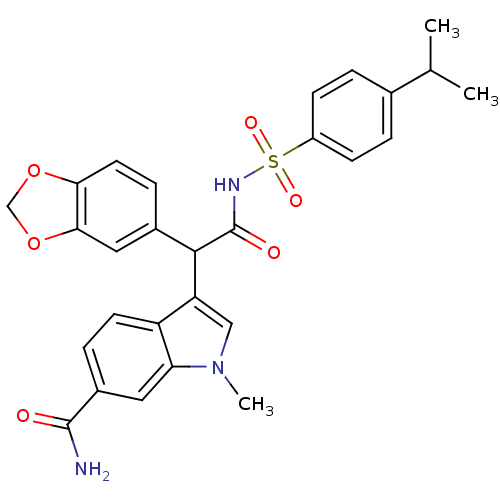 (3-[1-Benzo[1,3]dioxol-5-yl-2-(4-isopropyl-benzenes...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Displacement of [125I]-labeled ET-1 from human cloned endothelin A (ETA) receptor | Bioorg Med Chem Lett 12: 125-8 (2001) BindingDB Entry DOI: 10.7270/Q2JH3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50536209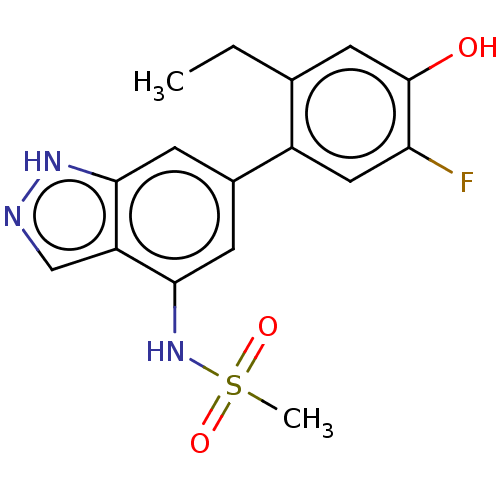 (CHEMBL4539949) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Design, In Vitro Biology, Skin PK and Early Safety, and Preformulation & Early Analytical Development, Global R&D, LEO Pharma A/S , Industriparken 55, DK-2750 Ballerup, Denmark. Curated by ChEMBL | Assay Description Inhibition of human His-tagged JAK3 expressed in baculovirus using biotin-synthetic peptide as substrate after 20 mins by HTRF assay | ACS Med Chem Lett 7: 641-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00087 BindingDB Entry DOI: 10.7270/Q2CC146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50536217 (CHEMBL4517645) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Design, In Vitro Biology, Skin PK and Early Safety, and Preformulation & Early Analytical Development, Global R&D, LEO Pharma A/S , Industriparken 55, DK-2750 Ballerup, Denmark. Curated by ChEMBL | Assay Description Inhibition of human His-tagged JAK3 expressed in baculovirus using biotin-synthetic peptide as substrate after 20 mins by HTRF assay | ACS Med Chem Lett 7: 641-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00087 BindingDB Entry DOI: 10.7270/Q2CC146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50536208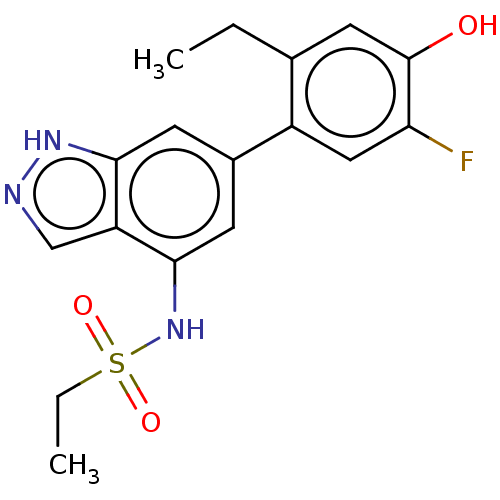 (CHEMBL4483364) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Design, In Vitro Biology, Skin PK and Early Safety, and Preformulation & Early Analytical Development, Global R&D, LEO Pharma A/S , Industriparken 55, DK-2750 Ballerup, Denmark. Curated by ChEMBL | Assay Description Inhibition of human His-tagged JAK3 expressed in baculovirus using biotin-synthetic peptide as substrate after 20 mins by HTRF assay | ACS Med Chem Lett 7: 641-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00087 BindingDB Entry DOI: 10.7270/Q2CC146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50108185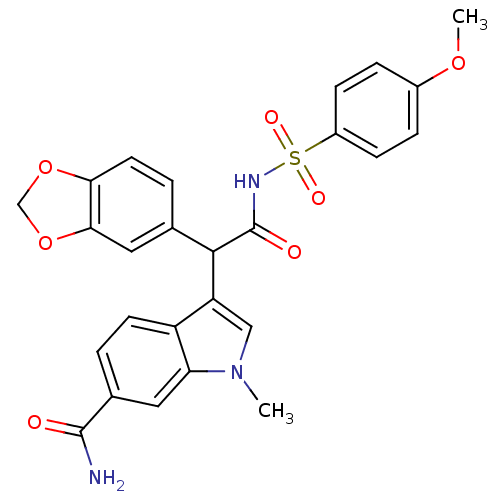 (3-[1-Benzo[1,3]dioxol-5-yl-2-(4-methoxy-benzenesul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Displacement of [125I]-labeled ET-1 from human cloned endothelin A (ETA) receptor | Bioorg Med Chem Lett 12: 125-8 (2001) BindingDB Entry DOI: 10.7270/Q2JH3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50354913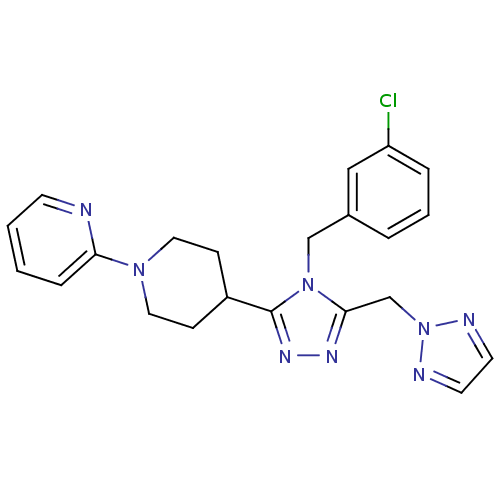 (CHEMBL1837028) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds... | Bioorg Med Chem Lett 21: 5684-7 (2011) Article DOI: 10.1016/j.bmcl.2011.08.038 BindingDB Entry DOI: 10.7270/Q2TB179T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50227733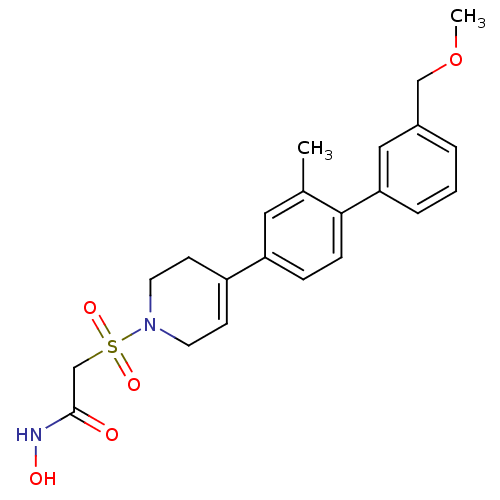 (CHEMBL255030 | N-hydroxy-2-[4-(3'-methoxymethyl-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP3 | Bioorg Med Chem Lett 17: 6750-3 (2008) Article DOI: 10.1016/j.bmcl.2007.10.042 BindingDB Entry DOI: 10.7270/Q20K289K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50227717 (CHEMBL251916 | N-hydroxy-2-{4-[3'-(2-methoxy-ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP3 | Bioorg Med Chem Lett 17: 6750-3 (2008) Article DOI: 10.1016/j.bmcl.2007.10.042 BindingDB Entry DOI: 10.7270/Q20K289K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50227728 (2-[4-(2-fluoro-biphenyl-4-yl)-3,6-dihydro-2H-pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP3 | Bioorg Med Chem Lett 17: 6750-3 (2008) Article DOI: 10.1016/j.bmcl.2007.10.042 BindingDB Entry DOI: 10.7270/Q20K289K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50108193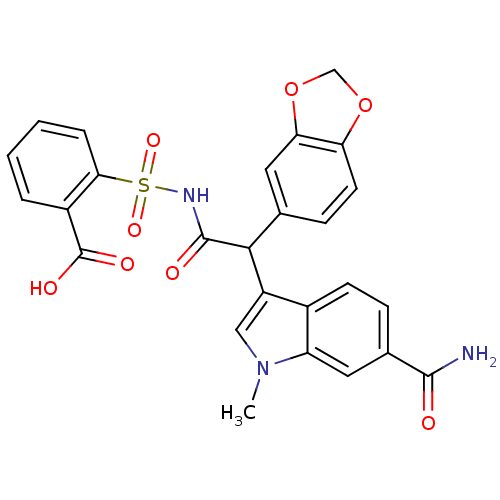 (2-[2-Benzo[1,3]dioxol-5-yl-2-(6-carbamoyl-1-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Displacement of [125I]-labeled ET-1 from human cloned endothelin A (ETA) receptor | Bioorg Med Chem Lett 12: 125-8 (2001) BindingDB Entry DOI: 10.7270/Q2JH3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50536221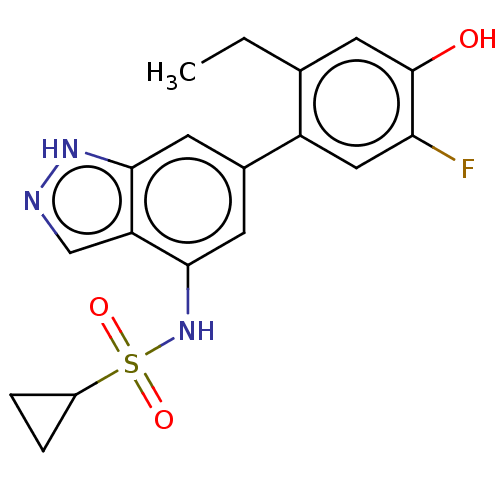 (CHEMBL4554460) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Design, In Vitro Biology, Skin PK and Early Safety, and Preformulation & Early Analytical Development, Global R&D, LEO Pharma A/S , Industriparken 55, DK-2750 Ballerup, Denmark. Curated by ChEMBL | Assay Description Inhibition of human His-tagged JAK3 expressed in baculovirus using biotin-synthetic peptide as substrate after 20 mins by HTRF assay | ACS Med Chem Lett 7: 641-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00087 BindingDB Entry DOI: 10.7270/Q2CC146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50536198 (CHEMBL4534573) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Design, In Vitro Biology, Skin PK and Early Safety, and Preformulation & Early Analytical Development, Global R&D, LEO Pharma A/S , Industriparken 55, DK-2750 Ballerup, Denmark. Curated by ChEMBL | Assay Description Inhibition of human His-tagged JAK3 expressed in baculovirus using biotin-synthetic peptide as substrate after 20 mins by HTRF assay | ACS Med Chem Lett 7: 641-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00087 BindingDB Entry DOI: 10.7270/Q2CC146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50536214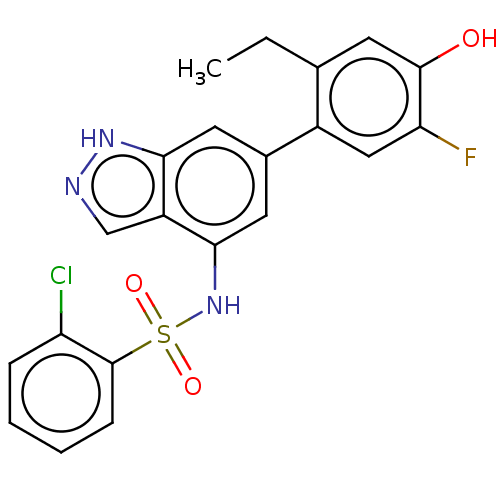 (CHEMBL4560212) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Design, In Vitro Biology, Skin PK and Early Safety, and Preformulation & Early Analytical Development, Global R&D, LEO Pharma A/S , Industriparken 55, DK-2750 Ballerup, Denmark. Curated by ChEMBL | Assay Description Inhibition of human His-tagged JAK3 expressed in baculovirus using biotin-synthetic peptide as substrate after 20 mins by HTRF assay | ACS Med Chem Lett 7: 641-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00087 BindingDB Entry DOI: 10.7270/Q2CC146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50536200 (CHEMBL4483513) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Design, In Vitro Biology, Skin PK and Early Safety, and Preformulation & Early Analytical Development, Global R&D, LEO Pharma A/S , Industriparken 55, DK-2750 Ballerup, Denmark. Curated by ChEMBL | Assay Description Inhibition of human His-tagged JAK3 expressed in baculovirus using biotin-synthetic peptide as substrate after 20 mins by HTRF assay | ACS Med Chem Lett 7: 641-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00087 BindingDB Entry DOI: 10.7270/Q2CC146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50354895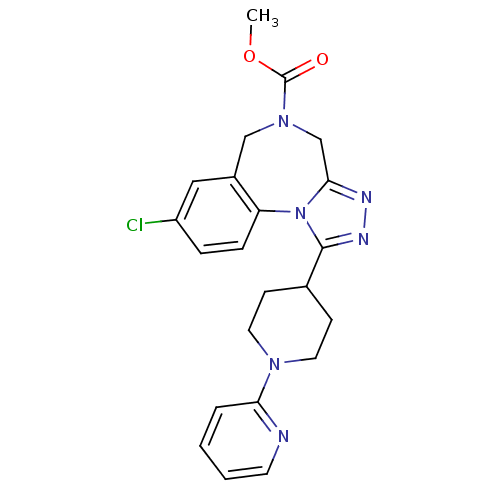 (CHEMBL1837038) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds... | Bioorg Med Chem Lett 21: 5684-7 (2011) Article DOI: 10.1016/j.bmcl.2011.08.038 BindingDB Entry DOI: 10.7270/Q2TB179T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Natriuretic peptides A (Homo sapiens (Human)) | BDBM50085452 ((S)-2-[((S)-6-Amino-2-methanesulfonylamino-hexanoy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of atrial natriuretic peptide | Bioorg Med Chem Lett 21: 3404-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.109 BindingDB Entry DOI: 10.7270/Q2PG1S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50108196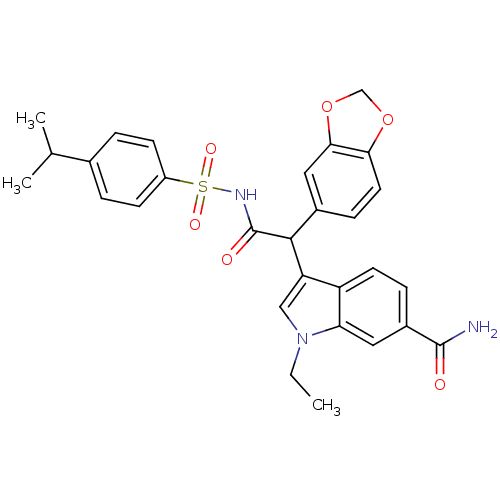 (3-[1-Benzo[1,3]dioxol-5-yl-2-(4-isopropyl-benzenes...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Displacement of [125I]-labeled ET-1 from human cloned endothelin A (ETA) receptor | Bioorg Med Chem Lett 12: 125-8 (2001) BindingDB Entry DOI: 10.7270/Q2JH3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50536197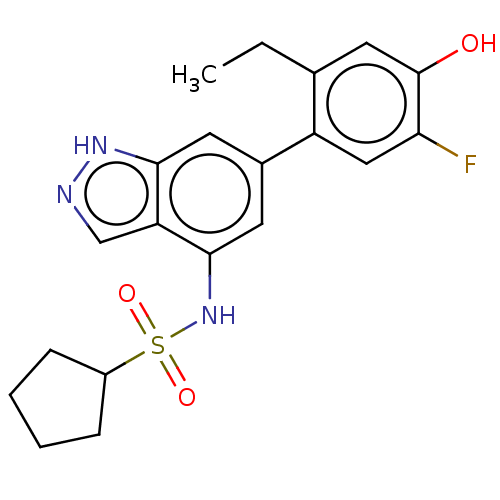 (CHEMBL4521312) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Design, In Vitro Biology, Skin PK and Early Safety, and Preformulation & Early Analytical Development, Global R&D, LEO Pharma A/S , Industriparken 55, DK-2750 Ballerup, Denmark. Curated by ChEMBL | Assay Description Inhibition of human His-tagged JAK3 expressed in baculovirus using biotin-synthetic peptide as substrate after 20 mins by HTRF assay | ACS Med Chem Lett 7: 641-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00087 BindingDB Entry DOI: 10.7270/Q2CC146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50536216 (CHEMBL4580425) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Design, In Vitro Biology, Skin PK and Early Safety, and Preformulation & Early Analytical Development, Global R&D, LEO Pharma A/S , Industriparken 55, DK-2750 Ballerup, Denmark. Curated by ChEMBL | Assay Description Inhibition of human His-tagged JAK3 expressed in baculovirus using biotin-synthetic peptide as substrate after 20 mins by HTRF assay | ACS Med Chem Lett 7: 641-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00087 BindingDB Entry DOI: 10.7270/Q2CC146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50536215 (CHEMBL4569613) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Design, In Vitro Biology, Skin PK and Early Safety, and Preformulation & Early Analytical Development, Global R&D, LEO Pharma A/S , Industriparken 55, DK-2750 Ballerup, Denmark. Curated by ChEMBL | Assay Description Inhibition of human His-tagged JAK3 expressed in baculovirus using biotin-synthetic peptide as substrate after 20 mins by HTRF assay | ACS Med Chem Lett 7: 641-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00087 BindingDB Entry DOI: 10.7270/Q2CC146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 438 total ) | Next | Last >> |