 Found 502 hits with Last Name = 'bertin' and Initial = 'l'
Found 502 hits with Last Name = 'bertin' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50049750

((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...)Show InChI InChI=1S/C9H12N2O/c1-2-9(6-10-4-1)12-7-8-3-5-11-8/h1-2,4,6,8,11H,3,5,7H2/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at alpha4beta2 nAChR (unknown origin) |
J Med Chem 57: 3966-83 (2014)
Article DOI: 10.1021/jm5004599
BindingDB Entry DOI: 10.7270/Q2CV4K8S |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50073179
(8-Chloro-11-piperidin-4-ylidene-6,11-dihydro-5H-be...)Show SMILES Clc1ccc2c(-[#6]-[#6]-c3cccnc3\[#6]-2=[#6]-2/[#6]-[#6]-[#7]-[#6]-[#6]-2)c1 Show InChI InChI=1S/C19H19ClN2/c20-16-5-6-17-15(12-16)4-3-14-2-1-9-22-19(14)18(17)13-7-10-21-11-8-13/h1-2,5-6,9,12,21H,3-4,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 5591-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.060
BindingDB Entry DOI: 10.7270/Q28C9VRH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50155336
(1-{4-[4-(4-{4-[bis(4-fluorophenyl)methyl]piperazin...)Show SMILES NC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C32H36F2N4O3/c33-28-12-8-26(9-13-28)31(27-10-14-29(34)15-11-27)37-22-20-36(21-23-37)18-3-4-24-41-30-16-6-25(7-17-30)5-1-2-19-38(40)32(35)39/h6-17,31,40H,2-4,18-24H2,(H2,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human Histamine H1 receptor expressed in CHO K1 cells |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50144624

((5-{4-[(R)-(4-Chloro-phenyl)-phenyl-methyl]-pipera...)Show SMILES NC(=O)N(O)Cc1ccc(CN2CCN(CC2)[C@H](c2ccccc2)c2ccc(Cl)cc2)o1 Show InChI InChI=1S/C24H27ClN4O3/c25-20-8-6-19(7-9-20)23(18-4-2-1-3-5-18)28-14-12-27(13-15-28)16-21-10-11-22(32-21)17-29(31)24(26)30/h1-11,23,31H,12-17H2,(H2,26,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 2265-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.005
BindingDB Entry DOI: 10.7270/Q2BV7G2P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50160828

(CHEMBL366925 | N-hydroxycarbamate derivative)Show SMILES COC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C33H37F2N3O4/c1-41-33(39)38(40)20-3-2-6-26-7-17-31(18-8-26)42-25-5-4-19-36-21-23-37(24-22-36)32(27-9-13-29(34)14-10-27)28-11-15-30(35)16-12-28/h7-18,32,40H,3-5,19-25H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Histamine H1 receptor in CHO K1 cells |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50160827
(CHEMBL179590 | N-hydroxycarbamate derivative)Show SMILES CCOC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C34H39F2N3O4/c1-2-42-34(40)39(41)21-4-3-7-27-8-18-32(19-9-27)43-26-6-5-20-37-22-24-38(25-23-37)33(28-10-14-30(35)15-11-28)29-12-16-31(36)17-13-29/h8-19,33,41H,2,4-6,20-26H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Histamine H1 receptor in CHO K1 cells |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22890

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show SMILES OC(=O)COCCN1CCN(CC1)C(c1ccccc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClN2O3/c22-19-8-6-18(7-9-19)21(17-4-2-1-3-5-17)24-12-10-23(11-13-24)14-15-27-16-20(25)26/h1-9,21H,10-16H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 5.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Histamine H1 receptor in CHO K1 cells |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50160831

(CHEMBL181085 | N-hydroxycarbamate derivative)Show SMILES CC(C)COC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C36H43F2N3O4/c1-28(2)27-45-36(42)41(43)21-4-3-7-29-8-18-34(19-9-29)44-26-6-5-20-39-22-24-40(25-23-39)35(30-10-14-32(37)15-11-30)31-12-16-33(38)17-13-31/h8-19,28,35,43H,4-6,20-27H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Histamine H1 receptor in CHO K1 cells |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50155335
(1-{4-[4-(3-{4-[(R)-(4-chlorophenyl)(phenyl)methyl]...)Show SMILES NC(=O)N(O)CCC#Cc1ccc(OCCCN2CCN(CC2)[C@H](c2ccccc2)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C31H35ClN4O3/c32-28-14-12-27(13-15-28)30(26-8-2-1-3-9-26)35-22-20-34(21-23-35)18-6-24-39-29-16-10-25(11-17-29)7-4-5-19-36(38)31(33)37/h1-3,8-17,30,38H,5-6,18-24H2,(H2,33,37)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 5591-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.060
BindingDB Entry DOI: 10.7270/Q28C9VRH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50160829

(CHEMBL180233 | N-hydroxycarbamate derivative)Show SMILES CC(C)OC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C35H41F2N3O4/c1-27(2)44-35(41)40(42)21-4-3-7-28-8-18-33(19-9-28)43-26-6-5-20-38-22-24-39(25-23-38)34(29-10-14-31(36)15-11-29)30-12-16-32(37)17-13-30/h8-19,27,34,42H,4-6,20-26H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Histamine H1 receptor in CHO K1 cells |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22890

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show SMILES OC(=O)COCCN1CCN(CC1)C(c1ccccc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClN2O3/c22-19-8-6-18(7-9-19)21(17-4-2-1-3-5-17)24-12-10-23(11-13-24)14-15-27-16-20(25)26/h1-9,21H,10-16H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 5591-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.060
BindingDB Entry DOI: 10.7270/Q28C9VRH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22890

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show SMILES OC(=O)COCCN1CCN(CC1)C(c1ccccc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClN2O3/c22-19-8-6-18(7-9-19)21(17-4-2-1-3-5-17)24-12-10-23(11-13-24)14-15-27-16-20(25)26/h1-9,21H,10-16H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 2265-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.005
BindingDB Entry DOI: 10.7270/Q2BV7G2P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50155341

(1-(4-{4-[3-(4-{13-chloro-4-azatricyclo[9.4.0.0^{3,...)Show SMILES [#7]-[#6](=O)-[#7](-[#8])-[#6]-[#6]C#Cc1ccc(-[#8]-[#6]-[#6]-[#6]-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2\c3ccc(Cl)cc3-[#6]-[#6]-c3cccnc-23)cc1 Show InChI InChI=1S/C33H35ClN4O3/c34-28-11-14-30-27(23-28)10-9-26-6-3-17-36-32(26)31(30)25-15-20-37(21-16-25)18-4-22-41-29-12-7-24(8-13-29)5-1-2-19-38(40)33(35)39/h3,6-8,11-14,17,23,40H,2,4,9-10,15-16,18-22H2,(H2,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 5591-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.060
BindingDB Entry DOI: 10.7270/Q28C9VRH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50160830
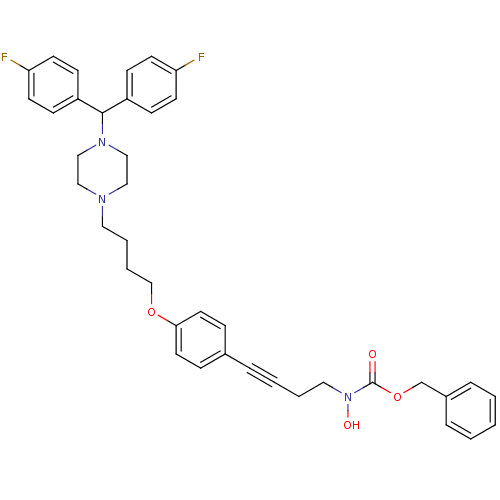
(CHEMBL360666 | N-hydroxycarbamate derivative)Show SMILES ON(CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1)C(=O)OCc1ccccc1 Show InChI InChI=1S/C39H41F2N3O4/c40-35-17-13-33(14-18-35)38(34-15-19-36(41)20-16-34)43-27-25-42(26-28-43)23-6-7-29-47-37-21-11-31(12-22-37)8-4-5-24-44(46)39(45)48-30-32-9-2-1-3-10-32/h1-3,9-22,38,46H,5-7,23-30H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Histamine H1 receptor in CHO K1 cells |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50155337
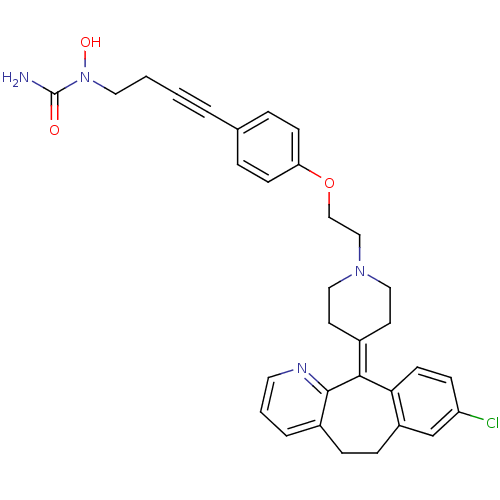
(1-(4-{4-[2-(4-{13-chloro-4-azatricyclo[9.4.0.0^{3,...)Show SMILES [#7]-[#6](=O)-[#7](-[#8])-[#6]-[#6]C#Cc1ccc(-[#8]-[#6]-[#6]-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2\c3ccc(Cl)cc3-[#6]-[#6]-c3cccnc-23)cc1 Show InChI InChI=1S/C32H33ClN4O3/c33-27-10-13-29-26(22-27)9-8-25-5-3-16-35-31(25)30(29)24-14-18-36(19-15-24)20-21-40-28-11-6-23(7-12-28)4-1-2-17-37(39)32(34)38/h3,5-7,10-13,16,22,39H,2,8-9,14-15,17-21H2,(H2,34,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 5591-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.060
BindingDB Entry DOI: 10.7270/Q28C9VRH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50155336

(1-{4-[4-(4-{4-[bis(4-fluorophenyl)methyl]piperazin...)Show SMILES NC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C32H36F2N4O3/c33-28-12-8-26(9-13-28)31(27-10-14-29(34)15-11-27)37-22-20-36(21-23-37)18-3-4-24-41-30-16-6-25(7-17-30)5-1-2-19-38(40)32(35)39/h6-17,31,40H,2-4,18-24H2,(H2,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 5591-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.060
BindingDB Entry DOI: 10.7270/Q28C9VRH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50155338
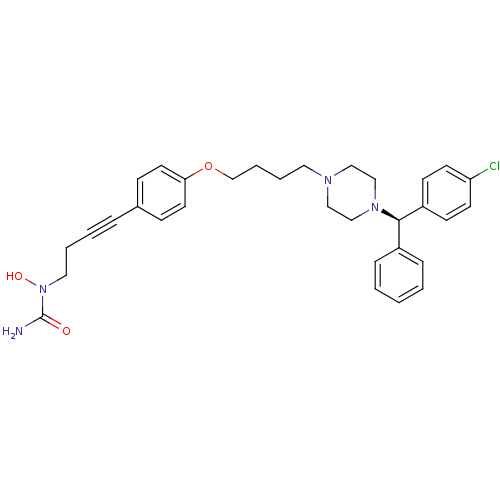
(1-{4-[4-(4-{4-[(R)-(4-chlorophenyl)(phenyl)methyl]...)Show SMILES NC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)[C@H](c2ccccc2)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C32H37ClN4O3/c33-29-15-13-28(14-16-29)31(27-9-2-1-3-10-27)36-23-21-35(22-24-36)19-6-7-25-40-30-17-11-26(12-18-30)8-4-5-20-37(39)32(34)38/h1-3,9-18,31,39H,5-7,19-25H2,(H2,34,38)/t31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 5591-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.060
BindingDB Entry DOI: 10.7270/Q28C9VRH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50144626
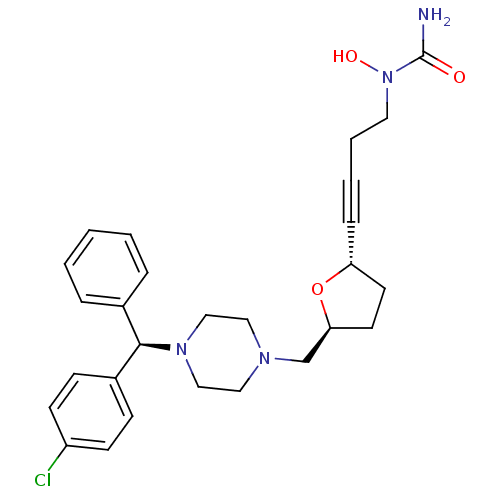
(CHEMBL305662 | [4-((2S,5S)-5-{4-[(R)-(4-Chloro-phe...)Show SMILES NC(=O)N(O)CCC#C[C@@H]1CC[C@@H](CN2CCN(CC2)[C@H](c2ccccc2)c2ccc(Cl)cc2)O1 Show InChI InChI=1S/C27H33ClN4O3/c28-23-11-9-22(10-12-23)26(21-6-2-1-3-7-21)31-18-16-30(17-19-31)20-25-14-13-24(35-25)8-4-5-15-32(34)27(29)33/h1-3,6-7,9-12,24-26,34H,5,13-20H2,(H2,29,33)/t24-,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 2265-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.005
BindingDB Entry DOI: 10.7270/Q2BV7G2P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50155334

(1-(4-{4-[4-(4-{13-chloro-4-azatricyclo[9.4.0.0^{3,...)Show SMILES [#7]-[#6](=O)-[#7](-[#8])-[#6]-[#6]C#Cc1ccc(-[#8]-[#6]-[#6]-[#6]-[#6]-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2\c3ccc(Cl)cc3-[#6]-[#6]-c3cccnc-23)cc1 Show InChI InChI=1S/C34H37ClN4O3/c35-29-12-15-31-28(24-29)11-10-27-7-5-18-37-33(27)32(31)26-16-21-38(22-17-26)19-3-4-23-42-30-13-8-25(9-14-30)6-1-2-20-39(41)34(36)40/h5,7-9,12-15,18,24,41H,2-4,10-11,16-17,19-23H2,(H2,36,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 5591-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.060
BindingDB Entry DOI: 10.7270/Q28C9VRH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50155339
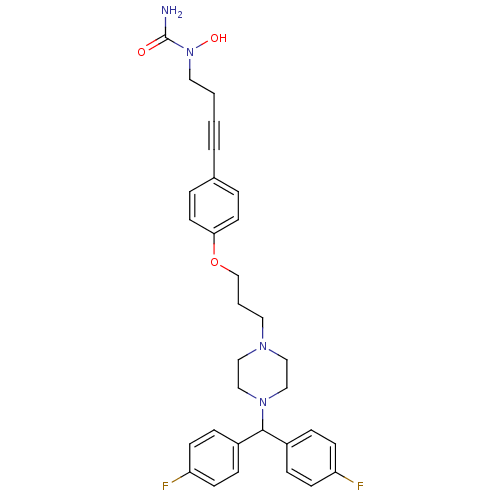
(1-{4-[4-(3-{4-[bis(4-fluorophenyl)methyl]piperazin...)Show SMILES NC(=O)N(O)CCC#Cc1ccc(OCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C31H34F2N4O3/c32-27-11-7-25(8-12-27)30(26-9-13-28(33)14-10-26)36-21-19-35(20-22-36)17-3-23-40-29-15-5-24(6-16-29)4-1-2-18-37(39)31(34)38/h5-16,30,39H,2-3,17-23H2,(H2,34,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 5591-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.060
BindingDB Entry DOI: 10.7270/Q28C9VRH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50049750

((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...)Show InChI InChI=1S/C9H12N2O/c1-2-9(6-10-4-1)12-7-8-3-5-11-8/h1-2,4,6,8,11H,3,5,7H2/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at alpha7 nAChR (unknown origin) |
J Med Chem 57: 3966-83 (2014)
Article DOI: 10.1021/jm5004599
BindingDB Entry DOI: 10.7270/Q2CV4K8S |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50144625
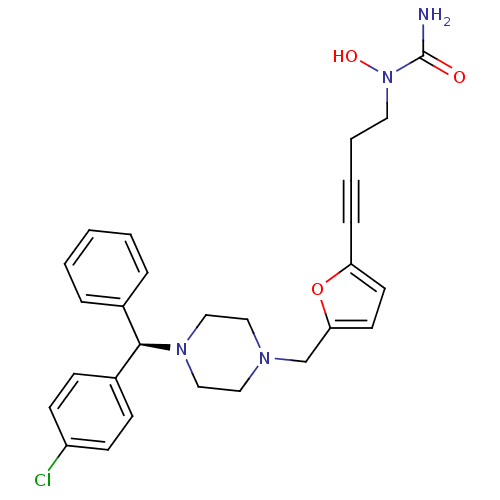
(1-[4-(5-{4-[(R)-(4-Chloro-phenyl)-phenyl-methyl]-p...)Show SMILES NC(=O)N(O)CCC#Cc1ccc(CN2CCN(CC2)[C@H](c2ccccc2)c2ccc(Cl)cc2)o1 Show InChI InChI=1S/C27H29ClN4O3/c28-23-11-9-22(10-12-23)26(21-6-2-1-3-7-21)31-18-16-30(17-19-31)20-25-14-13-24(35-25)8-4-5-15-32(34)27(29)33/h1-3,6-7,9-14,26,34H,5,15-20H2,(H2,29,33)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 2265-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.005
BindingDB Entry DOI: 10.7270/Q2BV7G2P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50144627
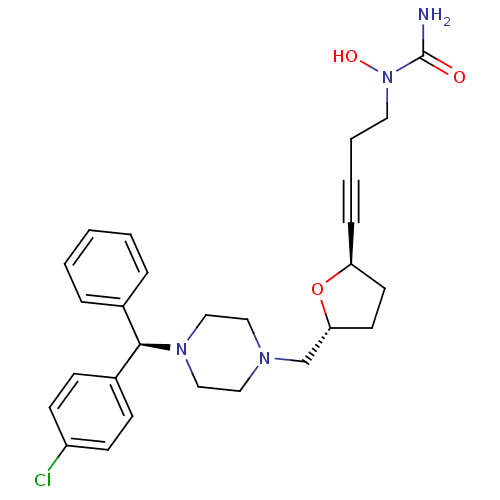
(CHEMBL74688 | [4-((2R,5R)-5-{4-[(R)-(4-Chloro-phen...)Show SMILES NC(=O)N(O)CCC#C[C@H]1CC[C@H](CN2CCN(CC2)[C@H](c2ccccc2)c2ccc(Cl)cc2)O1 Show InChI InChI=1S/C27H33ClN4O3/c28-23-11-9-22(10-12-23)26(21-6-2-1-3-7-21)31-18-16-30(17-19-31)20-25-14-13-24(35-25)8-4-5-15-32(34)27(29)33/h1-3,6-7,9-12,24-26,34H,5,13-20H2,(H2,29,33)/t24-,25+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 2265-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.005
BindingDB Entry DOI: 10.7270/Q2BV7G2P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50144622

(CHEMBL312082 | [3-(5-{4-[(R)-(4-Chloro-phenyl)-phe...)Show SMILES CC(C#Cc1ccc(CN2CCN(CC2)[C@H](c2ccccc2)c2ccc(Cl)cc2)o1)N(O)C(N)=O Show InChI InChI=1S/C27H29ClN4O3/c1-20(32(34)27(29)33)7-12-24-13-14-25(35-24)19-30-15-17-31(18-16-30)26(21-5-3-2-4-6-21)22-8-10-23(28)11-9-22/h2-6,8-11,13-14,20,26,34H,15-19H2,1H3,(H2,29,33)/t20?,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 2265-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.005
BindingDB Entry DOI: 10.7270/Q2BV7G2P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50155340
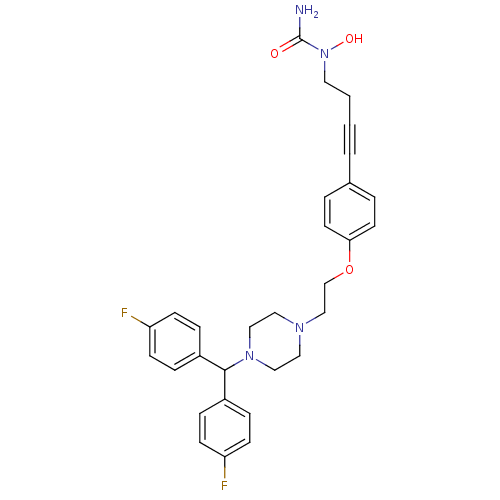
(1-{4-[4-(2-{4-[bis(4-fluorophenyl)methyl]piperazin...)Show SMILES NC(=O)N(O)CCC#Cc1ccc(OCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C30H32F2N4O3/c31-26-10-6-24(7-11-26)29(25-8-12-27(32)13-9-25)35-19-17-34(18-20-35)21-22-39-28-14-4-23(5-15-28)3-1-2-16-36(38)30(33)37/h4-15,29,38H,2,16-22H2,(H2,33,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 5591-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.060
BindingDB Entry DOI: 10.7270/Q28C9VRH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50155333
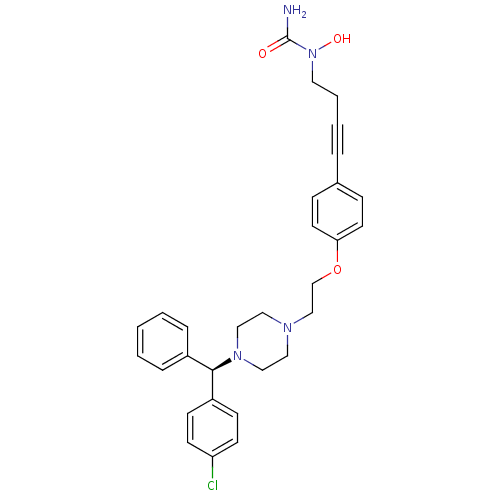
(1-{4-[4-(2-{4-[(R)-(4-chlorophenyl)(phenyl)methyl]...)Show SMILES NC(=O)N(O)CCC#Cc1ccc(OCCN2CCN(CC2)[C@H](c2ccccc2)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C30H33ClN4O3/c31-27-13-11-26(12-14-27)29(25-7-2-1-3-8-25)34-20-18-33(19-21-34)22-23-38-28-15-9-24(10-16-28)6-4-5-17-35(37)30(32)36/h1-3,7-16,29,37H,5,17-23H2,(H2,32,36)/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 5591-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.060
BindingDB Entry DOI: 10.7270/Q28C9VRH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22876
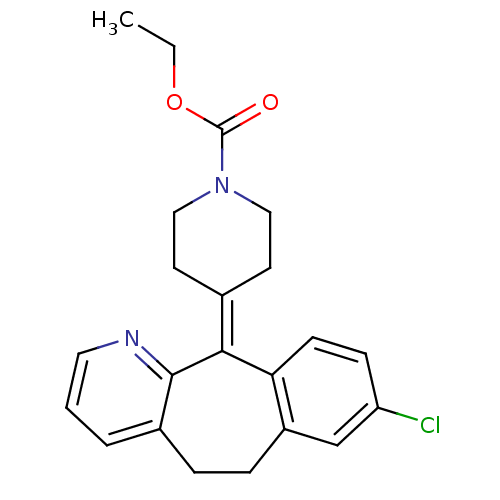
(CHEMBL998 | Claritin | Loratadine | Sch 29851 | US...)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C22H23ClN2O2/c1-2-27-22(26)25-12-9-15(10-13-25)20-19-8-7-18(23)14-17(19)6-5-16-4-3-11-24-21(16)20/h3-4,7-8,11,14H,2,5-6,9-10,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 414 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 5591-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.060
BindingDB Entry DOI: 10.7270/Q28C9VRH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50144620
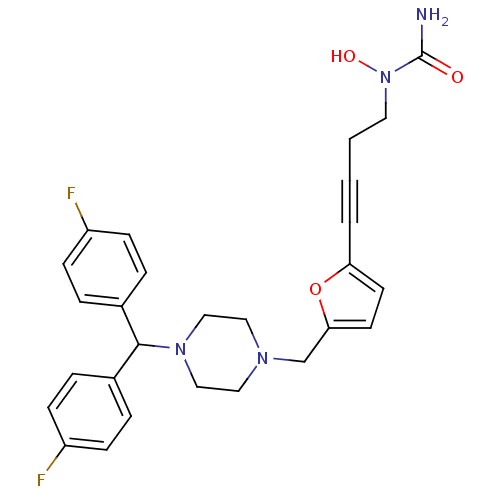
(CHEMBL73706 | [4-(5-{4-[Bis-(4-fluoro-phenyl)-meth...)Show SMILES NC(=O)N(O)CCC#Cc1ccc(CN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)o1 Show InChI InChI=1S/C27H28F2N4O3/c28-22-8-4-20(5-9-22)26(21-6-10-23(29)11-7-21)32-17-15-31(16-18-32)19-25-13-12-24(36-25)3-1-2-14-33(35)27(30)34/h4-13,26,35H,2,14-19H2,(H2,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 2265-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.005
BindingDB Entry DOI: 10.7270/Q2BV7G2P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50144623
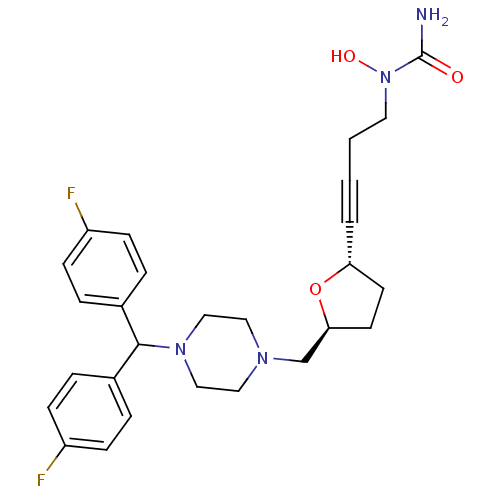
(CHEMBL74779 | [4-((2S,5S)-5-{4-[Bis-(4-fluoro-phen...)Show SMILES NC(=O)N(O)CCC#C[C@@H]1CC[C@@H](CN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)O1 Show InChI InChI=1S/C27H32F2N4O3/c28-22-8-4-20(5-9-22)26(21-6-10-23(29)11-7-21)32-17-15-31(16-18-32)19-25-13-12-24(36-25)3-1-2-14-33(35)27(30)34/h4-11,24-26,35H,2,12-19H2,(H2,30,34)/t24-,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards histamine H1 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 14: 2265-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.005
BindingDB Entry DOI: 10.7270/Q2BV7G2P |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50447088
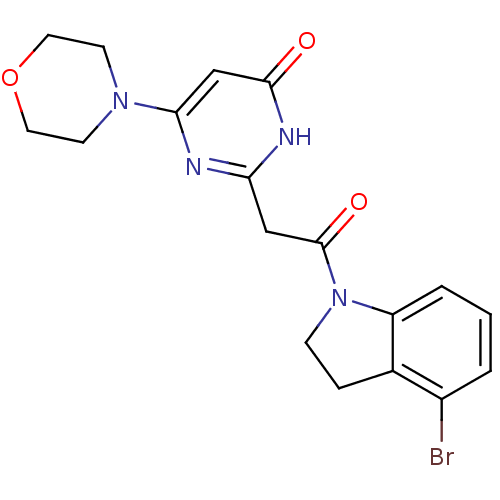
(CHEMBL3112850)Show SMILES Brc1cccc2N(CCc12)C(=O)Cc1nc(cc(=O)[nH]1)N1CCOCC1 Show InChI InChI=1S/C18H19BrN4O3/c19-13-2-1-3-14-12(13)4-5-23(14)18(25)10-15-20-16(11-17(24)21-15)22-6-8-26-9-7-22/h1-3,11H,4-10H2,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50447089
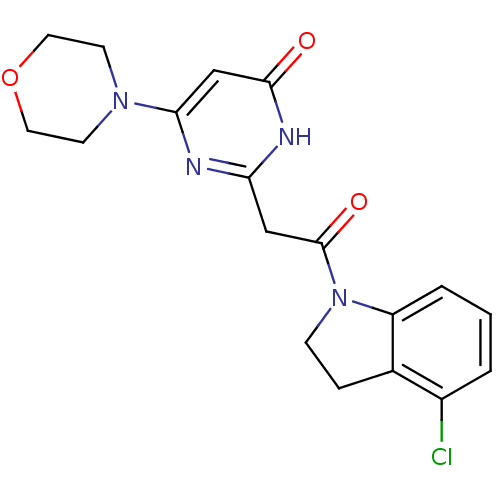
(CHEMBL3112849)Show SMILES Clc1cccc2N(CCc12)C(=O)Cc1nc(cc(=O)[nH]1)N1CCOCC1 Show InChI InChI=1S/C18H19ClN4O3/c19-13-2-1-3-14-12(13)4-5-23(14)18(25)10-15-20-16(11-17(24)21-15)22-6-8-26-9-7-22/h1-3,11H,4-10H2,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50447078
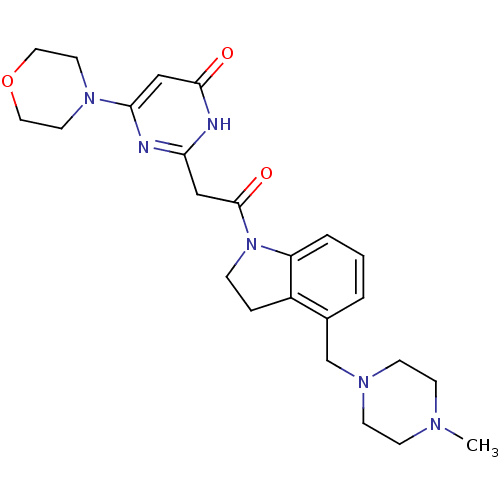
(CHEMBL3112860 | US9133168, Example 18d)Show SMILES CN1CCN(Cc2cccc3N(CCc23)C(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)CC1 Show InChI InChI=1S/C24H32N6O3/c1-27-7-9-28(10-8-27)17-18-3-2-4-20-19(18)5-6-30(20)24(32)15-21-25-22(16-23(31)26-21)29-11-13-33-14-12-29/h2-4,16H,5-15,17H2,1H3,(H,25,26,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kdelta expressed in baculovirus-infected sf9 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50408421

(CHEMBL2113725)Show SMILES CCCCN(O)C(=O)Nc1cc(cc(OC)c1OCCSc1c(F)c(F)cc(F)c1F)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C33H38F4N2O8S/c1-6-7-10-39(41)33(40)38-22-13-18(23-8-9-24(47-23)19-15-26(43-3)31(45-5)27(16-19)44-4)14-25(42-2)30(22)46-11-12-48-32-28(36)20(34)17-21(35)29(32)37/h13-17,23-24,41H,6-12H2,1-5H3,(H,38,40)/t23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PAF from PAF receptor of human platelet membranes |
J Med Chem 41: 1970-9 (1998)
Article DOI: 10.1021/jm980046r
BindingDB Entry DOI: 10.7270/Q2PV6JH2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50447087
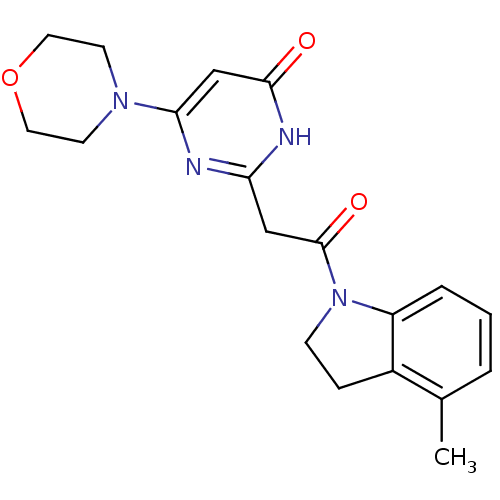
(CHEMBL3112851)Show SMILES Cc1cccc2N(CCc12)C(=O)Cc1nc(cc(=O)[nH]1)N1CCOCC1 Show InChI InChI=1S/C19H22N4O3/c1-13-3-2-4-15-14(13)5-6-23(15)19(25)11-16-20-17(12-18(24)21-16)22-7-9-26-10-8-22/h2-4,12H,5-11H2,1H3,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50447089

(CHEMBL3112849)Show SMILES Clc1cccc2N(CCc12)C(=O)Cc1nc(cc(=O)[nH]1)N1CCOCC1 Show InChI InChI=1S/C18H19ClN4O3/c19-13-2-1-3-14-12(13)4-5-23(14)18(25)10-15-20-16(11-17(24)21-15)22-6-8-26-9-7-22/h1-3,11H,4-10H2,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
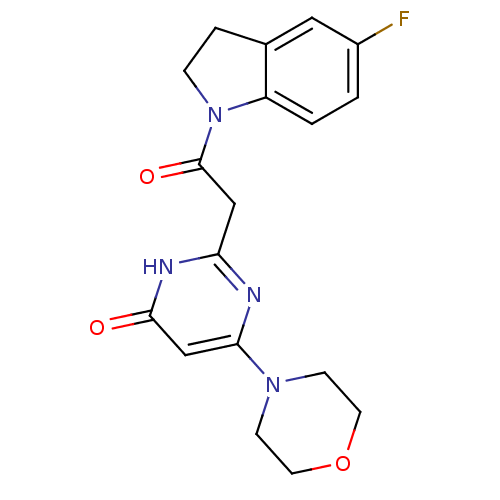
(Homo sapiens (Human)) | BDBM50447092
(CHEMBL3112847)Show SMILES Fc1ccc2N(CCc2c1)C(=O)Cc1nc(cc(=O)[nH]1)N1CCOCC1 Show InChI InChI=1S/C18H19FN4O3/c19-13-1-2-14-12(9-13)3-4-23(14)18(25)10-15-20-16(11-17(24)21-15)22-5-7-26-8-6-22/h1-2,9,11H,3-8,10H2,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
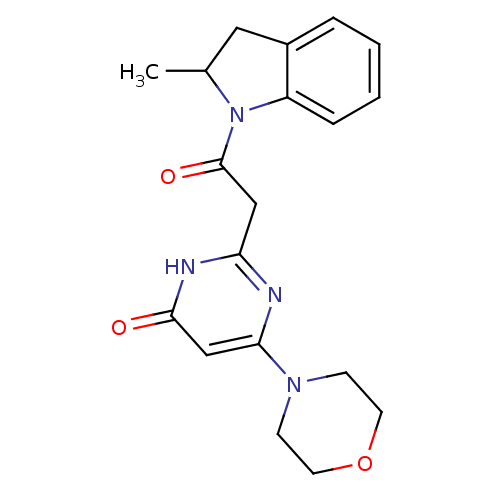
(Homo sapiens (Human)) | BDBM50447100
(CHEMBL3112862)Show SMILES CC1Cc2ccccc2N1C(=O)Cc1nc(cc(=O)[nH]1)N1CCOCC1 Show InChI InChI=1S/C19H22N4O3/c1-13-10-14-4-2-3-5-15(14)23(13)19(25)11-16-20-17(12-18(24)21-16)22-6-8-26-9-7-22/h2-5,12-13H,6-11H2,1H3,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
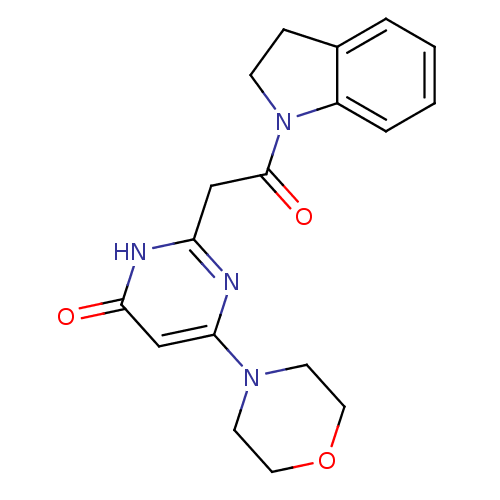
(Homo sapiens (Human)) | BDBM50447093
(CHEMBL3112690)Show SMILES O=C(Cc1nc(cc(=O)[nH]1)N1CCOCC1)N1CCc2ccccc12 Show InChI InChI=1S/C18H20N4O3/c23-17-12-16(21-7-9-25-10-8-21)19-15(20-17)11-18(24)22-6-5-13-3-1-2-4-14(13)22/h1-4,12H,5-11H2,(H,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50016184

(CHEMBL3262131)Show InChI InChI=1S/C16H17N5O2.ClH/c1-3-13(10-17-5-1)22-11-12-9-18-7-8-21(12)16-20-15-14(23-16)4-2-6-19-15;/h1-6,10,12,18H,7-9,11H2;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis |
J Med Chem 57: 3966-83 (2014)
Article DOI: 10.1021/jm5004599
BindingDB Entry DOI: 10.7270/Q2CV4K8S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50016200

(CHEMBL3262146)Show InChI InChI=1S/C16H16ClN5OS.ClH/c17-11-6-14-15(20-7-11)21-16(24-14)22-5-4-19-8-12(22)10-23-13-2-1-3-18-9-13;/h1-3,6-7,9,12,19H,4-5,8,10H2;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis |
J Med Chem 57: 3966-83 (2014)
Article DOI: 10.1021/jm5004599
BindingDB Entry DOI: 10.7270/Q2CV4K8S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
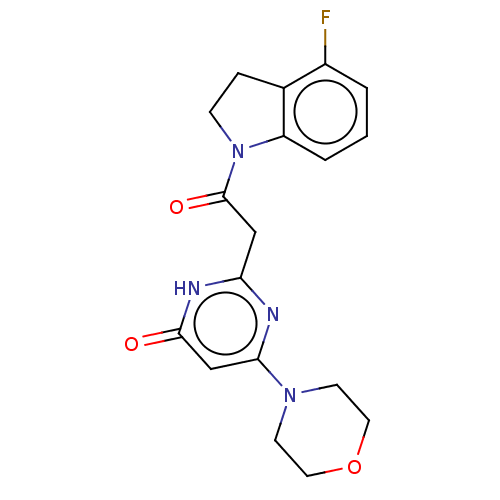
(Homo sapiens (Human)) | BDBM60629
(BDBM50447090 | US9133168, 16b)Show SMILES Fc1cccc2N(CCc12)C(=O)Cc1nc(cc(=O)[nH]1)N1CCOCC1 Show InChI InChI=1S/C18H19FN4O3/c19-13-2-1-3-14-12(13)4-5-23(14)18(25)10-15-20-16(11-17(24)21-15)22-6-8-26-9-7-22/h1-3,11H,4-10H2,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50447087

(CHEMBL3112851)Show SMILES Cc1cccc2N(CCc12)C(=O)Cc1nc(cc(=O)[nH]1)N1CCOCC1 Show InChI InChI=1S/C19H22N4O3/c1-13-3-2-4-15-14(13)5-6-23(15)19(25)11-16-20-17(12-18(24)21-16)22-7-9-26-10-8-22/h2-4,12H,5-11H2,1H3,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50016185
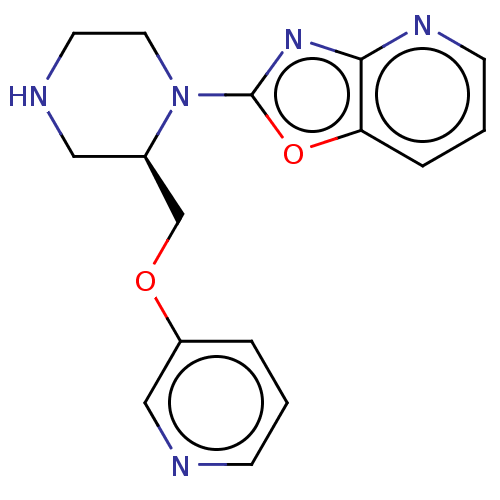
(CHEMBL3262132)Show SMILES Cl.C(Oc1cccnc1)[C@H]1CNCCN1c1nc2ncccc2o1 |r| Show InChI InChI=1S/C16H17N5O2.ClH/c1-3-13(10-17-5-1)22-11-12-9-18-7-8-21(12)16-20-15-14(23-16)4-2-6-19-15;/h1-6,10,12,18H,7-9,11H2;1H/t12-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated alpha-bungarotoxin binding to alpha7 nAChR in rat PC12 cells by FACS analysis |
J Med Chem 57: 3966-83 (2014)
Article DOI: 10.1021/jm5004599
BindingDB Entry DOI: 10.7270/Q2CV4K8S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50447100

(CHEMBL3112862)Show SMILES CC1Cc2ccccc2N1C(=O)Cc1nc(cc(=O)[nH]1)N1CCOCC1 Show InChI InChI=1S/C19H22N4O3/c1-13-10-14-4-2-3-5-15(14)23(13)19(25)11-16-20-17(12-18(24)21-16)22-6-8-26-9-7-22/h2-5,12-13H,6-11H2,1H3,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM60632
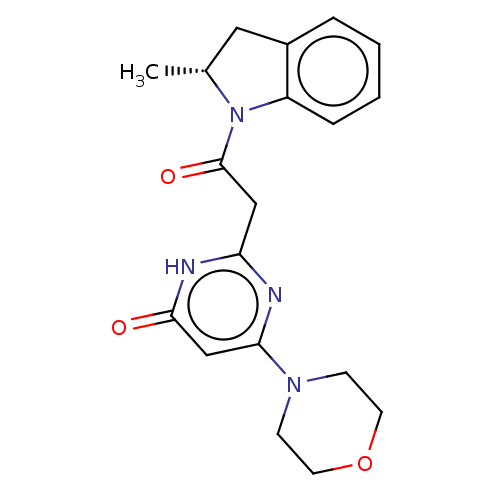
(BDBM50447095 | US9133168, 20b)Show SMILES C[C@@H]1Cc2ccccc2N1C(=O)Cc1nc(cc(=O)[nH]1)N1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kdelta expressed in baculovirus-infected sf9 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM60632

(BDBM50447095 | US9133168, 20b)Show SMILES C[C@@H]1Cc2ccccc2N1C(=O)Cc1nc(cc(=O)[nH]1)N1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50447088

(CHEMBL3112850)Show SMILES Brc1cccc2N(CCc12)C(=O)Cc1nc(cc(=O)[nH]1)N1CCOCC1 Show InChI InChI=1S/C18H19BrN4O3/c19-13-2-1-3-14-12(13)4-5-23(14)18(25)10-15-20-16(11-17(24)21-15)22-6-8-26-9-7-22/h1-3,11H,4-10H2,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002823
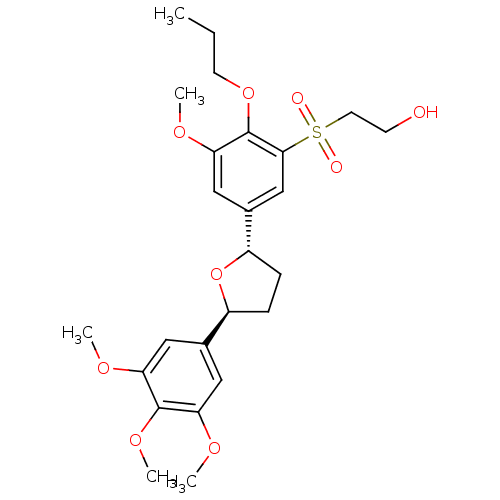
(2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...)Show SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)CCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C25H34O9S/c1-6-10-33-25-22(31-4)14-17(15-23(25)35(27,28)11-9-26)19-8-7-18(34-19)16-12-20(29-2)24(32-5)21(13-16)30-3/h12-15,18-19,26H,6-11H2,1-5H3/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PAF from PAF receptor of human platelet membranes |
J Med Chem 41: 1970-9 (1998)
Article DOI: 10.1021/jm980046r
BindingDB Entry DOI: 10.7270/Q2PV6JH2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM60629

(BDBM50447090 | US9133168, 16b)Show SMILES Fc1cccc2N(CCc12)C(=O)Cc1nc(cc(=O)[nH]1)N1CCOCC1 Show InChI InChI=1S/C18H19FN4O3/c19-13-2-1-3-14-12(13)4-5-23(14)18(25)10-15-20-16(11-17(24)21-15)22-6-8-26-9-7-22/h1-3,11H,4-10H2,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50447092

(CHEMBL3112847)Show SMILES Fc1ccc2N(CCc2c1)C(=O)Cc1nc(cc(=O)[nH]1)N1CCOCC1 Show InChI InChI=1S/C18H19FN4O3/c19-13-1-2-14-12(9-13)3-4-23(14)18(25)10-15-20-16(11-17(24)21-15)22-5-7-26-8-6-22/h1-2,9,11H,3-8,10H2,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... |
J Med Chem 57: 903-20 (2014)
Article DOI: 10.1021/jm401642q
BindingDB Entry DOI: 10.7270/Q2R212VJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data