 Found 208 hits with Last Name = 'rao' and Initial = 'l'
Found 208 hits with Last Name = 'rao' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50005232
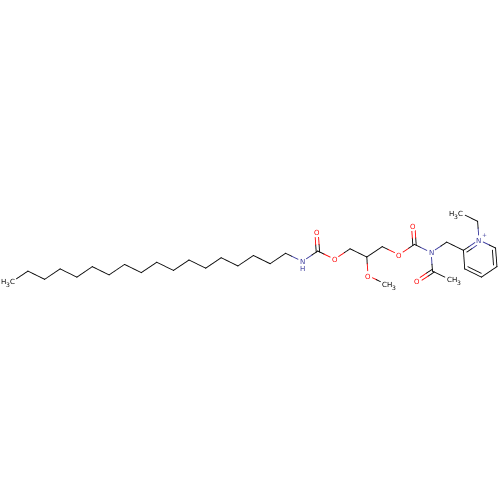
((R)-2-{[Acetyl-(2-methoxy-3-octadecylcarbamoyloxy-...)Show SMILES CCCCCCCCCCCCCCCCCCNC(=O)OCC(COC(=O)N(Cc1cccc[n+]1CC)C(C)=O)OC Show InChI InChI=1S/C34H59N3O6/c1-5-7-8-9-10-11-12-13-14-15-16-17-18-19-20-22-25-35-33(39)42-28-32(41-4)29-43-34(40)37(30(3)38)27-31-24-21-23-26-36(31)6-2/h21,23-24,26,32H,5-20,22,25,27-29H2,1-4H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50005232

((R)-2-{[Acetyl-(2-methoxy-3-octadecylcarbamoyloxy-...)Show SMILES CCCCCCCCCCCCCCCCCCNC(=O)OCC(COC(=O)N(Cc1cccc[n+]1CC)C(C)=O)OC Show InChI InChI=1S/C34H59N3O6/c1-5-7-8-9-10-11-12-13-14-15-16-17-18-19-20-22-25-35-33(39)42-28-32(41-4)29-43-34(40)37(30(3)38)27-31-24-21-23-26-36(31)6-2/h21,23-24,26,32H,5-20,22,25,27-29H2,1-4H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50038766
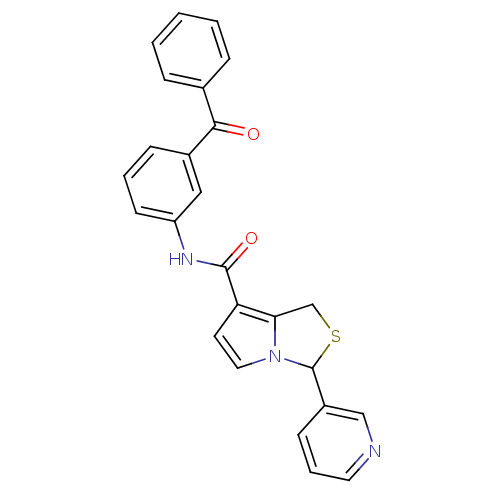
(3-Pyridin-3-yl-1H-pyrrolo[1,2-c]thiazole-7-carboxy...)Show SMILES O=C(Nc1cccc(c1)C(=O)c1ccccc1)c1ccn2C(SCc12)c1cccnc1 Show InChI InChI=1S/C25H19N3O2S/c29-23(17-6-2-1-3-7-17)18-8-4-10-20(14-18)27-24(30)21-11-13-28-22(21)16-31-25(28)19-9-5-12-26-15-19/h1-15,25H,16H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50038766

(3-Pyridin-3-yl-1H-pyrrolo[1,2-c]thiazole-7-carboxy...)Show SMILES O=C(Nc1cccc(c1)C(=O)c1ccccc1)c1ccn2C(SCc12)c1cccnc1 Show InChI InChI=1S/C25H19N3O2S/c29-23(17-6-2-1-3-7-17)18-8-4-10-20(14-18)27-24(30)21-11-13-28-22(21)16-31-25(28)19-9-5-12-26-15-19/h1-15,25H,16H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM81928
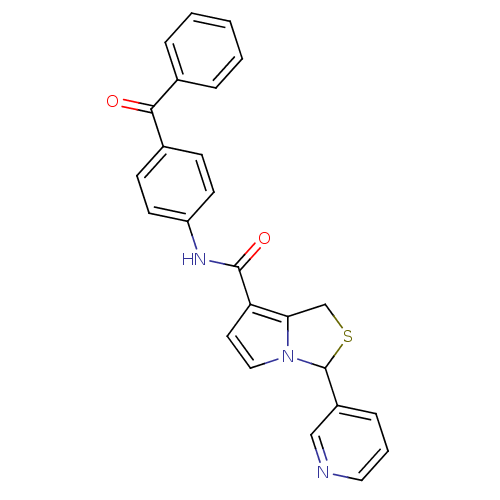
(1H,3H-Pyrrolo[1,2-c]thiazole-7-carboxamide,N-(3-be...)Show SMILES O=C(Nc1ccc(cc1)C(=O)c1ccccc1)c1ccn2C(SCc12)c1cccnc1 Show InChI InChI=1S/C25H19N3O2S/c29-23(17-5-2-1-3-6-17)18-8-10-20(11-9-18)27-24(30)21-12-14-28-22(21)16-31-25(28)19-7-4-13-26-15-19/h1-15,25H,16H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM471715
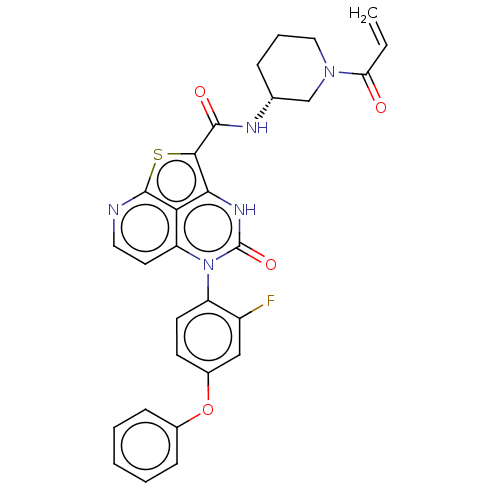
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(2-fluoro-4-phe...)Show SMILES Fc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wU:25.26,(-1.78,4.35,;-3.12,3.58,;-4.45,4.35,;-5.78,3.58,;-7.12,4.35,;-8.45,3.58,;-9.78,4.35,;-11.12,3.58,;-11.12,2.04,;-9.78,1.27,;-8.45,2.04,;-5.78,2.04,;-4.45,1.27,;-3.12,2.04,;-1.78,1.27,;-1.78,-.27,;-3.12,-1.04,;-3.12,-2.58,;-1.78,-3.35,;-.45,-2.58,;1.04,-2.98,;1.88,-1.68,;3.42,-1.68,;4.19,-.35,;4.19,-3.02,;5.73,-3.02,;6.5,-4.35,;8.04,-4.35,;8.81,-3.02,;8.04,-1.68,;6.5,-1.68,;8.81,-.35,;8.04,.98,;10.35,-.35,;11.12,-1.68,;.89,-.27,;.89,1.27,;-.45,2.04,;-.45,3.58,;-.45,-1.04,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM81928

(1H,3H-Pyrrolo[1,2-c]thiazole-7-carboxamide,N-(3-be...)Show SMILES O=C(Nc1ccc(cc1)C(=O)c1ccccc1)c1ccn2C(SCc12)c1cccnc1 Show InChI InChI=1S/C25H19N3O2S/c29-23(17-5-2-1-3-6-17)18-8-10-20(11-9-18)27-24(30)21-12-14-28-22(21)16-31-25(28)19-7-4-13-26-15-19/h1-15,25H,16H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 11.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50569791

(CHEMBL4846828)Show SMILES Cc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CC[C@@H](CC(=O)C=C)C4)c([nH]c1=O)c23 |r,wU:28.30,wD:25.26,(55.67,-29.88,;55.67,-28.34,;54.33,-27.57,;54.33,-26.03,;53,-25.26,;53,-23.72,;54.34,-22.95,;54.34,-21.41,;53,-20.64,;51.67,-21.42,;51.67,-22.95,;55.66,-25.25,;57,-26.02,;57,-27.57,;58.33,-28.34,;58.34,-29.88,;57.02,-30.64,;57.01,-32.16,;58.34,-32.93,;59.66,-32.16,;61.52,-32.32,;62.15,-30.91,;63.65,-30.59,;64.68,-31.74,;64.13,-29.13,;65.64,-28.81,;66.26,-27.4,;67.79,-27.56,;68.11,-29.07,;69.52,-29.7,;70.76,-28.79,;70.61,-27.26,;72.17,-29.42,;73.42,-28.52,;66.78,-29.84,;61,-29.88,;61,-28.34,;59.66,-27.56,;59.66,-26.02,;59.67,-30.65,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467364
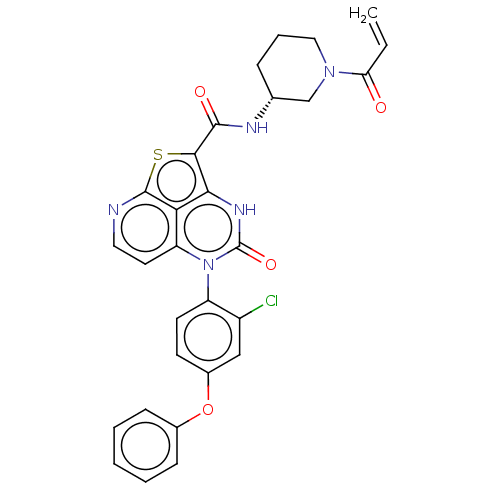
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(2-chloro-4-phe...)Show SMILES Clc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wU:25.26,(-1.95,3.85,;-3.29,3.08,;-4.62,3.85,;-5.95,3.08,;-7.29,3.85,;-8.62,3.08,;-9.95,3.85,;-11.29,3.08,;-11.29,1.54,;-9.95,.77,;-8.62,1.54,;-5.95,1.54,;-4.62,.77,;-3.29,1.54,;-1.95,.77,;-1.95,-.77,;-3.29,-1.54,;-3.29,-3.08,;-1.95,-3.85,;-.62,-3.08,;.72,-3.85,;2.05,-2.07,;3.59,-2.07,;4.36,-3.41,;4.36,-.74,;5.9,-.74,;6.67,-2.07,;8.21,-2.07,;8.98,-.74,;8.21,.59,;6.67,.59,;8.98,1.93,;8.21,3.26,;10.52,1.93,;11.29,.59,;.72,-.77,;.72,.77,;-.62,1.54,;-.62,3.08,;-.62,-1.54,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM485273
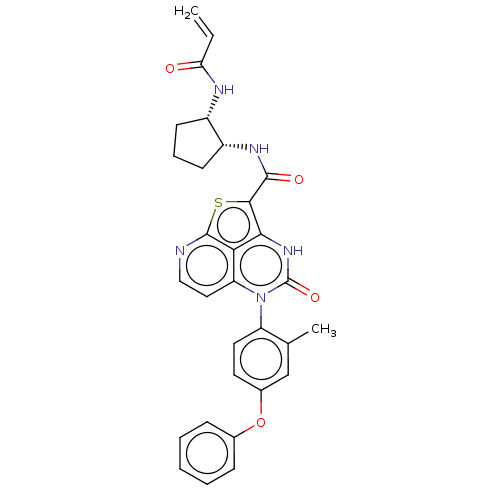
(N-((1R,2S)-2-Acrylamidocyclopentyl)-5-(*S)-(2-meth...)Show SMILES Cc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCC[C@@H]4NC(=O)C=C)c([nH]c1=O)c23 |r,wU:25.26,29.32,(-.48,2.9,;-1.82,2.13,;-3.15,2.9,;-4.48,2.13,;-5.82,2.9,;-7.15,2.13,;-8.49,2.9,;-9.82,2.13,;-9.82,.59,;-8.49,-.18,;-7.15,.59,;-4.48,.59,;-3.15,-.18,;-1.82,.59,;-.48,-.18,;-.48,-1.72,;-1.82,-2.49,;-1.82,-4.03,;-.48,-4.8,;.85,-4.03,;2.3,-4.48,;3,-3,;4.54,-3,;5.31,-4.33,;5.31,-1.66,;6.85,-1.66,;7.76,-2.91,;9.22,-2.43,;9.22,-.89,;7.76,-.42,;7.28,1.05,;8.31,2.19,;9.82,1.87,;7.84,3.66,;8.87,4.8,;2.18,-1.72,;2.18,-.18,;.85,.59,;.85,2.13,;.85,-2.49,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM81930
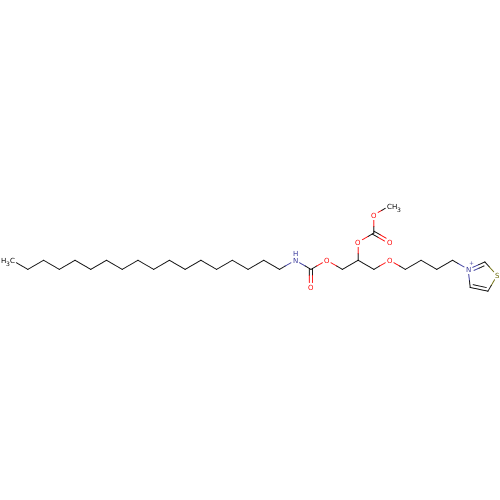
(CAS_129364 | NSC_129364 | RO 19-3704)Show SMILES CCCCCCCCCCCCCCCCCCNC(=O)OCC(COCCCC[n+]1ccsc1)OC(=O)OC Show InChI InChI=1S/C31H56N2O6S/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-21-32-30(34)38-27-29(39-31(35)36-2)26-37-24-20-19-22-33-23-25-40-28-33/h23,25,28-29H,3-22,24,26-27H2,1-2H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 16.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50011875
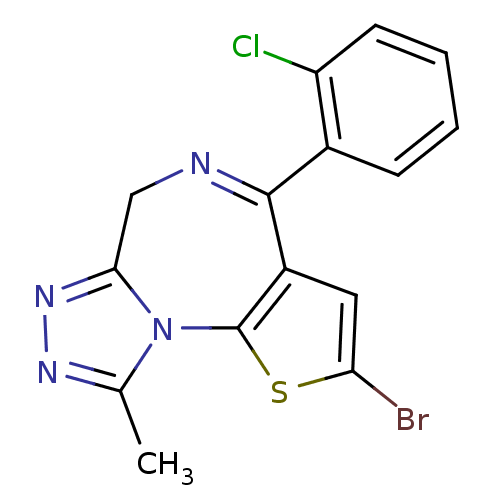
(2-Bromo-4-(2-chloro-phenyl)-9-methyl-6H-1-thia-5,7...)Show SMILES Cc1nnc2CN=C(c3cc(Br)sc3-n12)c1ccccc1Cl |c:6| Show InChI InChI=1S/C15H10BrClN4S/c1-8-19-20-13-7-18-14(9-4-2-3-5-11(9)17)10-6-12(16)22-15(10)21(8)13/h2-6H,7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468103
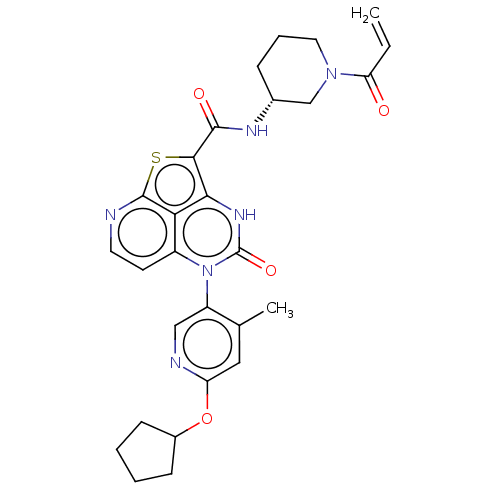
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(6-(cyclop...)Show SMILES Cc1cc(OC2CCCC2)ncc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:24.25,(-1.92,5.75,;-3.25,4.98,;-4.59,5.75,;-5.92,4.98,;-7.25,5.75,;-8.59,4.98,;-9.99,5.6,;-11.02,4.46,;-10.25,3.13,;-8.75,3.45,;-5.92,3.44,;-4.59,2.67,;-3.25,3.44,;-1.92,2.67,;-1.92,1.13,;-3.25,.36,;-3.25,-1.18,;-1.92,-1.95,;-.59,-1.18,;.88,-1.66,;1.78,-.41,;3.32,-.41,;4.09,.92,;4.09,-1.75,;5.63,-1.75,;6.4,-.41,;7.94,-.41,;8.71,-1.75,;7.94,-3.08,;6.4,-3.08,;8.71,-4.41,;7.94,-5.75,;10.25,-4.41,;11.02,-5.75,;.75,1.13,;.75,2.67,;-.59,3.44,;-.59,4.98,;-.59,.36,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50000714
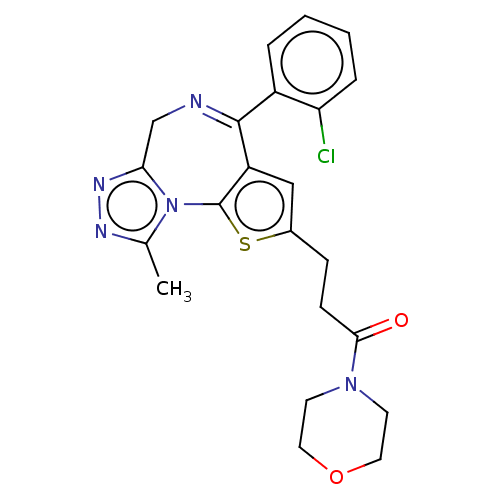
(3-[4-(2-Chloro-phenyl)-9-methyl-6H-1-thia-5,7,8,9a...)Show SMILES Cc1nnc2CN=C(c3cc(CCC(=O)N4CCOCC4)sc3-n12)c1ccccc1Cl |c:6| Show InChI InChI=1S/C22H22ClN5O2S/c1-14-25-26-19-13-24-21(16-4-2-3-5-18(16)23)17-12-15(31-22(17)28(14)19)6-7-20(29)27-8-10-30-11-9-27/h2-5,12H,6-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 23.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468000
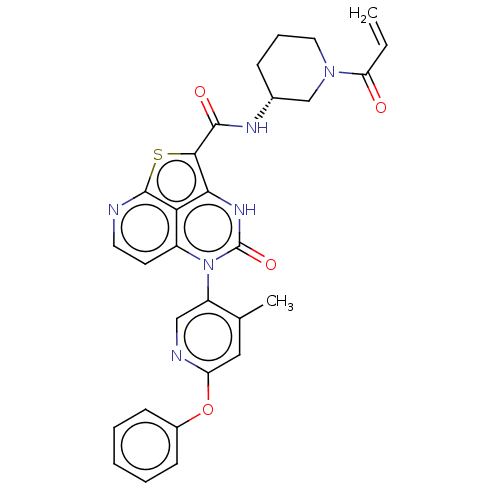
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(4-methyl-...)Show SMILES Cc1cc(Oc2ccccc2)ncc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wU:25.26,(-4.48,-.77,;-4.48,.77,;-5.82,1.54,;-5.82,3.08,;-7.15,3.85,;-8.48,3.08,;-9.82,3.85,;-11.15,3.08,;-11.15,1.54,;-9.82,.77,;-8.48,1.54,;-4.48,3.85,;-3.15,3.08,;-3.15,1.54,;-1.82,.77,;-1.82,-.77,;-3.15,-1.54,;-3.15,-3.08,;-1.82,-3.85,;-.48,-3.08,;1.01,-3.48,;1.94,-1.86,;3.48,-1.86,;4.25,-3.19,;4.25,-.53,;5.79,-.53,;6.56,-1.86,;8.1,-1.86,;8.87,-.53,;8.1,.81,;6.56,.81,;8.87,2.14,;8.1,3.48,;10.41,2.14,;11.15,3.43,;.85,-.77,;.85,.77,;-.48,1.54,;-.48,3.08,;-.48,-1.54,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467718
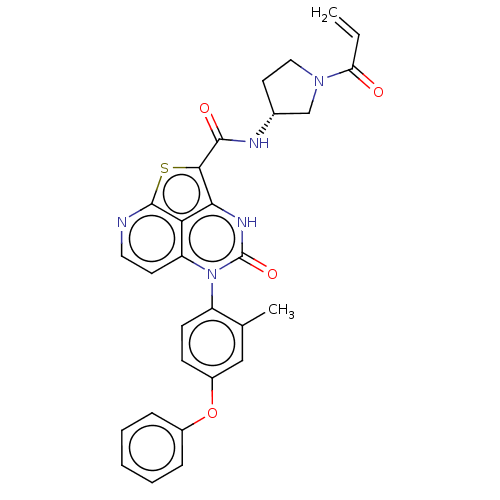
((R)-N-(1-Acryloylpyrrolidin-3-yl)-5-(2-methyl-4-(2...)Show SMILES Cc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:25.26,(-1,4.5,;-2.28,3.77,;-3.62,4.54,;-4.95,3.77,;-6.28,4.54,;-7.62,3.77,;-8.95,4.54,;-10.28,3.77,;-10.28,2.23,;-8.95,1.46,;-7.62,2.23,;-4.95,2.23,;-3.62,1.46,;-2.28,2.23,;-.95,1.46,;-.95,-.08,;-2.28,-.85,;-2.28,-2.39,;-.95,-3.16,;.39,-2.39,;1.85,-2.87,;2.76,-1.62,;4.29,-1.65,;5.09,-.33,;5.04,-3,;6.58,-3,;7.49,-1.75,;8.95,-2.23,;8.95,-3.77,;7.49,-4.24,;10.28,-4.54,;10.28,-6.08,;11.62,-3.77,;11.62,-2.23,;1.72,-.08,;1.72,1.46,;.39,2.23,;.39,3.77,;.39,-.85,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467718

((R)-N-(1-Acryloylpyrrolidin-3-yl)-5-(2-methyl-4-(2...)Show SMILES Cc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:25.26,(-1,4.5,;-2.28,3.77,;-3.62,4.54,;-4.95,3.77,;-6.28,4.54,;-7.62,3.77,;-8.95,4.54,;-10.28,3.77,;-10.28,2.23,;-8.95,1.46,;-7.62,2.23,;-4.95,2.23,;-3.62,1.46,;-2.28,2.23,;-.95,1.46,;-.95,-.08,;-2.28,-.85,;-2.28,-2.39,;-.95,-3.16,;.39,-2.39,;1.85,-2.87,;2.76,-1.62,;4.29,-1.65,;5.09,-.33,;5.04,-3,;6.58,-3,;7.49,-1.75,;8.95,-2.23,;8.95,-3.77,;7.49,-4.24,;10.28,-4.54,;10.28,-6.08,;11.62,-3.77,;11.62,-2.23,;1.72,-.08,;1.72,1.46,;.39,2.23,;.39,3.77,;.39,-.85,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467367
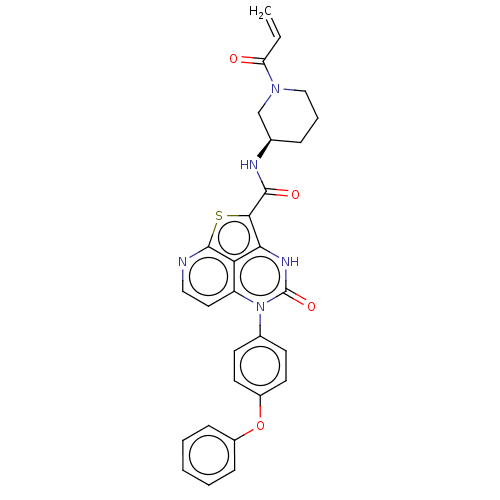
((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-(4-phenox...)Show SMILES C=CC(=O)N1CCC[C@H](C1)NC(=O)c1sc2nccc3n(-c4ccc(Oc5ccccc5)cc4)c(=O)[nH]c1c23 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM81930

(CAS_129364 | NSC_129364 | RO 19-3704)Show SMILES CCCCCCCCCCCCCCCCCCNC(=O)OCC(COCCCC[n+]1ccsc1)OC(=O)OC Show InChI InChI=1S/C31H56N2O6S/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-21-32-30(34)38-27-29(39-31(35)36-2)26-37-24-20-19-22-33-23-25-40-28-33/h23,25,28-29H,3-22,24,26-27H2,1-2H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 38.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50000714

(3-[4-(2-Chloro-phenyl)-9-methyl-6H-1-thia-5,7,8,9a...)Show SMILES Cc1nnc2CN=C(c3cc(CCC(=O)N4CCOCC4)sc3-n12)c1ccccc1Cl |c:6| Show InChI InChI=1S/C22H22ClN5O2S/c1-14-25-26-19-13-24-21(16-4-2-3-5-18(16)23)17-12-15(31-22(17)28(14)19)6-7-20(29)27-8-10-30-11-9-27/h2-5,12H,6-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 39.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50601991
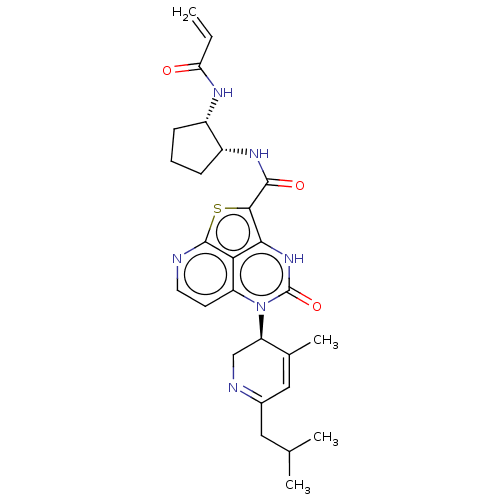
(CHEMBL5206366)Show SMILES CC(C)CC1=NC[C@H](C(C)=C1)n1c2ccnc3sc(C(=O)N[C@@H]4CCC[C@@H]4NC(=O)C=C)c([nH]c1=O)c23 |r,c:9,t:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50569790
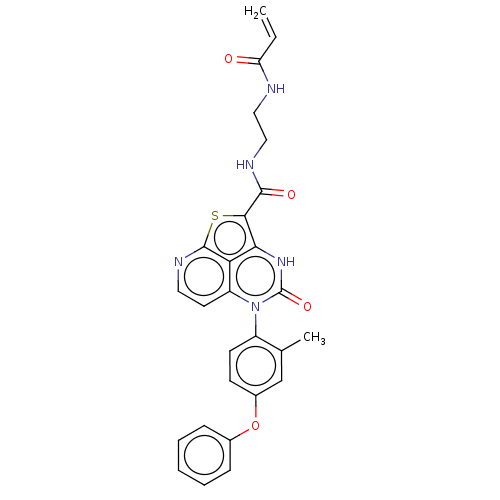
(CHEMBL4852459)Show SMILES Cc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)NCCNC(=O)C=C)c([nH]c1=O)c23 |(6.38,-11.63,;6.38,-10.09,;5.04,-9.32,;5.05,-7.78,;3.71,-7.01,;3.71,-5.47,;5.05,-4.7,;5.05,-3.16,;3.71,-2.39,;2.38,-3.17,;2.38,-4.71,;6.37,-7.01,;7.71,-7.77,;7.71,-9.32,;9.05,-10.09,;9.05,-11.63,;7.73,-12.39,;7.73,-13.92,;9.06,-14.68,;10.38,-13.91,;12.23,-14.07,;12.86,-12.67,;14.36,-12.35,;15.39,-13.49,;14.84,-10.88,;16.35,-10.57,;16.83,-9.1,;18.33,-8.78,;18.81,-7.32,;17.78,-6.17,;20.32,-7,;20.79,-5.54,;11.71,-11.63,;11.71,-10.09,;10.37,-9.31,;10.37,-7.77,;10.38,-12.4,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468010

((R)-N-(1-Acryloylpiperidin-3-yl)-5-(6-cyclobutoxy-...)Show SMILES Cc1cc(OC2CCC2)ncc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:23.24,(-1.8,6.31,;-3.13,5.54,;-4.47,6.31,;-5.8,5.54,;-7.14,6.31,;-8.47,5.54,;-9.96,5.93,;-10.36,4.45,;-8.87,4.05,;-5.8,4,;-4.47,3.23,;-3.13,4,;-1.8,3.23,;-1.8,1.69,;-3.13,.92,;-3.13,-.62,;-1.8,-1.39,;-.47,-.62,;1.14,-.89,;1.88,.36,;3.42,.36,;4.19,1.7,;4.19,-.97,;5.73,-.97,;6.5,.36,;8.04,.36,;8.82,-.97,;8.04,-2.3,;6.5,-2.3,;8.82,-3.64,;10.36,-3.64,;8.04,-4.97,;8.82,-6.31,;.87,1.69,;.87,3.23,;-.47,4,;-.47,5.54,;-.47,.92,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467836
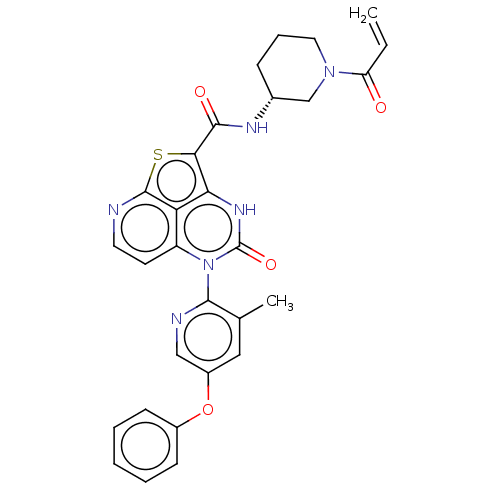
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(3-methyl-5-phe...)Show SMILES Cc1cc(Oc2ccccc2)cnc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:25.26,(-4.18,1.77,;-4.18,3.31,;-5.51,4.08,;-5.51,5.62,;-6.85,6.39,;-8.18,5.62,;-9.51,6.39,;-10.85,5.62,;-10.85,4.08,;-9.51,3.31,;-8.18,4.08,;-4.18,6.39,;-2.84,5.62,;-2.84,4.08,;-1.51,3.31,;-1.51,1.77,;-2.84,1,;-2.84,-.54,;-1.51,-1.31,;-.18,-.54,;1.16,-1.31,;2.37,.26,;3.91,.27,;4.66,1.61,;4.69,-1.06,;6.23,-1.06,;7,.28,;8.54,.28,;9.31,-1.06,;8.54,-2.39,;7,-2.39,;9.31,-3.72,;10.85,-3.72,;8.54,-5.06,;9.31,-6.39,;1.16,1.77,;1.16,3.31,;-.18,4.08,;-.18,5.62,;-.18,1,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50011875

(2-Bromo-4-(2-chloro-phenyl)-9-methyl-6H-1-thia-5,7...)Show SMILES Cc1nnc2CN=C(c3cc(Br)sc3-n12)c1ccccc1Cl |c:6| Show InChI InChI=1S/C15H10BrClN4S/c1-8-19-20-13-7-18-14(9-4-2-3-5-11(9)17)10-6-12(16)22-15(10)21(8)13/h2-6H,7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 80.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM81929
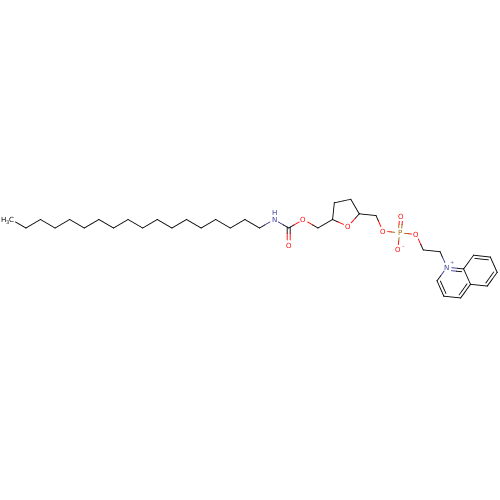
(CAS_108044 | NSC_108044 | SRI 63-441)Show SMILES CCCCCCCCCCCCCCCCCCNC(=O)OCC1CCC(COP([O-])(=O)OCC[n+]2cccc3ccccc23)O1 Show InChI InChI=1S/C36H59N2O7P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-19-26-37-36(39)42-30-33-24-25-34(45-33)31-44-46(40,41)43-29-28-38-27-20-22-32-21-17-18-23-35(32)38/h17-18,20-23,27,33-34H,2-16,19,24-26,28-31H2,1H3,(H-,37,39,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM81929

(CAS_108044 | NSC_108044 | SRI 63-441)Show SMILES CCCCCCCCCCCCCCCCCCNC(=O)OCC1CCC(COP([O-])(=O)OCC[n+]2cccc3ccccc23)O1 Show InChI InChI=1S/C36H59N2O7P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-19-26-37-36(39)42-30-33-24-25-34(45-33)31-44-46(40,41)43-29-28-38-27-20-22-32-21-17-18-23-35(32)38/h17-18,20-23,27,33-34H,2-16,19,24-26,28-31H2,1H3,(H-,37,39,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467457
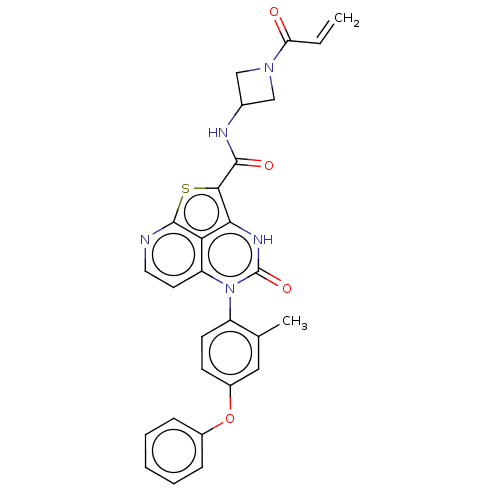
(N-(1-Acryloylazetidin-3-yl)-5-(2-methyl-4-phenoxyp...)Show SMILES Cc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)NC4CN(C4)C(=O)C=C)c([nH]c1=O)c23 |(-1.18,3.85,;-2.51,3.08,;-3.85,3.85,;-5.18,3.08,;-6.52,3.85,;-7.85,3.08,;-9.18,3.85,;-10.52,3.08,;-10.52,1.54,;-9.18,.77,;-7.85,1.54,;-5.18,1.54,;-3.85,.77,;-2.51,1.54,;-1.18,.77,;-1.18,-.77,;-2.51,-1.54,;-2.51,-3.08,;-1.18,-3.85,;.15,-3.08,;1.62,-3.56,;2.18,-2.24,;3.72,-2.24,;4.49,-3.57,;4.49,-.9,;6.03,-.9,;7.12,-1.99,;8.21,-.9,;7.12,.18,;9.75,-.9,;10.52,.43,;10.52,-2.24,;9.75,-3.57,;1.49,-.77,;1.49,.77,;.15,1.54,;.15,3.08,;.15,-1.54,)| Show InChI InChI=1S/C28H23N5O4S/c1-3-22(34)32-14-17(15-32)30-26(35)25-24-23-21(11-12-29-27(23)38-25)33(28(36)31-24)20-10-9-19(13-16(20)2)37-18-7-5-4-6-8-18/h3-13,17H,1,14-15H2,2H3,(H,30,35)(H,31,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468007
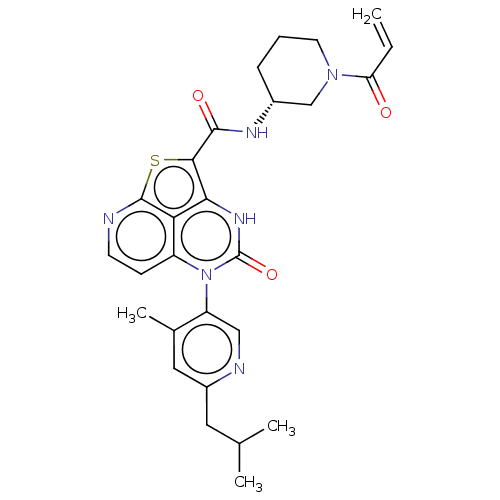
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(6-isobuty...)Show SMILES CC(C)Cc1cc(C)c(cn1)-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:22.22,(-9.16,1.88,;-9.16,3.42,;-10.5,4.19,;-7.83,4.19,;-6.5,3.42,;-6.5,1.88,;-5.16,1.11,;-5.16,-.49,;-3.83,1.88,;-3.83,3.42,;-5.16,4.19,;-2.5,1.11,;-2.5,-.43,;-3.83,-1.2,;-3.83,-2.74,;-2.5,-3.51,;-1.16,-2.74,;.32,-3.14,;1.26,-1.52,;2.8,-1.52,;3.57,-2.85,;3.57,-.19,;5.11,-.19,;5.88,1.15,;7.42,1.15,;8.19,-.19,;7.42,-1.52,;5.88,-1.52,;8.19,-2.85,;7.42,-4.19,;9.73,-2.85,;10.5,-1.52,;.17,-.43,;.17,1.11,;-1.16,1.88,;-1.16,3.42,;-1.16,-1.2,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467435
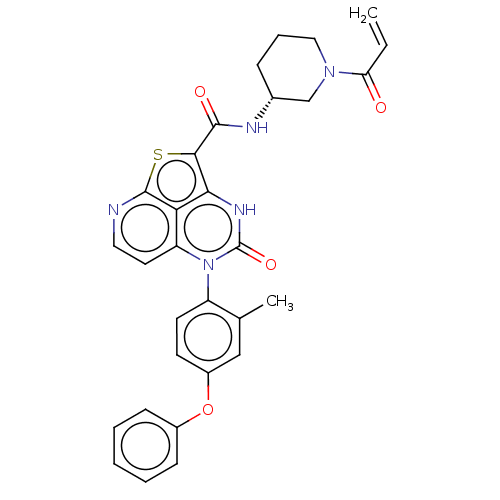
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*R)-(2-methyl-...)Show SMILES Cc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:25.26,(-1.68,4.44,;-3.01,3.67,;-4.34,4.44,;-5.68,3.67,;-7.01,4.44,;-8.34,3.67,;-9.68,4.44,;-11.01,3.67,;-11.01,2.13,;-9.68,1.36,;-8.34,2.13,;-5.68,2.13,;-4.34,1.36,;-3.01,2.13,;-1.68,1.36,;-1.68,-.18,;-3.01,-.95,;-3.01,-2.49,;-1.68,-3.26,;-.34,-2.49,;.99,-3.26,;1.76,-1.75,;3.3,-1.76,;4.06,-3.1,;4.08,-.44,;5.62,-.44,;6.39,.9,;7.93,.9,;8.7,-.44,;7.93,-1.77,;6.39,-1.77,;8.7,-3.1,;7.93,-4.44,;10.24,-3.1,;11.01,-1.77,;.99,-.18,;.99,1.36,;-.34,2.13,;-.34,3.67,;-.34,-.95,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467435

((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*R)-(2-methyl-...)Show SMILES Cc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:25.26,(-1.68,4.44,;-3.01,3.67,;-4.34,4.44,;-5.68,3.67,;-7.01,4.44,;-8.34,3.67,;-9.68,4.44,;-11.01,3.67,;-11.01,2.13,;-9.68,1.36,;-8.34,2.13,;-5.68,2.13,;-4.34,1.36,;-3.01,2.13,;-1.68,1.36,;-1.68,-.18,;-3.01,-.95,;-3.01,-2.49,;-1.68,-3.26,;-.34,-2.49,;.99,-3.26,;1.76,-1.75,;3.3,-1.76,;4.06,-3.1,;4.08,-.44,;5.62,-.44,;6.39,.9,;7.93,.9,;8.7,-.44,;7.93,-1.77,;6.39,-1.77,;8.7,-3.1,;7.93,-4.44,;10.24,-3.1,;11.01,-1.77,;.99,-.18,;.99,1.36,;-.34,2.13,;-.34,3.67,;-.34,-.95,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468124
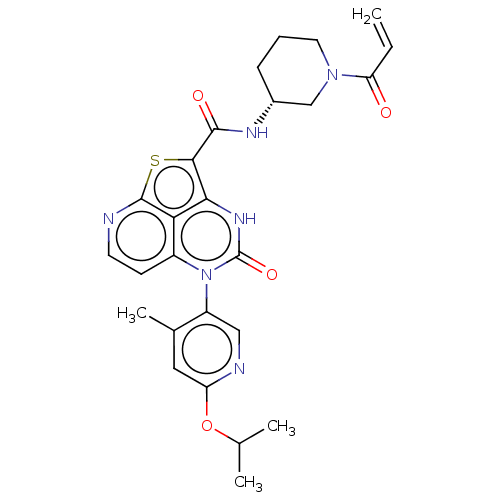
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(6-isoprop...)Show SMILES CC(C)Oc1cc(C)c(cn1)-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:22.22,(-10.22,5.69,;-8.88,4.92,;-8.88,3.38,;-7.55,5.69,;-6.22,4.92,;-4.88,5.69,;-3.55,4.92,;-2.22,5.69,;-3.55,3.38,;-4.88,2.61,;-6.22,3.38,;-2.22,2.61,;-2.22,1.07,;-3.55,.3,;-3.55,-1.24,;-2.22,-2.01,;-.88,-1.24,;.49,-1.84,;1.22,-.35,;2.76,-.35,;3.53,.98,;3.53,-1.69,;4.83,-1.69,;5.6,-.35,;7.14,-.35,;7.91,-1.69,;7.14,-3.02,;5.6,-3.02,;7.91,-4.35,;7.14,-5.69,;9.45,-4.35,;10.22,-5.69,;.45,1.07,;.45,2.61,;-.88,3.38,;-.88,4.92,;-.88,.3,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002831
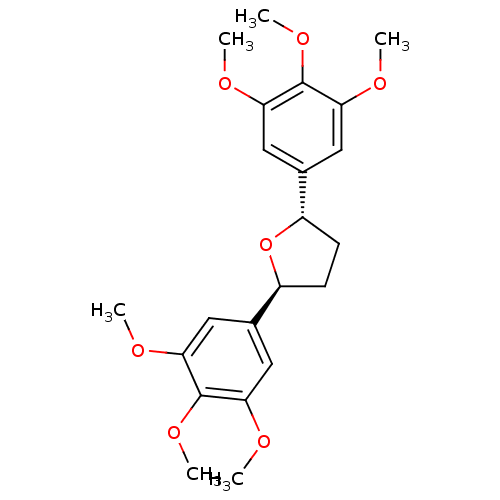
((2R,5R)-2,5-Bis-(3,4,5-trimethoxy-phenyl)-tetrahyd...)Show SMILES COc1cc(cc(OC)c1OC)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C22H28O7/c1-23-17-9-13(10-18(24-2)21(17)27-5)15-7-8-16(29-15)14-11-19(25-3)22(28-6)20(12-14)26-4/h9-12,15-16H,7-8H2,1-6H3/t15-,16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467857

((S)-N-(1-Acryloylpyrrolidin-3-yl)-5-(*S)-(2-methyl...)Show SMILES Cc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@H]4CCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wU:25.26,(-2.22,5.29,;-3.55,4.52,;-4.89,5.29,;-6.22,4.52,;-7.56,5.29,;-8.89,4.52,;-10.22,5.29,;-11.56,4.52,;-11.56,2.98,;-10.22,2.21,;-8.89,2.98,;-6.22,2.98,;-4.89,2.21,;-3.55,2.98,;-2.22,2.21,;-2.22,.67,;-3.55,-.1,;-3.55,-1.64,;-2.22,-2.41,;-.89,-1.64,;.6,-2.04,;1.44,-.74,;2.98,-.74,;3.75,.59,;3.75,-2.08,;5.29,-2.08,;6.19,-.83,;7.66,-1.31,;7.66,-2.85,;6.19,-3.32,;8.9,-3.75,;8.9,-5.29,;10.31,-3.13,;11.56,-4.03,;.45,.67,;.45,2.21,;-.89,2.98,;-.89,4.52,;-.89,-.1,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 217 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50001747
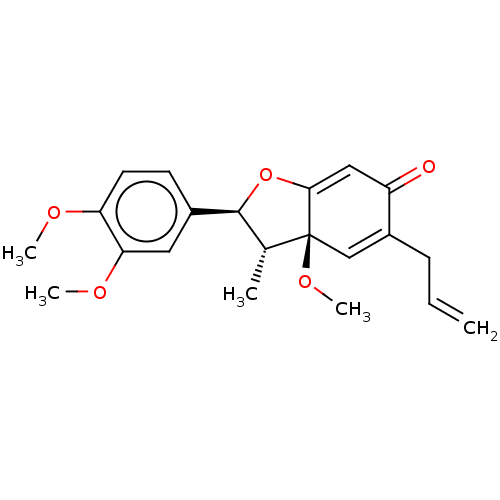
((+)-Kadsurenone | (Kadsurenone)5-Allyl-2-(3,4-dime...)Show SMILES COc1ccc(cc1OC)[C@H]1OC2=CC(=O)C(CC=C)=C[C@]2(OC)[C@@H]1C |c:20,t:13| Show InChI InChI=1S/C21H24O5/c1-6-7-15-12-21(25-5)13(2)20(26-19(21)11-16(15)22)14-8-9-17(23-3)18(10-14)24-4/h6,8-13,20H,1,7H2,2-5H3/t13-,20+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50001747

((+)-Kadsurenone | (Kadsurenone)5-Allyl-2-(3,4-dime...)Show SMILES COc1ccc(cc1OC)[C@H]1OC2=CC(=O)C(CC=C)=C[C@]2(OC)[C@@H]1C |c:20,t:13| Show InChI InChI=1S/C21H24O5/c1-6-7-15-12-21(25-5)13(2)20(26-19(21)11-16(15)22)14-8-9-17(23-3)18(10-14)24-4/h6,8-13,20H,1,7H2,2-5H3/t13-,20+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50002831

((2R,5R)-2,5-Bis-(3,4,5-trimethoxy-phenyl)-tetrahyd...)Show SMILES COc1cc(cc(OC)c1OC)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C22H28O7/c1-23-17-9-13(10-18(24-2)21(17)27-5)15-7-8-16(29-15)14-11-19(25-3)22(28-6)20(12-14)26-4/h9-12,15-16H,7-8H2,1-6H3/t15-,16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 268 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467759
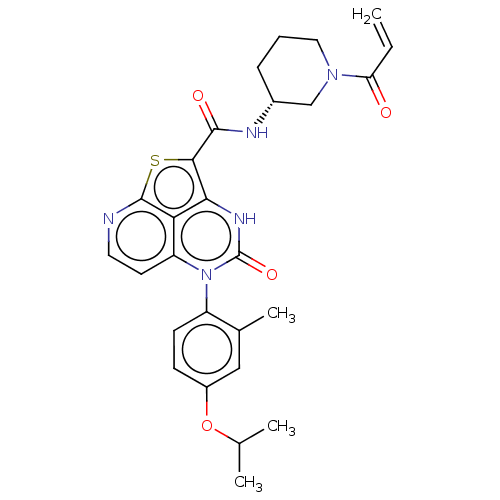
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(4-isoprop...)Show SMILES CC(C)Oc1ccc(c(C)c1)-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wU:22.22,(-10.5,3.85,;-9.17,3.08,;-9.17,1.54,;-7.83,3.85,;-6.5,3.08,;-5.16,3.85,;-3.83,3.08,;-3.83,1.54,;-5.16,.77,;-5.16,-.77,;-6.5,1.54,;-2.5,.77,;-2.5,-.77,;-3.83,-1.54,;-3.83,-3.08,;-2.5,-3.85,;-1.16,-3.08,;.36,-3.43,;1.26,-1.97,;2.8,-1.97,;3.57,-3.3,;3.57,-.63,;5.11,-.63,;5.88,-1.97,;7.42,-1.97,;8.19,-.63,;7.42,.7,;5.88,.7,;8.19,2.03,;7.42,3.37,;9.73,2.03,;10.5,3.37,;.17,-.77,;.17,.77,;-1.16,1.54,;-1.16,3.08,;-1.16,-1.54,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 337 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00044
BindingDB Entry DOI: 10.7270/Q25D8WM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467845

((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-(6-phenox...)Show SMILES C=CC(=O)N1CCC[C@H](C1)NC(=O)c1sc2nccc3n(-c4ccc(Oc5ccccc5)nc4)c(=O)[nH]c1c23 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 354 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467852

((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-(5-phenox...)Show SMILES C=CC(=O)N1CCC[C@H](C1)NC(=O)c1sc2nccc3n(-c4ccc(Oc5ccccc5)cn4)c(=O)[nH]c1c23 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50006048
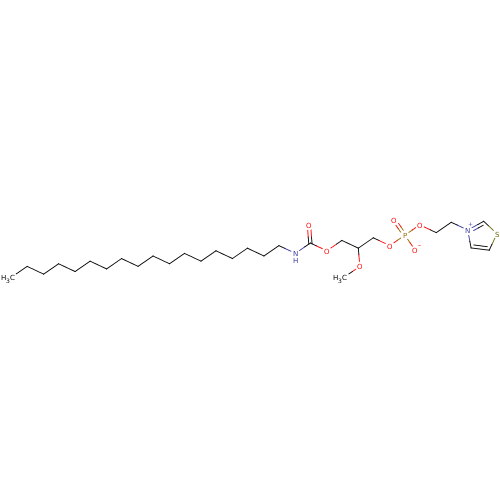
(2-methoxy-3-(octadecylcarbamoyloxy)propyl 2-(thiaz...)Show SMILES CCCCCCCCCCCCCCCCCCNC(=O)OCC(COP([O-])(=O)OCC[n+]1ccsc1)OC Show InChI InChI=1S/C28H53N2O7PS/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-29-28(31)35-24-27(34-2)25-37-38(32,33)36-22-20-30-21-23-39-26-30/h21,23,26-27H,3-20,22,24-25H2,1-2H3,(H-,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Fructose-bisphosphate aldolase class 2
(Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531933
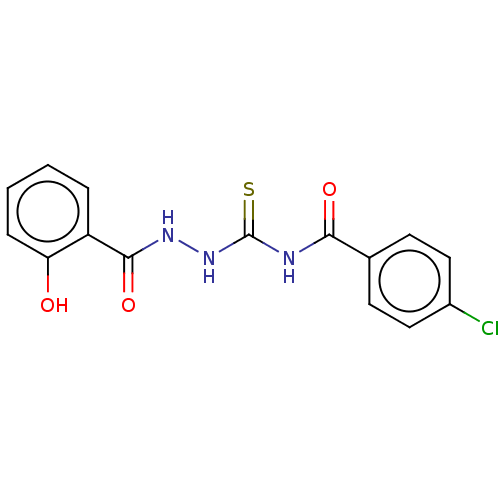
(CHEMBL4448356)Show InChI InChI=1S/C15H12ClN3O3S/c16-10-7-5-9(6-8-10)13(21)17-15(23)19-18-14(22)11-3-1-2-4-12(11)20/h1-8,20H,(H,18,22)(H2,17,19,21,23) | KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins |
Bioorg Med Chem 27: 805-812 (2019)
Article DOI: 10.1016/j.bmc.2019.01.023
BindingDB Entry DOI: 10.7270/Q20P13HV |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50070319
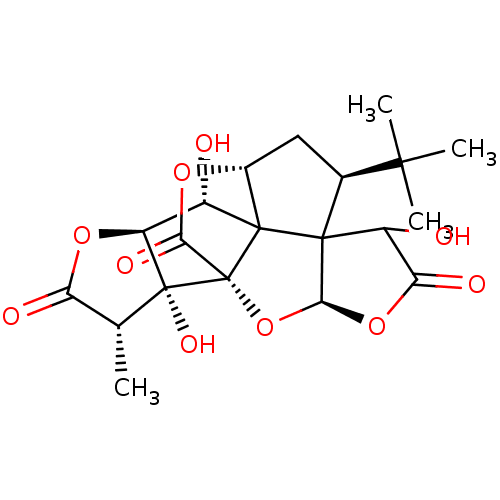
(8-(tert-butyl)-6,12,17-trihydroxy-16-methyl-2,4,14...)Show SMILES C[C@@H]1C(=O)O[C@H]2[C@H](O)C34[C@H]5C[C@@H](C(C)(C)C)C33C(O)C(=O)O[C@H]3O[C@@]4(C(=O)O5)[C@@]12O Show InChI InChI=1S/C20H24O10/c1-6-12(23)28-11-9(21)18-8-5-7(16(2,3)4)17(18)10(22)13(24)29-15(17)30-20(18,14(25)27-8)19(6,11)26/h6-11,15,21-22,26H,5H2,1-4H3/t6-,7+,8-,9+,10?,11+,15+,17?,18?,19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 643 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Fructose-bisphosphate aldolase class 2
(Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531928
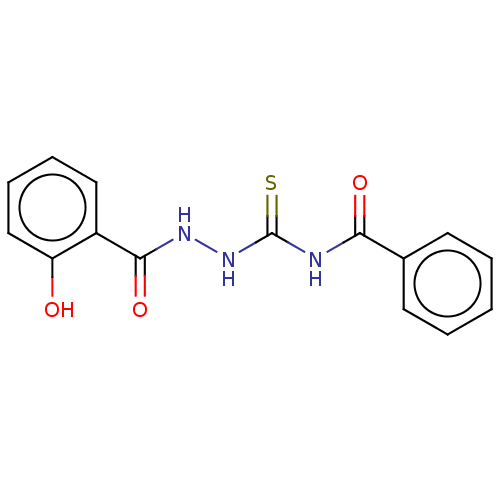
(CHEMBL1551854)Show InChI InChI=1S/C15H13N3O3S/c19-12-9-5-4-8-11(12)14(21)17-18-15(22)16-13(20)10-6-2-1-3-7-10/h1-9,19H,(H,17,21)(H2,16,18,20,22) | KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins |
Bioorg Med Chem 27: 805-812 (2019)
Article DOI: 10.1016/j.bmc.2019.01.023
BindingDB Entry DOI: 10.7270/Q20P13HV |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50006048

(2-methoxy-3-(octadecylcarbamoyloxy)propyl 2-(thiaz...)Show SMILES CCCCCCCCCCCCCCCCCCNC(=O)OCC(COP([O-])(=O)OCC[n+]1ccsc1)OC Show InChI InChI=1S/C28H53N2O7PS/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-29-28(31)35-24-27(34-2)25-37-38(32,33)36-22-20-30-21-23-39-26-30/h21,23,26-27H,3-20,22,24-25H2,1-2H3,(H-,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 686 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50001765

(8-chloro-6-(2-chlorophenyl)-1-methyl-4H-[1,2,4]tri...)Show SMILES Cc1nnc2CN=C(c3ccccc3Cl)c3cc(Cl)ccc3-n12 |t:6| Show InChI InChI=1S/C17H12Cl2N4/c1-10-21-22-16-9-20-17(12-4-2-3-5-14(12)19)13-8-11(18)6-7-15(13)23(10)16/h2-8H,9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 699 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50038766

(3-Pyridin-3-yl-1H-pyrrolo[1,2-c]thiazole-7-carboxy...)Show SMILES O=C(Nc1cccc(c1)C(=O)c1ccccc1)c1ccn2C(SCc12)c1cccnc1 Show InChI InChI=1S/C25H19N3O2S/c29-23(17-6-2-1-3-7-17)18-8-4-10-20(14-18)27-24(30)21-11-13-28-22(21)16-31-25(28)19-9-5-12-26-15-19/h1-15,25H,16H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 869 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Fructose-bisphosphate aldolase class 2
(Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM75654
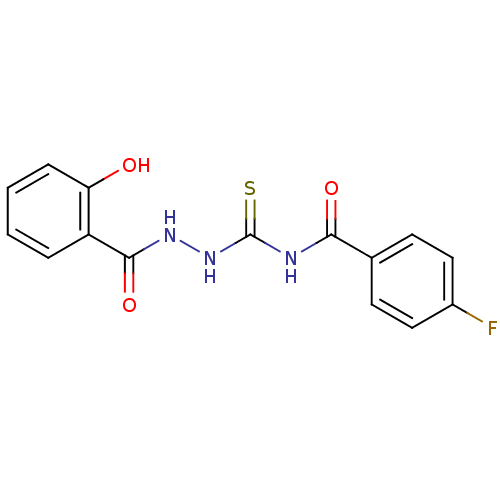
(4-Fluoro-N-[N'-(2-hydroxy-benzoyl)-hydrazinoca...)Show InChI InChI=1S/C15H12FN3O3S/c16-10-7-5-9(6-8-10)13(21)17-15(23)19-18-14(22)11-3-1-2-4-12(11)20/h1-8,20H,(H,18,22)(H2,17,19,21,23) | KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5 mins |
Bioorg Med Chem 27: 805-812 (2019)
Article DOI: 10.1016/j.bmc.2019.01.023
BindingDB Entry DOI: 10.7270/Q20P13HV |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50070319

(8-(tert-butyl)-6,12,17-trihydroxy-16-methyl-2,4,14...)Show SMILES C[C@@H]1C(=O)O[C@H]2[C@H](O)C34[C@H]5C[C@@H](C(C)(C)C)C33C(O)C(=O)O[C@H]3O[C@@]4(C(=O)O5)[C@@]12O Show InChI InChI=1S/C20H24O10/c1-6-12(23)28-11-9(21)18-8-5-7(16(2,3)4)17(18)10(22)13(24)29-15(17)30-20(18,14(25)27-8)19(6,11)26/h6-11,15,21-22,26H,5H2,1-4H3/t6-,7+,8-,9+,10?,11+,15+,17?,18?,19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 964 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Sante
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 252: 1221-7 (1990)
BindingDB Entry DOI: 10.7270/Q2JW8CC2 |
More data for this
Ligand-Target Pair | |
Fructose-bisphosphate aldolase class 2
(Synechocystis sp. (strain PCC 6803 / Kazusa)) | BDBM50531928

(CHEMBL1551854)Show InChI InChI=1S/C15H13N3O3S/c19-12-9-5-4-8-11(12)14(21)17-18-15(22)16-13(20)10-6-2-1-3-7-10/h1-9,19H,(H,17,21)(H2,16,18,20,22) | KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Cooperation Base of Pesticide and Green Synthesis (Hubei)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Synechocystis sp. PCC 6803 FBA2 S55A mutant expressed in Escherichia coli BL21 (DE3) using FBP as substrate measured over 5... |
Bioorg Med Chem 27: 805-812 (2019)
Article DOI: 10.1016/j.bmc.2019.01.023
BindingDB Entry DOI: 10.7270/Q20P13HV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data