

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427363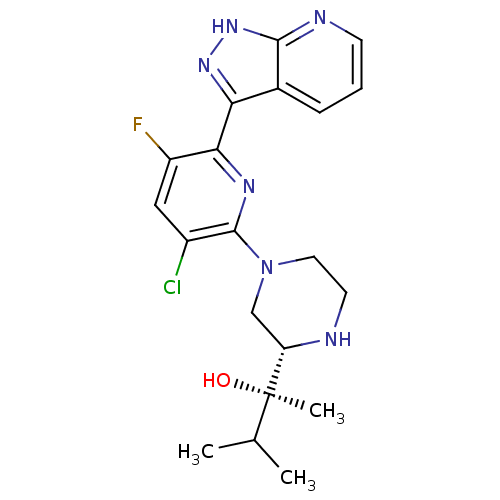 (CHEMBL2326002) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427364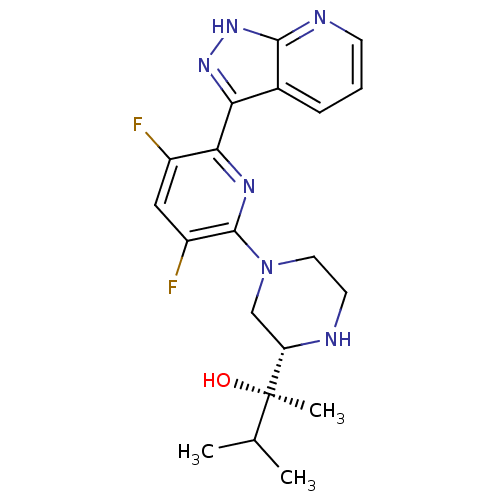 (CHEMBL2326001) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427365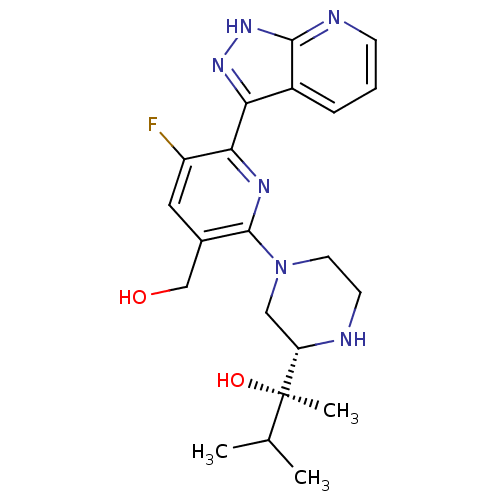 (CHEMBL2326000) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427367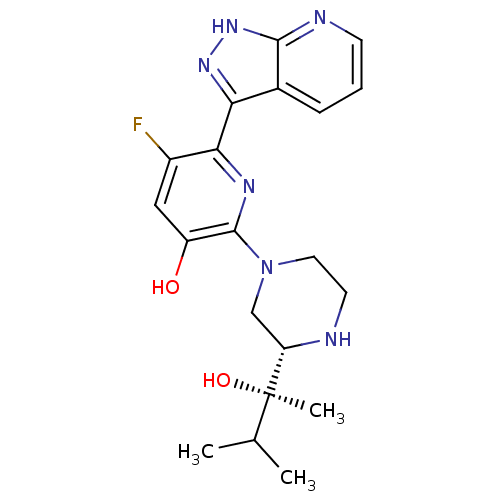 (CHEMBL2325998) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427370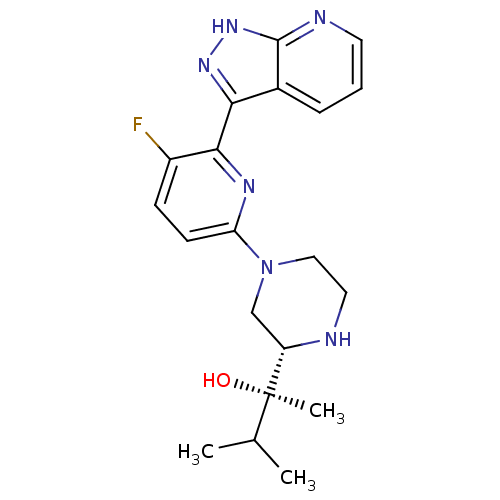 (CHEMBL2326007) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19854 (CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525600 (CHEMBL4469412) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410611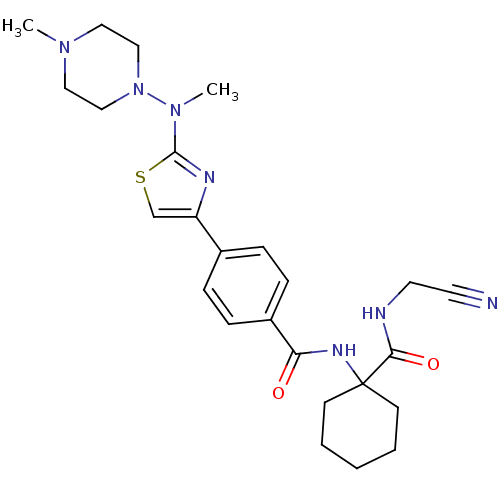 (CHEMBL414669) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410588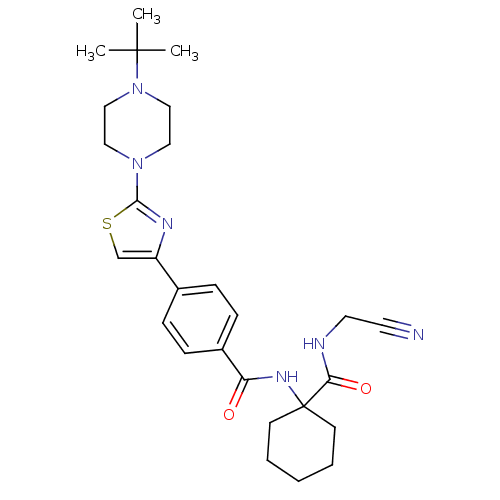 (CHEMBL200708) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525603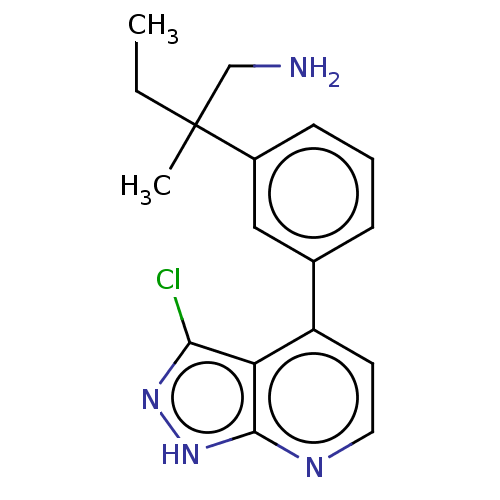 (CHEMBL4528271) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525602 (CHEMBL4569479) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410609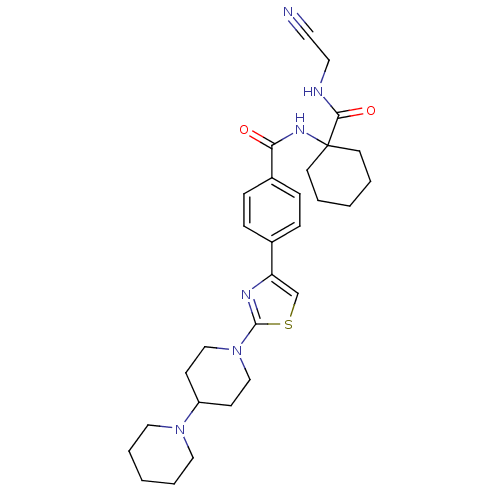 (CHEMBL198798) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410590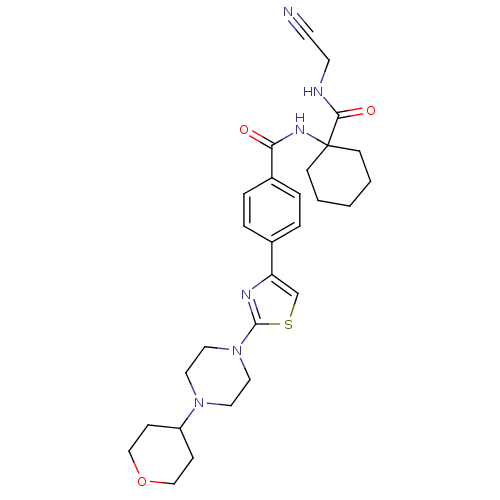 (CHEMBL200543) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410587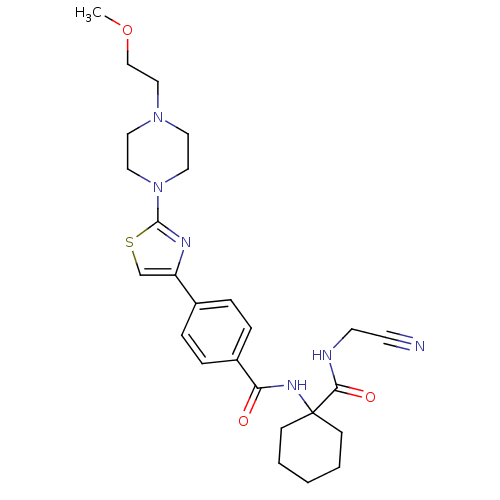 (CHEMBL200602) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19854 (CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50064172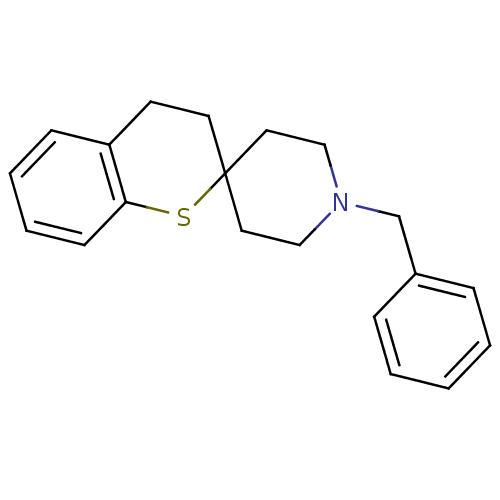 (1-benzylspiro[hexahydropyridine-4,2'-(3',4'-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards sigma 1 receptor using [3H]-(+)-pentazocine from guinea pig brain | J Med Chem 41: 1557-60 (1998) Article DOI: 10.1021/jm970740r BindingDB Entry DOI: 10.7270/Q2N29W3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410607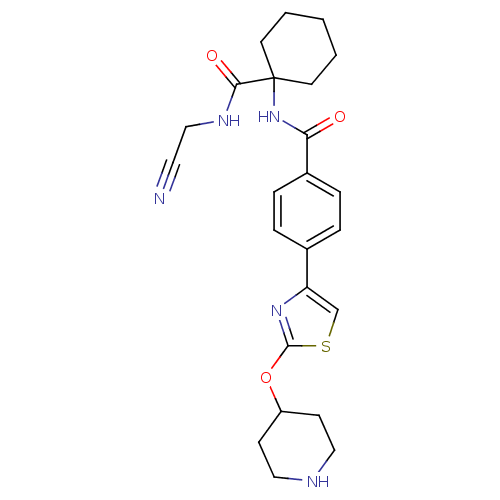 (CHEMBL200744) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410571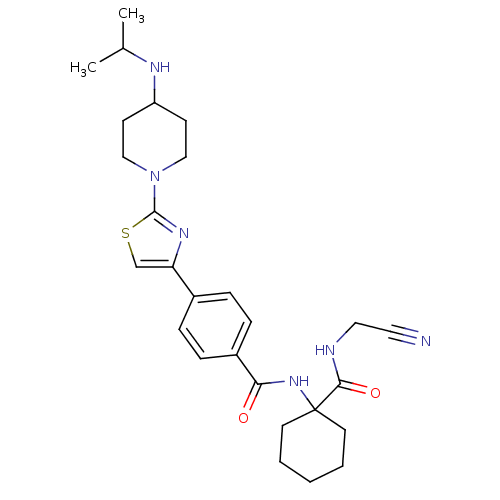 (CHEMBL200287) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50387328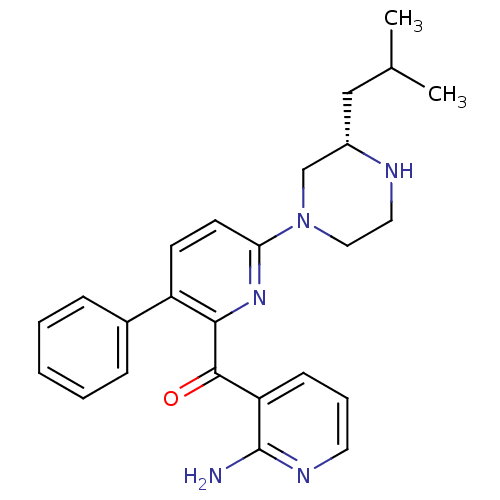 (CHEMBL2046649) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry at Vertex Pharmaceuticals (Europe) Ltd Curated by ChEMBL | Assay Description Inhibition of PKCtheta | Bioorg Med Chem Lett 22: 4645-9 (2012) Article DOI: 10.1016/j.bmcl.2012.05.114 BindingDB Entry DOI: 10.7270/Q2TM7C65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50064171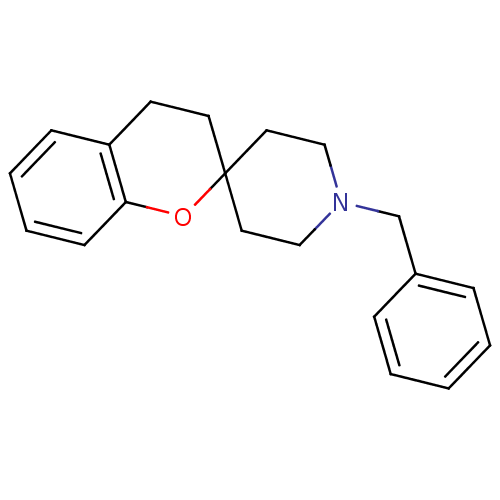 (1'-benzylspiro[3,4-dihydro-2H-chromene-2,4'-(hexah...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards sigma 1 receptor using [3H]-(+)-pentazocine from guinea pig brain | J Med Chem 41: 1557-60 (1998) Article DOI: 10.1021/jm970740r BindingDB Entry DOI: 10.7270/Q2N29W3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410580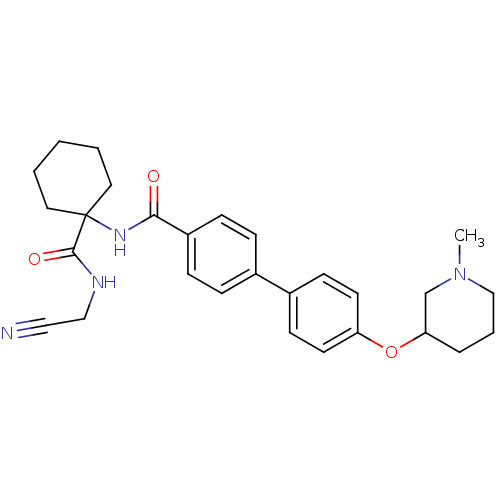 (CHEMBL435913) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410575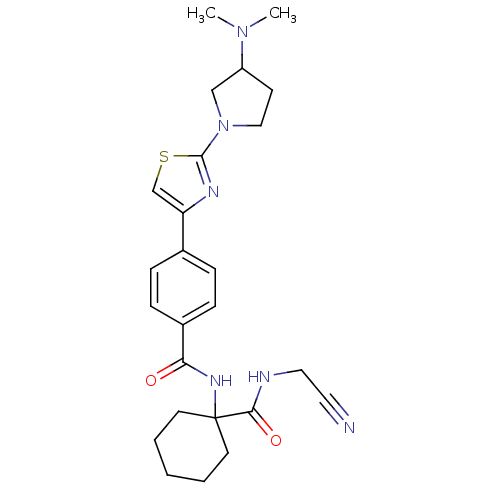 (CHEMBL199470) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50142480 ((E)-7-[(1R,2S,3R)-3-Hydroxy-2-((E)-(S)-3-hydroxy-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined against prostanoid EP4 receptor | Bioorg Med Chem Lett 14: 1655-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.063 BindingDB Entry DOI: 10.7270/Q2MK6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50188295 ((R,S)-4-methyl-2-[3'-(2-piperazin-1-yl-thiazol-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Bioorg Med Chem Lett 16: 4296-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.061 BindingDB Entry DOI: 10.7270/Q2KW5FNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410591 (CHEMBL200506) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410612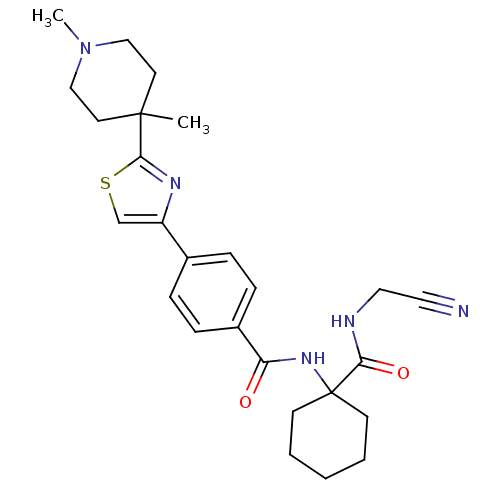 (CHEMBL200166) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50427363 (CHEMBL2326002) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of PKC epsilon (unknown origin) | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525604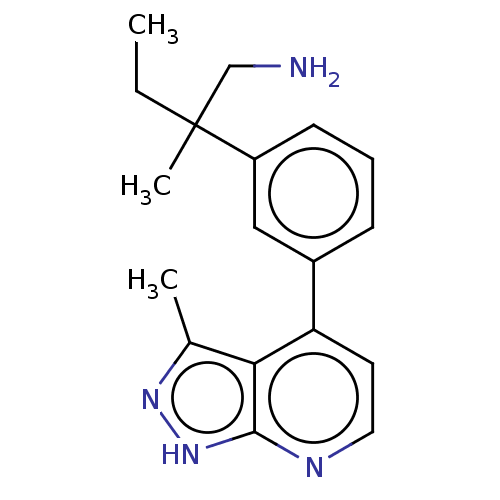 (CHEMBL4532737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427366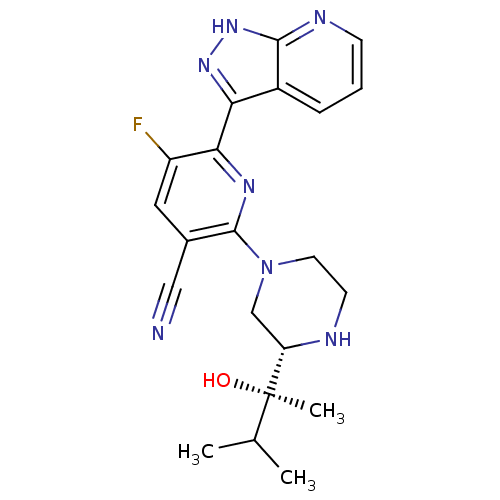 (CHEMBL2325999) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427374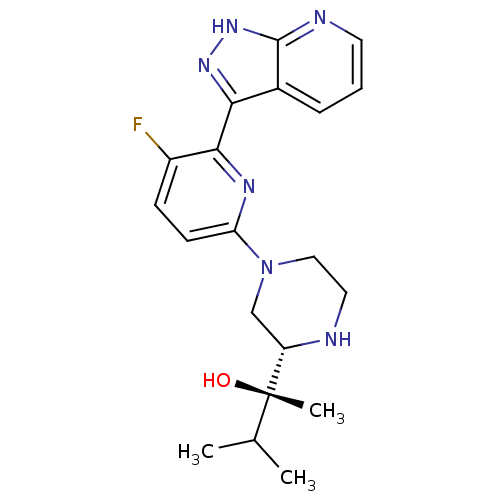 (CHEMBL2326009) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Alpha-1A adrenergic receptor using [3H]-prazosin from rat submaxillary gland | J Med Chem 41: 1557-60 (1998) Article DOI: 10.1021/jm970740r BindingDB Entry DOI: 10.7270/Q2N29W3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50188296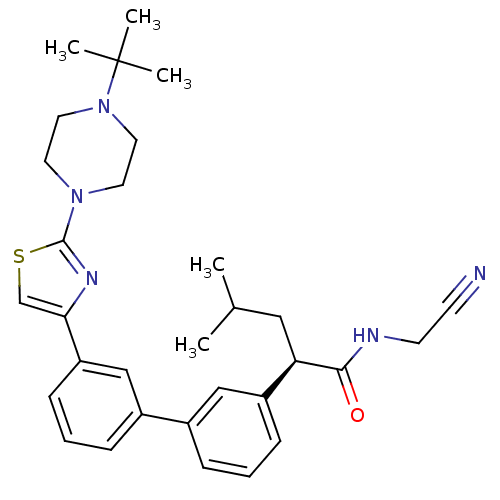 ((R)-2-{3'-[2-(4-tert-butyl-piperazin-1-yl)-thiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Bioorg Med Chem Lett 16: 4296-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.061 BindingDB Entry DOI: 10.7270/Q2KW5FNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50188297 ((R,S)-4-methyl-2-{3'-[2-(4-methyl-piperazin-1-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Bioorg Med Chem Lett 16: 4296-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.061 BindingDB Entry DOI: 10.7270/Q2KW5FNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50036764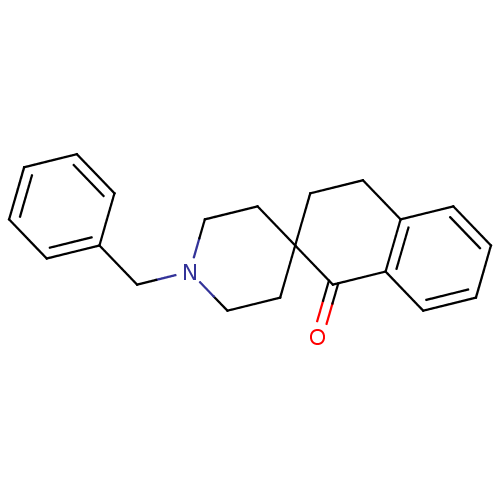 (1'-benzylspiro[1,2,3,4-tetrahydronaphthalene-2,4'-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards sigma 1 receptor using [3H]-(+)-pentazocine from guinea pig brain | J Med Chem 41: 1557-60 (1998) Article DOI: 10.1021/jm970740r BindingDB Entry DOI: 10.7270/Q2N29W3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19855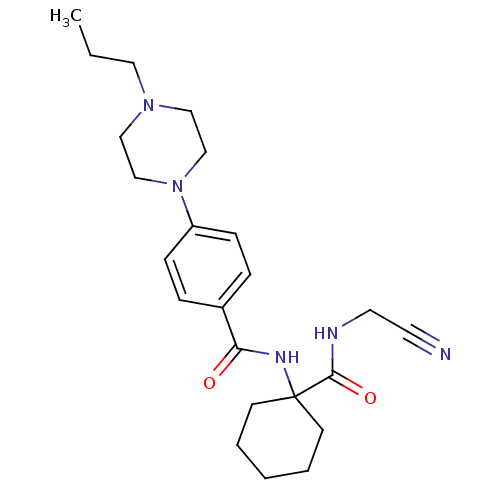 (Balicatib | CHEMBL371064 | N-[1-(cyanomethylcarbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410595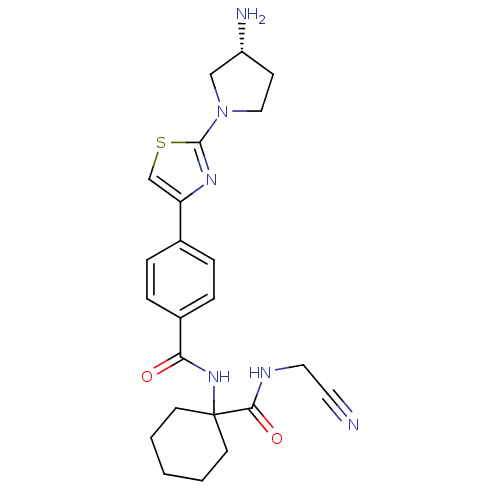 (CHEMBL200507) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410572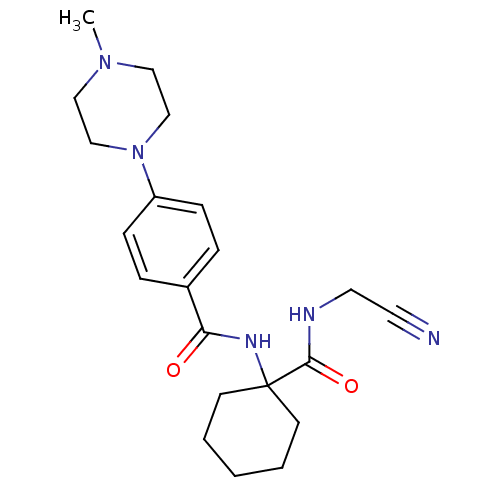 (CHEMBL440035) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410592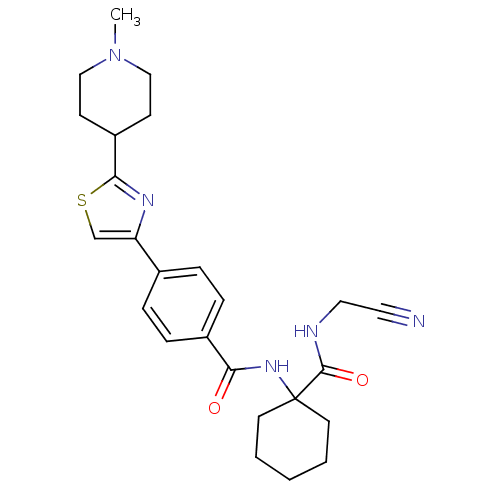 (CHEMBL200455) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410594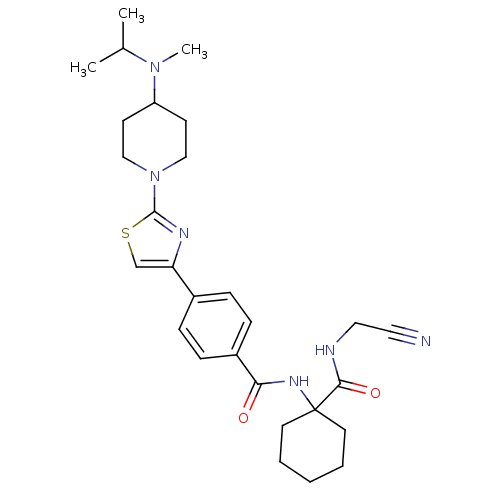 (CHEMBL200596) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50142482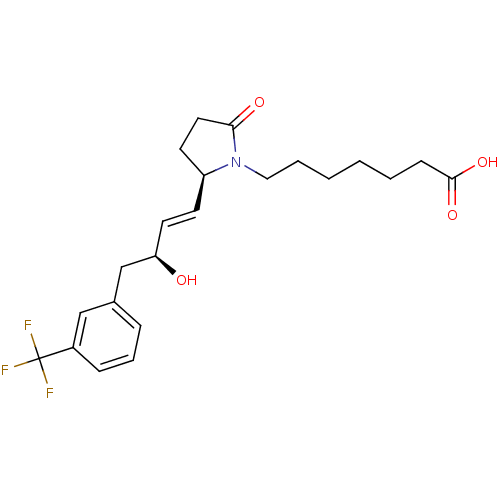 (7-{(R)-2-[(E)-(S)-3-Hydroxy-4-(3-trifluoromethyl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Agonist activity against recombinant prostanoid EP4 receptor stably transfected in CHO cells | Bioorg Med Chem Lett 14: 1655-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.063 BindingDB Entry DOI: 10.7270/Q2MK6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50387349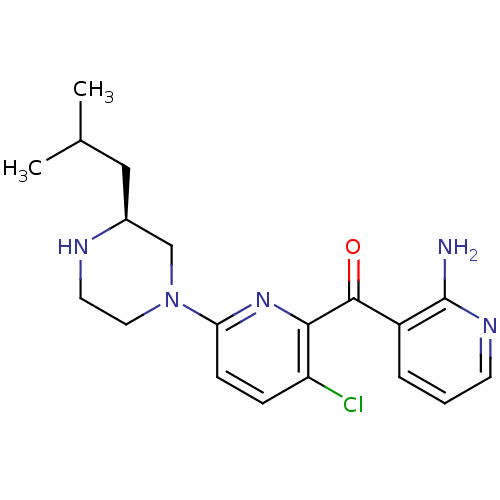 (CHEMBL2046644) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry at Vertex Pharmaceuticals (Europe) Ltd Curated by ChEMBL | Assay Description Inhibition of PKCtheta | Bioorg Med Chem Lett 22: 4645-9 (2012) Article DOI: 10.1016/j.bmcl.2012.05.114 BindingDB Entry DOI: 10.7270/Q2TM7C65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410606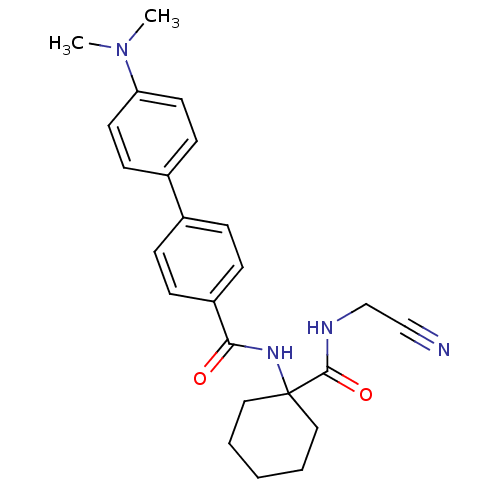 (CHEMBL383186) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525587 (CHEMBL4589844) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427382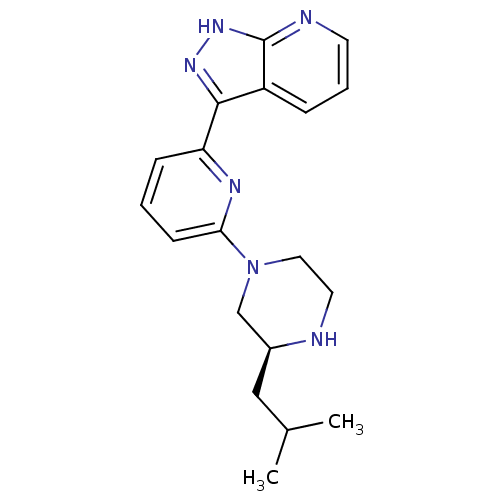 (CHEMBL2326019) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50129070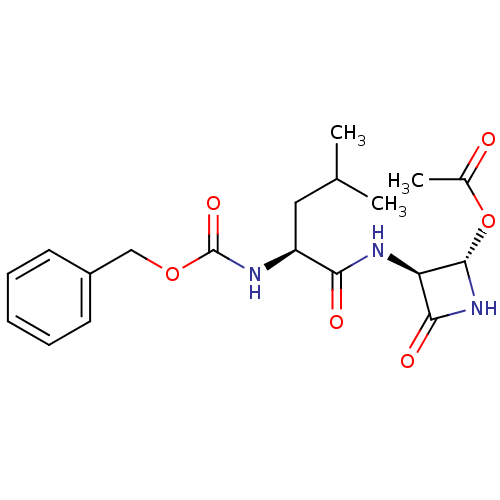 (Acetic acid (2S,3S)-3-((S)-2-benzyloxycarbonylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured against Cathepsin K | Bioorg Med Chem Lett 13: 2051-3 (2003) BindingDB Entry DOI: 10.7270/Q2BG2NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19857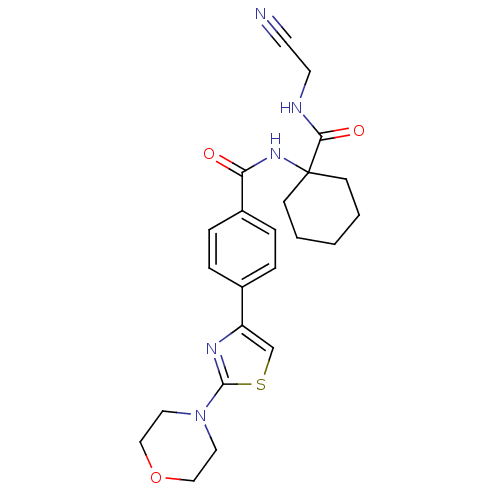 (N-{1-[(cyanomethyl)carbamoyl]cyclohexyl}-4-[2-(mor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Rattus norvegicus (rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Alpha-1B adrenergic receptor using [3H]-prazosin from rat liver | J Med Chem 41: 1557-60 (1998) Article DOI: 10.1021/jm970740r BindingDB Entry DOI: 10.7270/Q2N29W3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50387330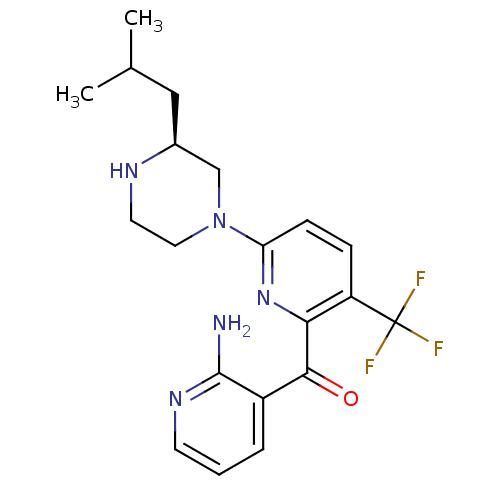 (CHEMBL2046643) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry at Vertex Pharmaceuticals (Europe) Ltd Curated by ChEMBL | Assay Description Inhibition of PKCtheta | Bioorg Med Chem Lett 22: 4645-9 (2012) Article DOI: 10.1016/j.bmcl.2012.05.114 BindingDB Entry DOI: 10.7270/Q2TM7C65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525584 (CHEMBL4453941) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525599 (CHEMBL4461370) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 9919 total ) | Next | Last >> |