 Found 363 hits with Last Name = 'wolfe' and Initial = 'l'
Found 363 hits with Last Name = 'wolfe' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190788
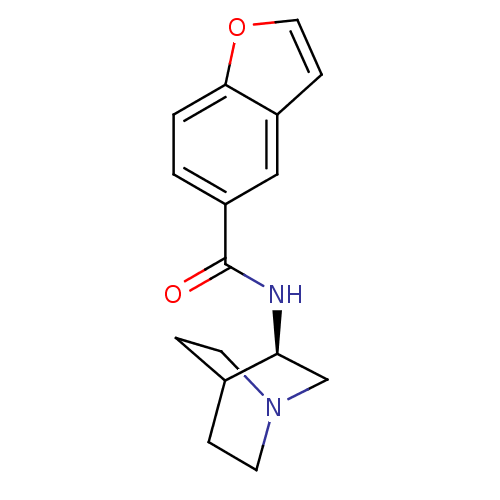
(CHEMBL378471 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2occc2c1 |wU:3.2,(-3.91,-15.15,;-3.91,-16.69,;-2.57,-17.45,;-1.24,-16.67,;-1.25,-15.14,;.09,-14.37,;1.43,-15.13,;1.43,-16.67,;.1,-17.45,;-.78,-16.3,;.04,-15.5,;-5.24,-17.46,;-5.23,-19.02,;-6.57,-19.79,;-7.91,-19.02,;-9.37,-19.49,;-10.28,-18.24,;-9.37,-17,;-7.9,-17.48,;-6.58,-16.7,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190785
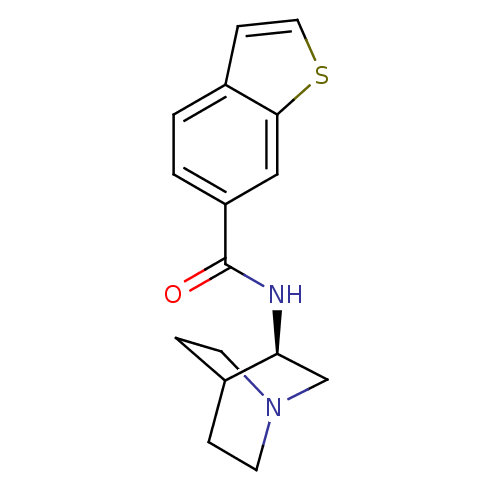
(CHEMBL378349 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccsc2c1 |wU:3.2,(28.02,-3.67,;28.02,-5.21,;29.36,-5.97,;30.69,-5.19,;30.68,-3.66,;32.02,-2.89,;33.36,-3.65,;33.36,-5.2,;32.03,-5.97,;31.15,-4.82,;31.97,-4.02,;26.69,-5.98,;26.7,-7.54,;25.35,-8.31,;24.02,-7.54,;22.56,-8.01,;21.65,-6.76,;22.56,-5.52,;24.03,-6,;25.35,-5.22,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50377050

(CHEMBL403858 | PH-709829)Show SMILES O=C(N[C@@H]1C[C@H]2CCN(C2)C1)c1cc2ccoc2cn1 |TLB:2:3:9:7.6| Show InChI InChI=1S/C15H17N3O2/c19-15(13-6-11-2-4-20-14(11)7-16-13)17-12-5-10-1-3-18(8-10)9-12/h2,4,6-7,10,12H,1,3,5,8-9H2,(H,17,19)/t10-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha-7 nACh receptor in rat brain |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190793
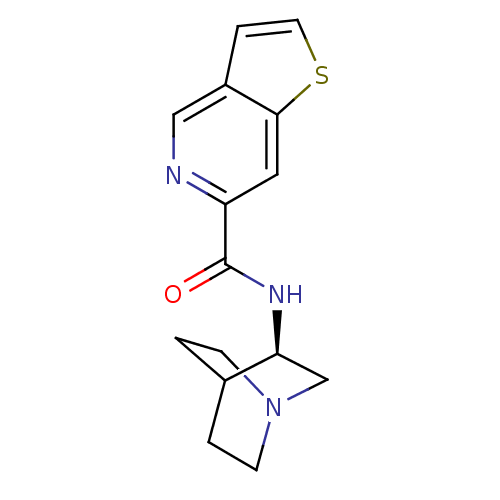
(CHEMBL268939 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2sccc2cn1 |wU:3.2,(26.77,-30.5,;26.77,-32.04,;28.11,-32.81,;29.44,-32.03,;29.43,-30.5,;30.77,-29.72,;32.11,-30.49,;32.11,-32.03,;30.78,-32.8,;29.9,-31.66,;30.72,-30.86,;25.44,-32.82,;24.1,-32.06,;22.78,-32.83,;21.31,-32.36,;20.4,-33.6,;21.31,-34.85,;22.77,-34.37,;24.1,-35.14,;25.45,-34.37,)| Show InChI InChI=1S/C15H17N3OS/c19-15(12-7-14-11(8-16-12)3-6-20-14)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50377049
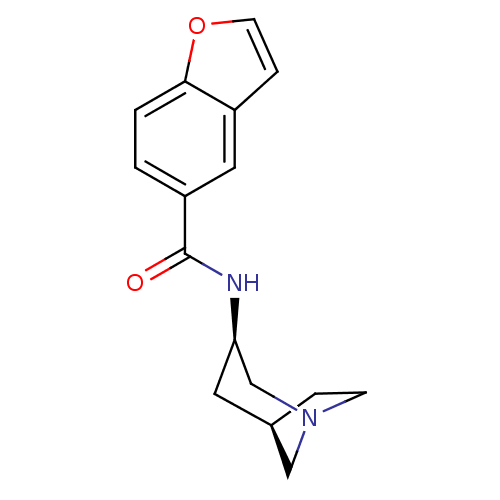
(CHEMBL258031)Show SMILES O=C(N[C@@H]1C[C@H]2CCN(C2)C1)c1ccc2occc2c1 |TLB:2:3:9:7.6| Show InChI InChI=1S/C16H18N2O2/c19-16(13-1-2-15-12(8-13)4-6-20-15)17-14-7-11-3-5-18(9-11)10-14/h1-2,4,6,8,11,14H,3,5,7,9-10H2,(H,17,19)/t11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha-7 nACh receptor in rat brain |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190791
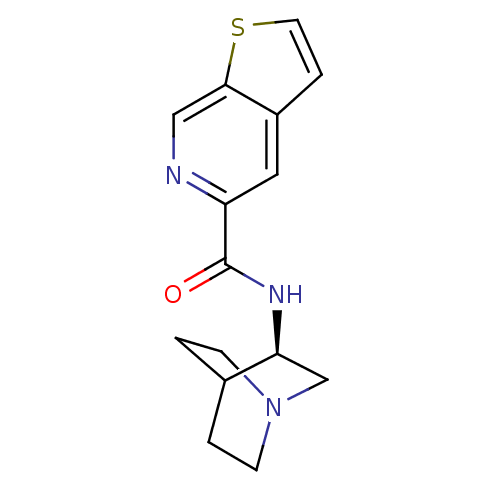
(CHEMBL210865 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccsc2cn1 |wU:3.2,(11.37,-32.25,;11.38,-33.79,;12.72,-34.55,;14.05,-33.78,;14.04,-32.24,;15.38,-31.47,;16.72,-32.24,;16.71,-33.78,;15.39,-34.55,;14.51,-33.4,;15.33,-32.61,;10.05,-34.57,;8.71,-33.8,;7.38,-34.58,;5.92,-34.1,;5.01,-35.35,;5.92,-36.59,;7.38,-36.12,;8.71,-36.89,;10.06,-36.12,)| Show InChI InChI=1S/C15H17N3OS/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190789
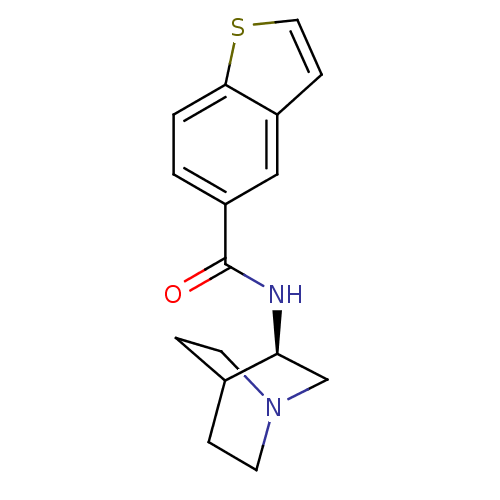
(CHEMBL208565 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2sccc2c1 |wU:3.2,(11.98,-5.28,;11.99,-6.82,;13.33,-7.58,;14.66,-6.8,;14.65,-5.27,;15.99,-4.5,;17.32,-5.26,;17.32,-6.8,;16,-7.58,;15.12,-6.43,;15.94,-5.63,;10.66,-7.59,;10.66,-9.15,;9.32,-9.92,;7.99,-9.15,;6.52,-9.62,;5.62,-8.37,;6.53,-7.13,;7.99,-7.61,;9.32,-6.83,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50377048

(CHEMBL403857)Show SMILES O=C(N[C@@H]1C[C@H]2CCN(C2)C1)c1cc2sccc2cn1 |TLB:2:3:9:7.6| Show InChI InChI=1S/C15H17N3OS/c19-15(13-6-14-11(7-16-13)2-4-20-14)17-12-5-10-1-3-18(8-10)9-12/h2,4,6-7,10,12H,1,3,5,8-9H2,(H,17,19)/t10-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha-7 nACh receptor in rat brain |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50190786
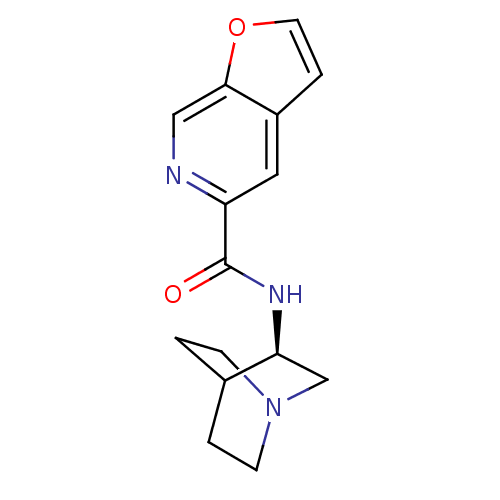
((R)-N-(quinuclidin-3-yl)furo[2,3-c]pyridine-5-carb...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccoc2cn1 |wU:3.2,(-5.16,-23.46,;-5.16,-25,;-3.82,-25.76,;-2.49,-24.99,;-2.5,-23.45,;-1.16,-22.68,;.18,-23.45,;.18,-24.99,;-1.15,-25.76,;-2.03,-24.61,;-1.21,-23.81,;-6.49,-25.78,;-7.83,-25.01,;-9.15,-25.79,;-10.62,-25.31,;-11.52,-26.55,;-10.62,-27.8,;-9.16,-27.33,;-7.82,-28.1,;-6.48,-27.33,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha-7 nACh receptor in human IMR32 cells |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190786

((R)-N-(quinuclidin-3-yl)furo[2,3-c]pyridine-5-carb...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccoc2cn1 |wU:3.2,(-5.16,-23.46,;-5.16,-25,;-3.82,-25.76,;-2.49,-24.99,;-2.5,-23.45,;-1.16,-22.68,;.18,-23.45,;.18,-24.99,;-1.15,-25.76,;-2.03,-24.61,;-1.21,-23.81,;-6.49,-25.78,;-7.83,-25.01,;-9.15,-25.79,;-10.62,-25.31,;-11.52,-26.55,;-10.62,-27.8,;-9.16,-27.33,;-7.82,-28.1,;-6.48,-27.33,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190786

((R)-N-(quinuclidin-3-yl)furo[2,3-c]pyridine-5-carb...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccoc2cn1 |wU:3.2,(-5.16,-23.46,;-5.16,-25,;-3.82,-25.76,;-2.49,-24.99,;-2.5,-23.45,;-1.16,-22.68,;.18,-23.45,;.18,-24.99,;-1.15,-25.76,;-2.03,-24.61,;-1.21,-23.81,;-6.49,-25.78,;-7.83,-25.01,;-9.15,-25.79,;-10.62,-25.31,;-11.52,-26.55,;-10.62,-27.8,;-9.16,-27.33,;-7.82,-28.1,;-6.48,-27.33,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha-7 nACh receptor in rat brain |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50377050

(CHEMBL403858 | PH-709829)Show SMILES O=C(N[C@@H]1C[C@H]2CCN(C2)C1)c1cc2ccoc2cn1 |TLB:2:3:9:7.6| Show InChI InChI=1S/C15H17N3O2/c19-15(13-6-11-2-4-20-14(11)7-16-13)17-12-5-10-1-3-18(8-10)9-12/h2,4,6-7,10,12H,1,3,5,8-9H2,(H,17,19)/t10-,12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha-7 nACh receptor in human IMR32 cells |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190783
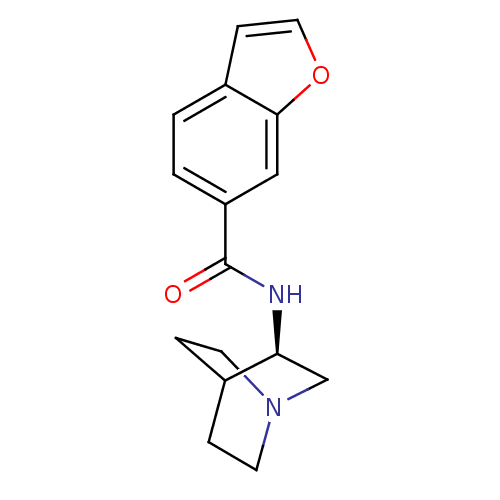
(CHEMBL379302 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccoc2c1 |wU:3.2,(27.6,-12.86,;27.61,-14.4,;28.95,-15.16,;30.28,-14.39,;30.27,-12.85,;31.61,-12.08,;32.95,-12.85,;32.94,-14.39,;31.62,-15.16,;30.74,-14.01,;31.56,-13.22,;26.28,-15.18,;26.29,-16.73,;24.94,-17.5,;23.61,-16.73,;22.15,-17.2,;21.24,-15.96,;22.15,-14.71,;23.61,-15.19,;24.94,-14.41,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50377047
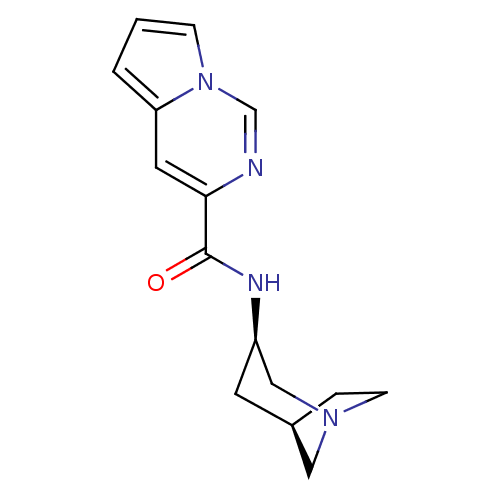
(CHEMBL404299)Show SMILES O=C(N[C@@H]1C[C@H]2CCN(C2)C1)c1cc2cccn2cn1 |TLB:2:3:9:7.6| Show InChI InChI=1S/C15H18N4O/c20-15(14-7-13-2-1-4-19(13)10-16-14)17-12-6-11-3-5-18(8-11)9-12/h1-2,4,7,10-12H,3,5-6,8-9H2,(H,17,20)/t11-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha-7 nACh receptor in rat brain |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190784
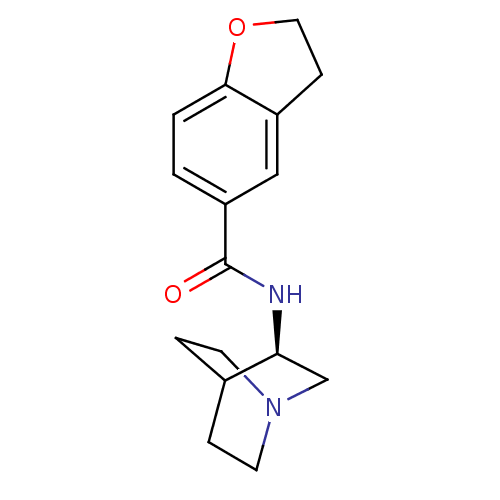
(CHEMBL378496 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2OCCc2c1 |wU:3.2,(11.67,-14.82,;11.67,-16.36,;13.01,-17.12,;14.34,-16.35,;14.33,-14.81,;15.67,-14.04,;17.01,-14.81,;17.01,-16.35,;15.68,-17.12,;14.8,-15.97,;15.62,-15.18,;10.34,-17.14,;10.35,-18.69,;9,-19.46,;7.67,-18.69,;6.21,-19.16,;5.3,-17.92,;6.21,-16.67,;7.68,-17.15,;9,-16.37,)| Show InChI InChI=1S/C16H20N2O2/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,9,11,14H,3-8,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50377051
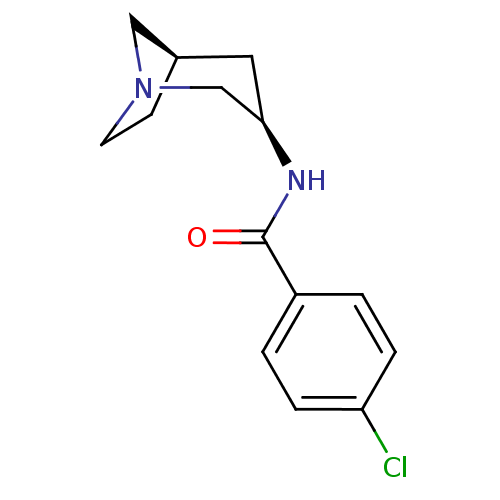
(CHEMBL256578)Show SMILES Clc1ccc(cc1)C(=O)N[C@@H]1C[C@H]2CCN(C2)C1 |TLB:9:10:16:14.13| Show InChI InChI=1S/C14H17ClN2O/c15-12-3-1-11(2-4-12)14(18)16-13-7-10-5-6-17(8-10)9-13/h1-4,10,13H,5-9H2,(H,16,18)/t10-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha-7 nACh receptor in rat brain |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50377053
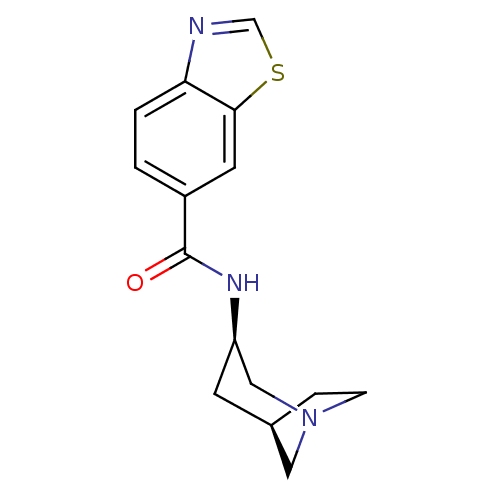
(CHEMBL258239)Show SMILES O=C(N[C@@H]1C[C@H]2CCN(C2)C1)c1ccc2ncsc2c1 |TLB:2:3:9:7.6| Show InChI InChI=1S/C15H17N3OS/c19-15(11-1-2-13-14(6-11)20-9-16-13)17-12-5-10-3-4-18(7-10)8-12/h1-2,6,9-10,12H,3-5,7-8H2,(H,17,19)/t10-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha-7 nACh receptor in rat brain |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50161764
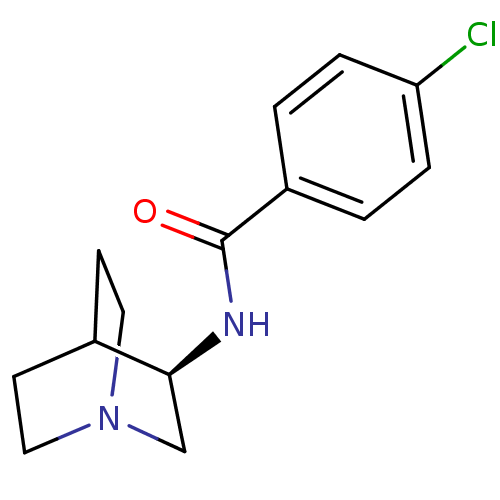
((R)-4-chloro-N-(quinuclidin-3-yl)benzamide | (R)-4...)Show SMILES Clc1ccc(cc1)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:10.10,TLB:9:10:14.13:16.17,(9.34,-31.61,;10.67,-32.38,;10.67,-33.92,;12,-34.69,;13.35,-33.92,;13.34,-32.37,;12,-31.6,;14.68,-34.69,;16.01,-33.92,;14.68,-36.23,;16.02,-37,;16.46,-35.89,;16.53,-37.53,;15.18,-38.13,;14.9,-39.53,;16.27,-38.89,;17.81,-39.55,;18,-38.17,)| Show InChI InChI=1S/C14H17ClN2O/c15-12-3-1-11(2-4-12)14(18)16-13-9-17-7-5-10(13)6-8-17/h1-4,10,13H,5-9H2,(H,16,18)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha-7 nACh receptor in rat brain |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50161764

((R)-4-chloro-N-(quinuclidin-3-yl)benzamide | (R)-4...)Show SMILES Clc1ccc(cc1)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:10.10,TLB:9:10:14.13:16.17,(9.34,-31.61,;10.67,-32.38,;10.67,-33.92,;12,-34.69,;13.35,-33.92,;13.34,-32.37,;12,-31.6,;14.68,-34.69,;16.01,-33.92,;14.68,-36.23,;16.02,-37,;16.46,-35.89,;16.53,-37.53,;15.18,-38.13,;14.9,-39.53,;16.27,-38.89,;17.81,-39.55,;18,-38.17,)| Show InChI InChI=1S/C14H17ClN2O/c15-12-3-1-11(2-4-12)14(18)16-13-9-17-7-5-10(13)6-8-17/h1-4,10,13H,5-9H2,(H,16,18)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50161753
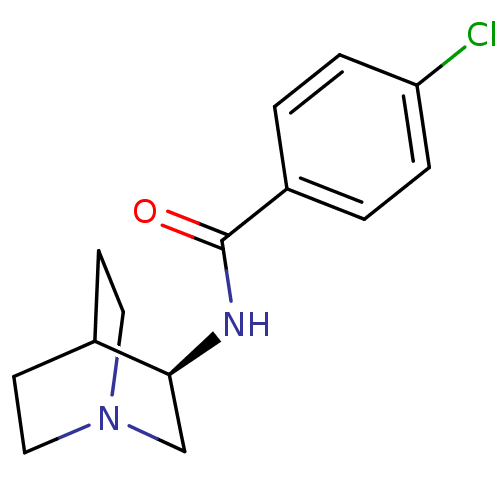
(CHEMBL554984 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES Clc1ccc(cc1)C(=O)N[C@H]1CN2CCC1CC2 |wU:10.10,(-6.01,-2.41,;-4.68,-1.64,;-3.35,-2.41,;-2.01,-1.64,;-2.01,-.08,;-3.35,.69,;-4.68,-.08,;-.68,.69,;-.69,2.23,;.66,-.08,;1.99,.69,;1.98,2.23,;3.25,3,;4.04,1.67,;2.55,1.28,;3.32,-.08,;4.65,.69,;4.65,2.23,)| Show InChI InChI=1S/C14H17ClN2O/c15-12-3-1-11(2-4-12)14(18)16-13-9-17-7-5-10(13)6-8-17/h1-4,10,13H,5-9H2,(H,16,18)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of methyllycaconitine (MLA) binding to Nicotinic acetylcholine receptor alpha-7 in rat brain homogenates |
J Med Chem 48: 905-8 (2005)
Article DOI: 10.1021/jm049363q
BindingDB Entry DOI: 10.7270/Q2ZC83MF |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50377052

(CHEMBL257145)Show SMILES O=C(N[C@@H]1C[C@H]2CCN(C2)C1)c1ccc2OCCOc2c1 |TLB:2:3:9:7.6| Show InChI InChI=1S/C16H20N2O3/c19-16(17-13-7-11-3-4-18(9-11)10-13)12-1-2-14-15(8-12)21-6-5-20-14/h1-2,8,11,13H,3-7,9-10H2,(H,17,19)/t11-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha-7 nACh receptor in rat brain |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190790
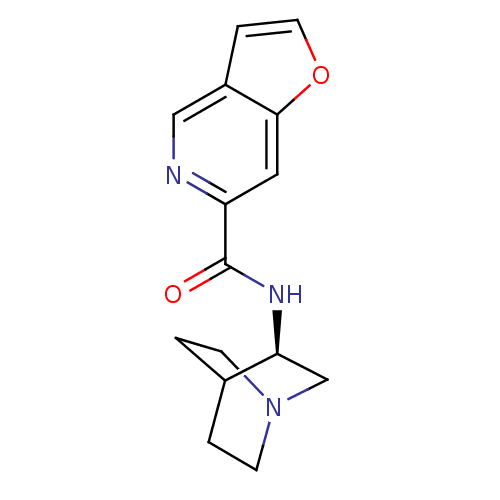
(CHEMBL214195 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2occc2cn1 |wU:3.2,(27.35,-21.97,;27.36,-23.51,;28.69,-24.27,;30.02,-23.5,;30.01,-21.96,;31.35,-21.19,;32.69,-21.96,;32.69,-23.5,;31.36,-24.27,;30.48,-23.12,;31.3,-22.32,;26.02,-24.29,;24.68,-23.52,;23.36,-24.3,;21.89,-23.82,;20.99,-25.07,;21.89,-26.31,;23.36,-25.84,;24.69,-26.61,;26.03,-25.84,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-14-11(8-16-12)3-6-20-14)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190794
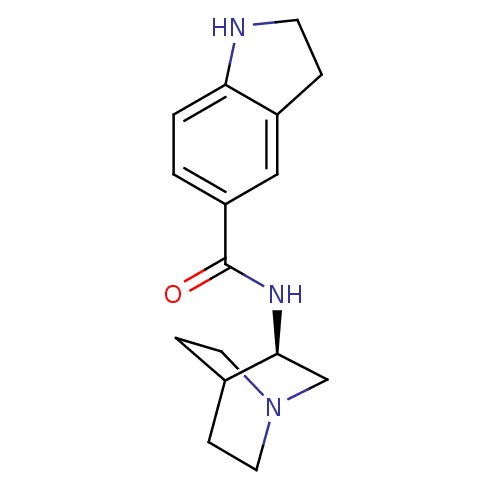
(CHEMBL211572 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2NCCc2c1 |wU:3.2,(-3.98,-5.4,;-3.98,-6.95,;-2.64,-7.71,;-1.31,-6.93,;-1.32,-5.4,;.02,-4.62,;1.36,-5.39,;1.36,-6.93,;.03,-7.7,;-.85,-6.56,;-.03,-5.76,;-5.31,-7.72,;-5.3,-9.27,;-6.64,-10.05,;-7.97,-9.28,;-9.44,-9.75,;-10.34,-8.5,;-9.44,-7.26,;-7.97,-7.74,;-6.65,-6.96,)| Show InChI InChI=1S/C16H21N3O/c20-16(13-1-2-14-12(9-13)3-6-17-14)18-15-10-19-7-4-11(15)5-8-19/h1-2,9,11,15,17H,3-8,10H2,(H,18,20)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190785

(CHEMBL378349 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccsc2c1 |wU:3.2,(28.02,-3.67,;28.02,-5.21,;29.36,-5.97,;30.69,-5.19,;30.68,-3.66,;32.02,-2.89,;33.36,-3.65,;33.36,-5.2,;32.03,-5.97,;31.15,-4.82,;31.97,-4.02,;26.69,-5.98,;26.7,-7.54,;25.35,-8.31,;24.02,-7.54,;22.56,-8.01,;21.65,-6.76,;22.56,-5.52,;24.03,-6,;25.35,-5.22,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT3 receptor |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190785

(CHEMBL378349 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccsc2c1 |wU:3.2,(28.02,-3.67,;28.02,-5.21,;29.36,-5.97,;30.69,-5.19,;30.68,-3.66,;32.02,-2.89,;33.36,-3.65,;33.36,-5.2,;32.03,-5.97,;31.15,-4.82,;31.97,-4.02,;26.69,-5.98,;26.7,-7.54,;25.35,-8.31,;24.02,-7.54,;22.56,-8.01,;21.65,-6.76,;22.56,-5.52,;24.03,-6,;25.35,-5.22,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Activity at 5HT3 receptor expressed in SHEP1 cells by FLIPR assay |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190783

(CHEMBL379302 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccoc2c1 |wU:3.2,(27.6,-12.86,;27.61,-14.4,;28.95,-15.16,;30.28,-14.39,;30.27,-12.85,;31.61,-12.08,;32.95,-12.85,;32.94,-14.39,;31.62,-15.16,;30.74,-14.01,;31.56,-13.22,;26.28,-15.18,;26.29,-16.73,;24.94,-17.5,;23.61,-16.73,;22.15,-17.2,;21.24,-15.96,;22.15,-14.71,;23.61,-15.19,;24.94,-14.41,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT3 receptor |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50377050

(CHEMBL403858 | PH-709829)Show SMILES O=C(N[C@@H]1C[C@H]2CCN(C2)C1)c1cc2ccoc2cn1 |TLB:2:3:9:7.6| Show InChI InChI=1S/C15H17N3O2/c19-15(13-6-11-2-4-20-14(11)7-16-13)17-12-5-10-1-3-18(8-10)9-12/h2,4,6-7,10,12H,1,3,5,8-9H2,(H,17,19)/t10-,12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to rat 5HT3 receptor |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190786

((R)-N-(quinuclidin-3-yl)furo[2,3-c]pyridine-5-carb...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccoc2cn1 |wU:3.2,(-5.16,-23.46,;-5.16,-25,;-3.82,-25.76,;-2.49,-24.99,;-2.5,-23.45,;-1.16,-22.68,;.18,-23.45,;.18,-24.99,;-1.15,-25.76,;-2.03,-24.61,;-1.21,-23.81,;-6.49,-25.78,;-7.83,-25.01,;-9.15,-25.79,;-10.62,-25.31,;-11.52,-26.55,;-10.62,-27.8,;-9.16,-27.33,;-7.82,-28.1,;-6.48,-27.33,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 511 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT3 receptor |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190788

(CHEMBL378471 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2occc2c1 |wU:3.2,(-3.91,-15.15,;-3.91,-16.69,;-2.57,-17.45,;-1.24,-16.67,;-1.25,-15.14,;.09,-14.37,;1.43,-15.13,;1.43,-16.67,;.1,-17.45,;-.78,-16.3,;.04,-15.5,;-5.24,-17.46,;-5.23,-19.02,;-6.57,-19.79,;-7.91,-19.02,;-9.37,-19.49,;-10.28,-18.24,;-9.37,-17,;-7.9,-17.48,;-6.58,-16.7,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Activity at 5HT3 receptor expressed in SHEP1 cells by FLIPR assay |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190786

((R)-N-(quinuclidin-3-yl)furo[2,3-c]pyridine-5-carb...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccoc2cn1 |wU:3.2,(-5.16,-23.46,;-5.16,-25,;-3.82,-25.76,;-2.49,-24.99,;-2.5,-23.45,;-1.16,-22.68,;.18,-23.45,;.18,-24.99,;-1.15,-25.76,;-2.03,-24.61,;-1.21,-23.81,;-6.49,-25.78,;-7.83,-25.01,;-9.15,-25.79,;-10.62,-25.31,;-11.52,-26.55,;-10.62,-27.8,;-9.16,-27.33,;-7.82,-28.1,;-6.48,-27.33,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 628 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Activity at 5HT3 receptor expressed in SHEP1 cells by FLIPR assay |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50190786

((R)-N-(quinuclidin-3-yl)furo[2,3-c]pyridine-5-carb...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccoc2cn1 |wU:3.2,(-5.16,-23.46,;-5.16,-25,;-3.82,-25.76,;-2.49,-24.99,;-2.5,-23.45,;-1.16,-22.68,;.18,-23.45,;.18,-24.99,;-1.15,-25.76,;-2.03,-24.61,;-1.21,-23.81,;-6.49,-25.78,;-7.83,-25.01,;-9.15,-25.79,;-10.62,-25.31,;-11.52,-26.55,;-10.62,-27.8,;-9.16,-27.33,;-7.82,-28.1,;-6.48,-27.33,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to rat 5HT3 receptor |
Bioorg Med Chem Lett 18: 3611-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.070
BindingDB Entry DOI: 10.7270/Q2KD1ZS9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190788

(CHEMBL378471 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2occc2c1 |wU:3.2,(-3.91,-15.15,;-3.91,-16.69,;-2.57,-17.45,;-1.24,-16.67,;-1.25,-15.14,;.09,-14.37,;1.43,-15.13,;1.43,-16.67,;.1,-17.45,;-.78,-16.3,;.04,-15.5,;-5.24,-17.46,;-5.23,-19.02,;-6.57,-19.79,;-7.91,-19.02,;-9.37,-19.49,;-10.28,-18.24,;-9.37,-17,;-7.9,-17.48,;-6.58,-16.7,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 663 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT3 receptor |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190783

(CHEMBL379302 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccoc2c1 |wU:3.2,(27.6,-12.86,;27.61,-14.4,;28.95,-15.16,;30.28,-14.39,;30.27,-12.85,;31.61,-12.08,;32.95,-12.85,;32.94,-14.39,;31.62,-15.16,;30.74,-14.01,;31.56,-13.22,;26.28,-15.18,;26.29,-16.73,;24.94,-17.5,;23.61,-16.73,;22.15,-17.2,;21.24,-15.96,;22.15,-14.71,;23.61,-15.19,;24.94,-14.41,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 962 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Activity at 5HT3 receptor expressed in SHEP1 cells by FLIPR assay |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50161764

((R)-4-chloro-N-(quinuclidin-3-yl)benzamide | (R)-4...)Show SMILES Clc1ccc(cc1)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:10.10,TLB:9:10:14.13:16.17,(9.34,-31.61,;10.67,-32.38,;10.67,-33.92,;12,-34.69,;13.35,-33.92,;13.34,-32.37,;12,-31.6,;14.68,-34.69,;16.01,-33.92,;14.68,-36.23,;16.02,-37,;16.46,-35.89,;16.53,-37.53,;15.18,-38.13,;14.9,-39.53,;16.27,-38.89,;17.81,-39.55,;18,-38.17,)| Show InChI InChI=1S/C14H17ClN2O/c15-12-3-1-11(2-4-12)14(18)16-13-9-17-7-5-10(13)6-8-17/h1-4,10,13H,5-9H2,(H,16,18)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT3 receptor |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50161753

(CHEMBL554984 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES Clc1ccc(cc1)C(=O)N[C@H]1CN2CCC1CC2 |wU:10.10,(-6.01,-2.41,;-4.68,-1.64,;-3.35,-2.41,;-2.01,-1.64,;-2.01,-.08,;-3.35,.69,;-4.68,-.08,;-.68,.69,;-.69,2.23,;.66,-.08,;1.99,.69,;1.98,2.23,;3.25,3,;4.04,1.67,;2.55,1.28,;3.32,-.08,;4.65,.69,;4.65,2.23,)| Show InChI InChI=1S/C14H17ClN2O/c15-12-3-1-11(2-4-12)14(18)16-13-9-17-7-5-10(13)6-8-17/h1-4,10,13H,5-9H2,(H,16,18)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]GR-65630 binding to 5-hydroxytryptamine 3 receptor from rat brain homogenates |
J Med Chem 48: 905-8 (2005)
Article DOI: 10.1021/jm049363q
BindingDB Entry DOI: 10.7270/Q2ZC83MF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50161764

((R)-4-chloro-N-(quinuclidin-3-yl)benzamide | (R)-4...)Show SMILES Clc1ccc(cc1)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:10.10,TLB:9:10:14.13:16.17,(9.34,-31.61,;10.67,-32.38,;10.67,-33.92,;12,-34.69,;13.35,-33.92,;13.34,-32.37,;12,-31.6,;14.68,-34.69,;16.01,-33.92,;14.68,-36.23,;16.02,-37,;16.46,-35.89,;16.53,-37.53,;15.18,-38.13,;14.9,-39.53,;16.27,-38.89,;17.81,-39.55,;18,-38.17,)| Show InChI InChI=1S/C14H17ClN2O/c15-12-3-1-11(2-4-12)14(18)16-13-9-17-7-5-10(13)6-8-17/h1-4,10,13H,5-9H2,(H,16,18)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Activity at 5HT3 receptor expressed in SHEP1 cells by FLIPR assay |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM23400
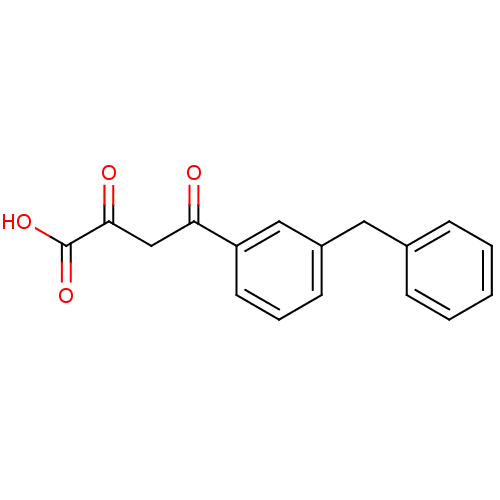
((2Z)-4-(3-benzylphenyl)-2-hydroxy-4-oxobut-2-enoic...)Show InChI InChI=1S/C17H14O4/c18-15(11-16(19)17(20)21)14-8-4-7-13(10-14)9-12-5-2-1-3-6-12/h1-8,10H,9,11H2,(H,20,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit the strand transfer of the integration process catalyzed by HIV integrase |
J Med Chem 46: 453-6 (2003)
Article DOI: 10.1021/jm025553u
BindingDB Entry DOI: 10.7270/Q24T6HRG |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM304727

(3-(7-methoxy-1-methyl-1H-benzo[d][1,2,3]triazol-5-...)Show SMILES COc1cc(cc2nnn(C)c12)C(CC(O)=O)c1ccc(C)c(CN2C[C@@H](C)Oc3ccccc3S2(=O)=O)c1 |r| Show InChI InChI=1S/C28H30N4O6S/c1-17-9-10-19(22(14-27(33)34)20-12-23-28(25(13-20)37-4)31(3)30-29-23)11-21(17)16-32-15-18(2)38-24-7-5-6-8-26(24)39(32,35)36/h5-13,18,22H,14-16H2,1-4H3,(H,33,34)/t18-,22?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 5'-TAMRA-labelled NRF2 peptide from human N-terminal His6-tagged KEAP1 Kelch domain (321 to 609 residues) expressed in baculovirus in... |
J Med Chem 62: 4683-4702 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00279
BindingDB Entry DOI: 10.7270/Q2PK0KKB |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50123468
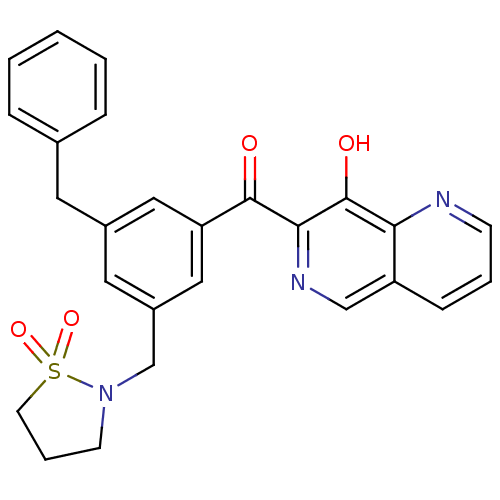
(CHEMBL32865 | [3-Benzyl-5-(1,1-dioxo-1lambda 6 -is...)Show SMILES Oc1c(ncc2cccnc12)C(=O)c1cc(CN2CCCS2(=O)=O)cc(Cc2ccccc2)c1 Show InChI InChI=1S/C26H23N3O4S/c30-25(24-26(31)23-21(16-28-24)8-4-9-27-23)22-14-19(12-18-6-2-1-3-7-18)13-20(15-22)17-29-10-5-11-34(29,32)33/h1-4,6-9,13-16,31H,5,10-12,17H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit the strand transfer of the integration process catalyzed by HIV integrase |
J Med Chem 46: 453-6 (2003)
Article DOI: 10.1021/jm025553u
BindingDB Entry DOI: 10.7270/Q24T6HRG |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM304721
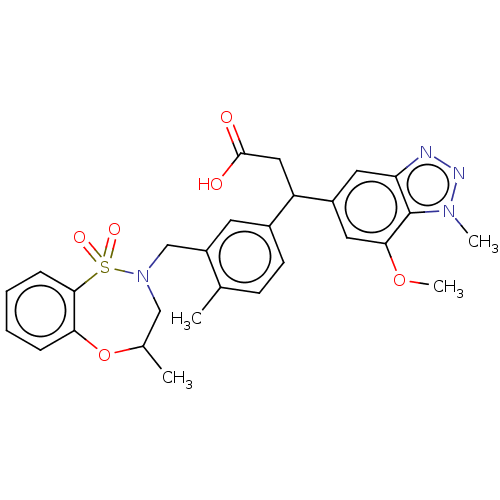
(3-(7-methoxy-1-methyl-1H-benzo[d][1,2,3]triazol-5-...)Show SMILES COc1cc(cc2nnn(C)c12)C(CC(O)=O)c1ccc(C)c(CN2CC(C)Oc3ccccc3S2(=O)=O)c1 Show InChI InChI=1S/C28H30N4O6S/c1-17-9-10-19(22(14-27(33)34)20-12-23-28(25(13-20)37-4)31(3)30-29-23)11-21(17)16-32-15-18(2)38-24-7-5-6-8-26(24)39(32,35)36/h5-13,18,22H,14-16H2,1-4H3,(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 5'-TAMRA-labelled NRF2 peptide from human N-terminal His6-tagged KEAP1 Kelch domain (321 to 609 residues) expressed in baculovirus in... |
J Med Chem 62: 4683-4702 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00279
BindingDB Entry DOI: 10.7270/Q2PK0KKB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM304748
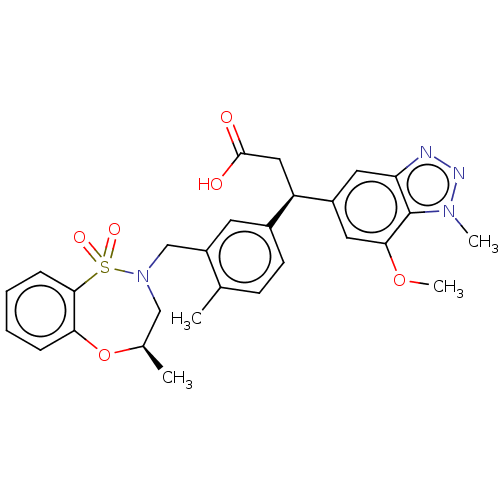
(BDBM304749 | US10144731, Example 34a)Show SMILES COc1cc(cc2nnn(C)c12)[C@H](CC(O)=O)c1ccc(C)c(CN2C[C@@H](C)Oc3ccccc3S2(=O)=O)c1 |r| Show InChI InChI=1S/C28H30N4O6S/c1-17-9-10-19(22(14-27(33)34)20-12-23-28(25(13-20)37-4)31(3)30-29-23)11-21(17)16-32-15-18(2)38-24-7-5-6-8-26(24)39(32,35)36/h5-13,18,22H,14-16H2,1-4H3,(H,33,34)/t18-,22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 5'-TAMRA-labelled NRF2 peptide from human N-terminal His6-tagged KEAP1 Kelch domain (321 to 609 residues) expressed in baculovirus in... |
J Med Chem 62: 4683-4702 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00279
BindingDB Entry DOI: 10.7270/Q2PK0KKB |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM304414

(3-(7-methoxy-1-methyl-1H-benzo[d][1,2,3]triazol-5-...)Show SMILES COc1cc(cc2nnn(C)c12)C(CC(O)=O)c1ccc(C)c(CN2CCN(C)c3ccccc3S2(=O)=O)c1 Show InChI InChI=1S/C28H31N5O5S/c1-18-9-10-19(22(16-27(34)35)20-14-23-28(25(15-20)38-4)32(3)30-29-23)13-21(18)17-33-12-11-31(2)24-7-5-6-8-26(24)39(33,36)37/h5-10,13-15,22H,11-12,16-17H2,1-4H3,(H,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 5'-TAMRA-labelled NRF2 peptide from human N-terminal His6-tagged KEAP1 Kelch domain (321 to 609 residues) expressed in baculovirus in... |
J Med Chem 62: 4683-4702 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00279
BindingDB Entry DOI: 10.7270/Q2PK0KKB |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50123469
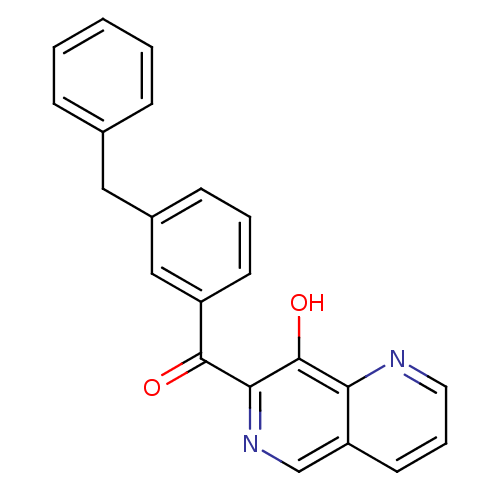
((3-Benzyl-phenyl)-(8-hydroxy-[1,6]naphthyridin-7-y...)Show InChI InChI=1S/C22H16N2O2/c25-21(20-22(26)19-18(14-24-20)10-5-11-23-19)17-9-4-8-16(13-17)12-15-6-2-1-3-7-15/h1-11,13-14,26H,12H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit the strand transfer of the integration process catalyzed by HIV integrase |
J Med Chem 46: 453-6 (2003)
Article DOI: 10.1021/jm025553u
BindingDB Entry DOI: 10.7270/Q24T6HRG |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50601802
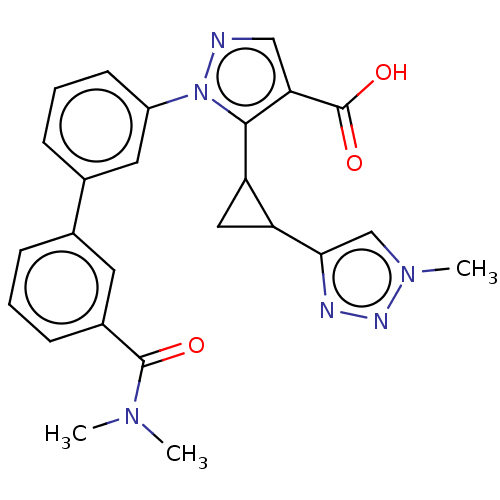
(CHEMBL5203783)Show SMILES CN(C)C(=O)c1cccc(c1)-c1cccc(c1)-n1ncc(C(O)=O)c1C1CC1c1cn(C)nn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01351
BindingDB Entry DOI: 10.7270/Q2JS9VJF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50601811
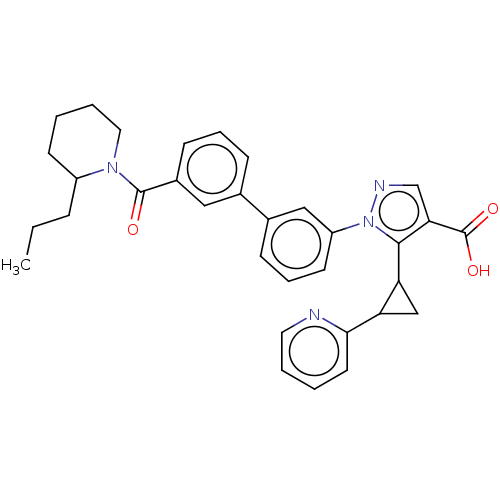
(CHEMBL5189627)Show SMILES CCCC1CCCCN1C(=O)c1cccc(c1)-c1cccc(c1)-n1ncc(C(O)=O)c1C1CC1c1ccccn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01351
BindingDB Entry DOI: 10.7270/Q2JS9VJF |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM304728

(3-(7-methoxy-1-methyl-1H-benzo[d][1,2,3]triazol-5-...)Show SMILES COc1cc(cc2nnn(C)c12)C(CC(O)=O)c1ccc(C)c(CN2C[C@H](C)Oc3ccccc3S2(=O)=O)c1 |r| Show InChI InChI=1S/C28H30N4O6S/c1-17-9-10-19(22(14-27(33)34)20-12-23-28(25(13-20)37-4)31(3)30-29-23)11-21(17)16-32-15-18(2)38-24-7-5-6-8-26(24)39(32,35)36/h5-13,18,22H,14-16H2,1-4H3,(H,33,34)/t18-,22?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 5'-TAMRA-labelled NRF2 peptide from human N-terminal His6-tagged KEAP1 Kelch domain (321 to 609 residues) expressed in baculovirus in... |
J Med Chem 62: 4683-4702 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00279
BindingDB Entry DOI: 10.7270/Q2PK0KKB |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM304768
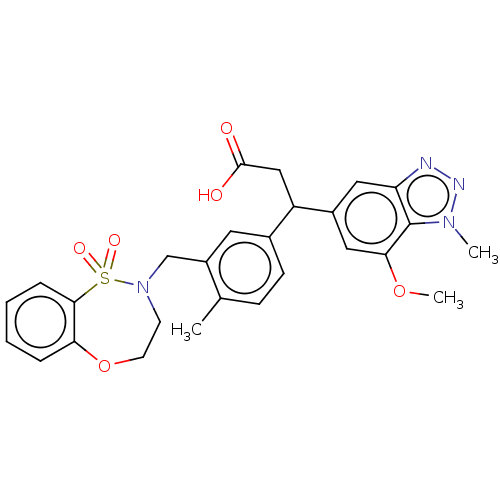
(3-(3-((1,1-dioxido-3,4-dihydro-2H-benzo[b][1,4,5]o...)Show SMILES COc1cc(cc2nnn(C)c12)C(CC(O)=O)c1ccc(C)c(CN2CCOc3ccccc3S2(=O)=O)c1 Show InChI InChI=1S/C27H28N4O6S/c1-17-8-9-18(12-20(17)16-31-10-11-37-23-6-4-5-7-25(23)38(31,34)35)21(15-26(32)33)19-13-22-27(24(14-19)36-3)30(2)29-28-22/h4-9,12-14,21H,10-11,15-16H2,1-3H3,(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 5'-TAMRA-labelled NRF2 peptide from human N-terminal His6-tagged KEAP1 Kelch domain (321 to 609 residues) expressed in baculovirus in... |
J Med Chem 62: 4683-4702 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00279
BindingDB Entry DOI: 10.7270/Q2PK0KKB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrase
(Human immunodeficiency virus 1) | BDBM50123471
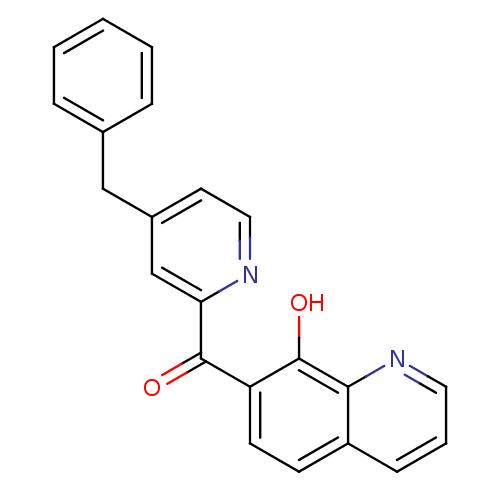
((4-Benzyl-pyridin-2-yl)-(8-hydroxy-quinolin-7-yl)-...)Show InChI InChI=1S/C22H16N2O2/c25-21(18-9-8-17-7-4-11-24-20(17)22(18)26)19-14-16(10-12-23-19)13-15-5-2-1-3-6-15/h1-12,14,26H,13H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit the strand transfer of the integration process catalyzed by HIV integrase |
J Med Chem 46: 453-6 (2003)
Article DOI: 10.1021/jm025553u
BindingDB Entry DOI: 10.7270/Q24T6HRG |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50601805
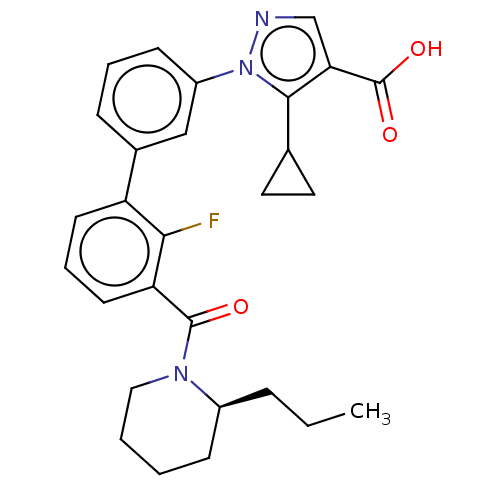
(CHEMBL5208399)Show SMILES CCC[C@H]1CCCCN1C(=O)c1cccc(c1F)-c1cccc(c1)-n1ncc(C(O)=O)c1C1CC1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01351
BindingDB Entry DOI: 10.7270/Q2JS9VJF |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM304837
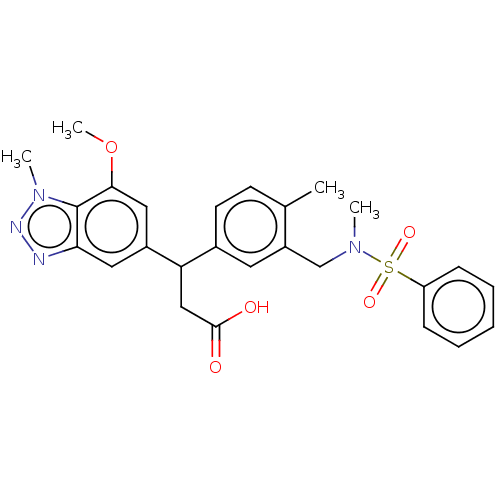
(3-(7-methoxy-1-methyl-1H-1,2,3-benzotriazol-5-yl)-...)Show SMILES COc1cc(cc2nnn(C)c12)C(CC(O)=O)c1ccc(C)c(CN(C)S(=O)(=O)c2ccccc2)c1 Show InChI InChI=1S/C26H28N4O5S/c1-17-10-11-18(12-20(17)16-29(2)36(33,34)21-8-6-5-7-9-21)22(15-25(31)32)19-13-23-26(24(14-19)35-4)30(3)28-27-23/h5-14,22H,15-16H2,1-4H3,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 5'-TAMRA-labelled NRF2 peptide from human N-terminal His6-tagged KEAP1 Kelch domain (321 to 609 residues) expressed in baculovirus in... |
J Med Chem 62: 4683-4702 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00279
BindingDB Entry DOI: 10.7270/Q2PK0KKB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data