 Found 2206 hits with Last Name = 'yin' and Initial = 'l'
Found 2206 hits with Last Name = 'yin' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserine from 5-HT2A receptor in rat cerebral cortex homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT2A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50285668
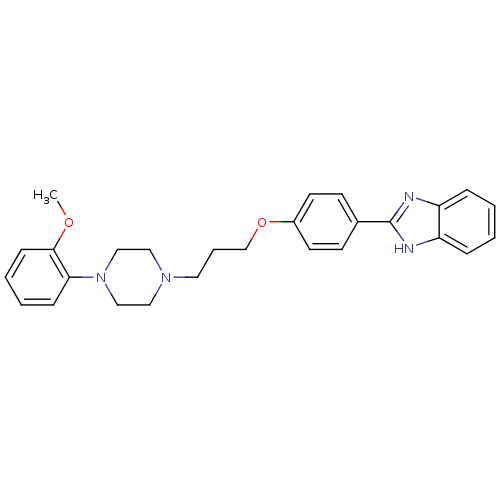
(2-(4-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-prop...)Show SMILES COc1ccccc1N1CCN(CCCOc2ccc(cc2)-c2nc3ccccc3[nH]2)CC1 Show InChI InChI=1S/C27H30N4O2/c1-32-26-10-5-4-9-25(26)31-18-16-30(17-19-31)15-6-20-33-22-13-11-21(12-14-22)27-28-23-7-2-3-8-24(23)29-27/h2-5,7-14H,6,15-20H2,1H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to dopaminergic D3 receptor (unknown origin) |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50561093

(CHEMBL4784373)Show SMILES CC(=O)Nc1ccc(OCCCN2CCC(CC2)c2noc3cc(F)ccc23)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to D2 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50133171

(CHEMBL3634821)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCOc2ccc(Cc3nc4ccccc4o3)cc2)CC1 Show InChI InChI=1S/C29H28FN3O3/c30-22-8-11-24-27(19-22)36-32-29(24)21-12-15-33(16-13-21)14-3-17-34-23-9-6-20(7-10-23)18-28-31-25-4-1-2-5-26(25)35-28/h1-2,4-11,19,21H,3,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserine from 5-HT2A receptor in rat cerebral cortex homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from 5-HT7 receptor in rat hypothalamus homogenates after 120 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50561093

(CHEMBL4784373)Show SMILES CC(=O)Nc1ccc(OCCCN2CCC(CC2)c2noc3cc(F)ccc23)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT1A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from dopaminergic D2 receptor in rat striatum homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to D2 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600600
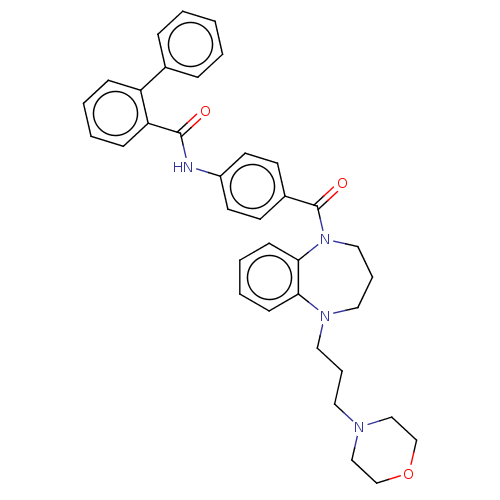
(CHEMBL5207885)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12)c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50561093

(CHEMBL4784373)Show SMILES CC(=O)Nc1ccc(OCCCN2CCC(CC2)c2noc3cc(F)ccc23)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT2A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50133181
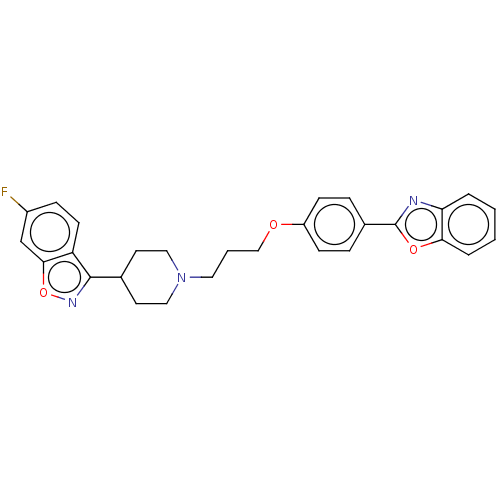
(CHEMBL3634813)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCOc2ccc(cc2)-c2nc3ccccc3o2)CC1 Show InChI InChI=1S/C28H26FN3O3/c29-21-8-11-23-26(18-21)35-31-27(23)19-12-15-32(16-13-19)14-3-17-33-22-9-6-20(7-10-22)28-30-24-4-1-2-5-25(24)34-28/h1-2,4-11,18-19H,3,12-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserine from 5-HT2A receptor in rat cerebral cortex homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50285668

(2-(4-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-prop...)Show SMILES COc1ccccc1N1CCN(CCCOc2ccc(cc2)-c2nc3ccccc3[nH]2)CC1 Show InChI InChI=1S/C27H30N4O2/c1-32-26-10-5-4-9-25(26)31-18-16-30(17-19-31)15-6-20-33-22-13-11-21(12-14-22)27-28-23-7-2-3-8-24(23)29-27/h2-5,7-14H,6,15-20H2,1H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Binding affinity to dopaminergic D2 receptor (unknown origin) |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600605
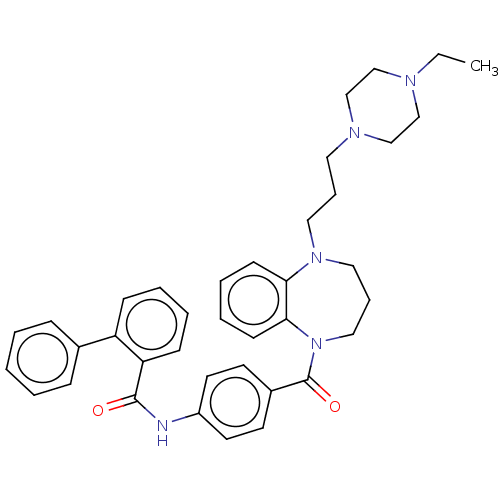
(CHEMBL5208751)Show SMILES CCN1CCN(CCCN2CCCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc23)CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600607

(CHEMBL5207405)Show SMILES CCN(CC)CCCN1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3-c3ccccc3)cc2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50133171

(CHEMBL3634821)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCOc2ccc(Cc3nc4ccccc4o3)cc2)CC1 Show InChI InChI=1S/C29H28FN3O3/c30-22-8-11-24-27(19-22)36-32-29(24)21-12-15-33(16-13-21)14-3-17-34-23-9-6-20(7-10-23)18-28-31-25-4-1-2-5-26(25)35-28/h1-2,4-11,19,21H,3,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from dopaminergic D2 receptor in rat striatum homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600611
(CHEMBL5199628)Show SMILES Cc1cc(NC(=O)c2ccccc2-c2ccccc2)ccc1C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600603

(CHEMBL5192961)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCCCC2)c2ccccc12)c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600604
(CHEMBL5193621)Show SMILES CN1CCN(CCCN2CCCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc23)CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50561093

(CHEMBL4784373)Show SMILES CC(=O)Nc1ccc(OCCCN2CCC(CC2)c2noc3cc(F)ccc23)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT6 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50561101

(CHEMBL4792709)Show SMILES CN(C(C)=O)c1ccc(OCCCN2CCC(CC2)c2noc3cc(F)ccc23)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT2A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserine from 5-HT2A receptor in rat cerebral cortex homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50285668

(2-(4-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-prop...)Show SMILES COc1ccccc1N1CCN(CCCOc2ccc(cc2)-c2nc3ccccc3[nH]2)CC1 Show InChI InChI=1S/C27H30N4O2/c1-32-26-10-5-4-9-25(26)31-18-16-30(17-19-31)15-6-20-33-22-13-11-21(12-14-22)27-28-23-7-2-3-8-24(23)29-27/h2-5,7-14H,6,15-20H2,1H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from dopaminergic D2 receptor in rat striatum homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50561095

(CHEMBL4764692)Show SMILES CC(=O)Nc1ccc(OCCCN2CCN(CC2)c2cccc3ccsc23)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to D2 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50561093

(CHEMBL4784373)Show SMILES CC(=O)Nc1ccc(OCCCN2CCC(CC2)c2noc3cc(F)ccc23)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to alpha2 adrenergic receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50133179
(CHEMBL3634814)Show SMILES C(COc1ccc(cc1)-c1nc2ccccc2o1)CN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C27H26N4O2S/c1-4-9-25-22(6-1)26(29-34-25)31-17-15-30(16-18-31)14-5-19-32-21-12-10-20(11-13-21)27-28-23-7-2-3-8-24(23)33-27/h1-4,6-13H,5,14-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserine from 5-HT2A receptor in rat cerebral cortex homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600601
(CHEMBL5181318)Show SMILES Cc1ccc(cc1)-c1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50133171

(CHEMBL3634821)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCOc2ccc(Cc3nc4ccccc4o3)cc2)CC1 Show InChI InChI=1S/C29H28FN3O3/c30-22-8-11-24-27(19-22)36-32-29(24)21-12-15-33(16-13-21)14-3-17-34-23-9-6-20(7-10-23)18-28-31-25-4-1-2-5-26(25)35-28/h1-2,4-11,19,21H,3,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from 5-HT7 receptor in rat hypothalamus homogenates after 120 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50561101

(CHEMBL4792709)Show SMILES CN(C(C)=O)c1ccc(OCCCN2CCC(CC2)c2noc3cc(F)ccc23)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to D2 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50561101

(CHEMBL4792709)Show SMILES CN(C(C)=O)c1ccc(OCCCN2CCC(CC2)c2noc3cc(F)ccc23)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT1A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50133171

(CHEMBL3634821)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCOc2ccc(Cc3nc4ccccc4o3)cc2)CC1 Show InChI InChI=1S/C29H28FN3O3/c30-22-8-11-24-27(19-22)36-32-29(24)21-12-15-33(16-13-21)14-3-17-34-23-9-6-20(7-10-23)18-28-31-25-4-1-2-5-26(25)35-28/h1-2,4-11,19,21H,3,12-18H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat cerebral cortex homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50133181

(CHEMBL3634813)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCOc2ccc(cc2)-c2nc3ccccc3o2)CC1 Show InChI InChI=1S/C28H26FN3O3/c29-21-8-11-23-26(18-21)35-31-27(23)19-12-15-32(16-13-19)14-3-17-33-22-9-6-20(7-10-22)28-30-24-4-1-2-5-25(24)34-28/h1-2,4-11,18-19H,3,12-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from dopaminergic D2 receptor in rat striatum homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to alpha1 adrenergic receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600610
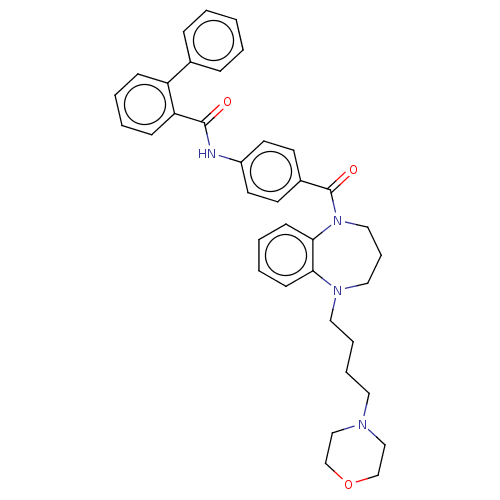
(CHEMBL5179440)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCN(CCCCN2CCOCC2)c2ccccc12)c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50133169

(CHEMBL3634819)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCOc2ccc(-c3nc4ccccc4o3)c(F)c2)CC1 Show InChI InChI=1S/C28H25F2N3O3/c29-19-6-8-22-26(16-19)36-32-27(22)18-10-13-33(14-11-18)12-3-15-34-20-7-9-21(23(30)17-20)28-31-24-4-1-2-5-25(24)35-28/h1-2,4-9,16-18H,3,10-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserine from 5-HT2A receptor in rat cerebral cortex homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600609

(CHEMBL5172250)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCN(CCN2CCOCC2)c2ccccc12)c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to alpha2 adrenergic receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50133203

(CHEMBL3634810)Show SMILES COc1ccccc1N1CCN(CCCOc2ccc(cc2)-c2nc3ccccc3o2)CC1 Show InChI InChI=1S/C27H29N3O3/c1-31-26-10-5-3-8-24(26)30-18-16-29(17-19-30)15-6-20-32-22-13-11-21(12-14-22)27-28-23-7-2-4-9-25(23)33-27/h2-5,7-14H,6,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserine from 5-HT2A receptor in rat cerebral cortex homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from 5-HT2C receptor in rat cerebral cortex homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50133174
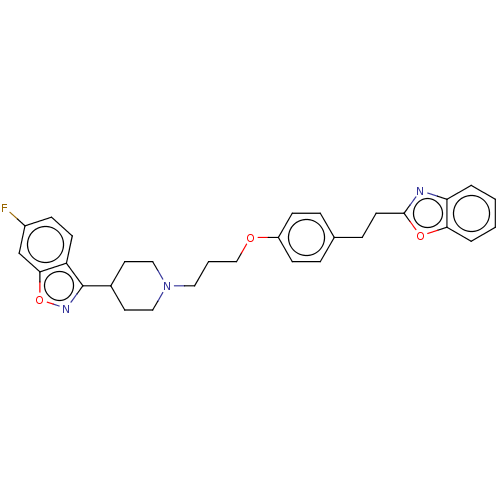
(CHEMBL3634824)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCOc2ccc(CCc3nc4ccccc4o3)cc2)CC1 Show InChI InChI=1S/C30H30FN3O3/c31-23-9-12-25-28(20-23)37-33-30(25)22-14-17-34(18-15-22)16-3-19-35-24-10-6-21(7-11-24)8-13-29-32-26-4-1-2-5-27(26)36-29/h1-2,4-7,9-12,20,22H,3,8,13-19H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat cerebral cortex homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50133175
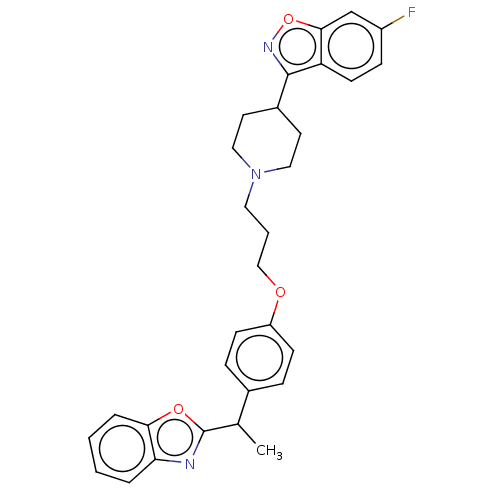
(CHEMBL3634825)Show SMILES CC(c1nc2ccccc2o1)c1ccc(OCCCN2CCC(CC2)c2noc3cc(F)ccc23)cc1 Show InChI InChI=1S/C30H30FN3O3/c1-20(30-32-26-5-2-3-6-27(26)36-30)21-7-10-24(11-8-21)35-18-4-15-34-16-13-22(14-17-34)29-25-12-9-23(31)19-28(25)37-33-29/h2-3,5-12,19-20,22H,4,13-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserine from 5-HT2A receptor in rat cerebral cortex homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600612

(CHEMBL5187046)Show SMILES Clc1ccc2N(CCCN3CCOCC3)CCCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c2c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50133174

(CHEMBL3634824)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCOc2ccc(CCc3nc4ccccc4o3)cc2)CC1 Show InChI InChI=1S/C30H30FN3O3/c31-23-9-12-25-28(20-23)37-33-30(25)22-14-17-34(18-15-22)16-3-19-35-24-10-6-21(7-11-24)8-13-29-32-26-4-1-2-5-27(26)36-29/h1-2,4-7,9-12,20,22H,3,8,13-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserine from 5-HT2A receptor in rat cerebral cortex homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT2C receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50133183

(CHEMBL3634811)Show SMILES C(COc1ccc(cc1)-c1nc2ccccc2o1)CN1CCN(CC1)c1ncccn1 Show InChI InChI=1S/C24H25N5O2/c1-2-6-22-21(5-1)27-23(31-22)19-7-9-20(10-8-19)30-18-4-13-28-14-16-29(17-15-28)24-25-11-3-12-26-24/h1-3,5-12H,4,13-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserine from 5-HT2A receptor in rat cerebral cortex homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50133177
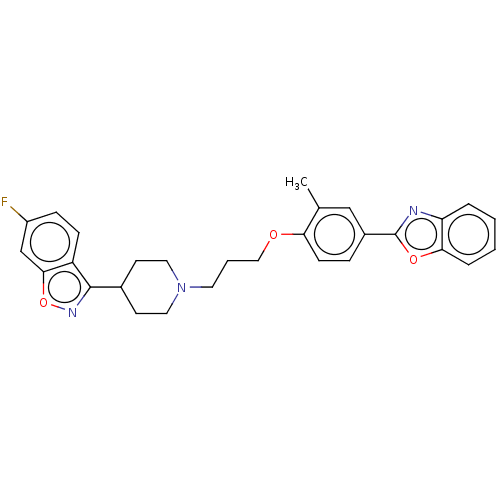
(CHEMBL3634817)Show SMILES Cc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)-c1nc2ccccc2o1 Show InChI InChI=1S/C29H28FN3O3/c1-19-17-21(29-31-24-5-2-3-6-26(24)35-29)7-10-25(19)34-16-4-13-33-14-11-20(12-15-33)28-23-9-8-22(30)18-27(23)36-32-28/h2-3,5-10,17-18,20H,4,11-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserine from 5-HT2A receptor in rat cerebral cortex homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50561098

(CHEMBL4790699)Show SMILES CC(=O)Nc1ccc(OCCCN2CCC(CC2)c2noc3cc(F)ccc23)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT2A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data