

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296336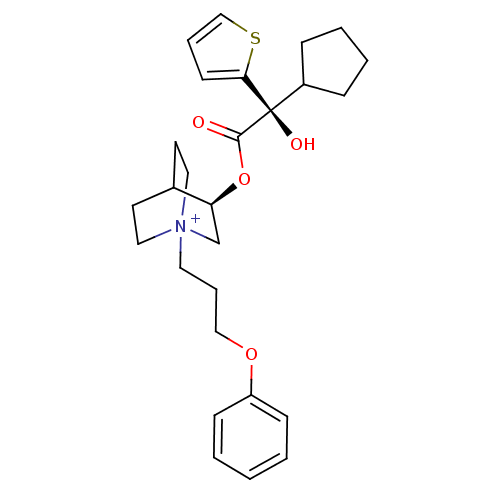 ((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296336 ((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296331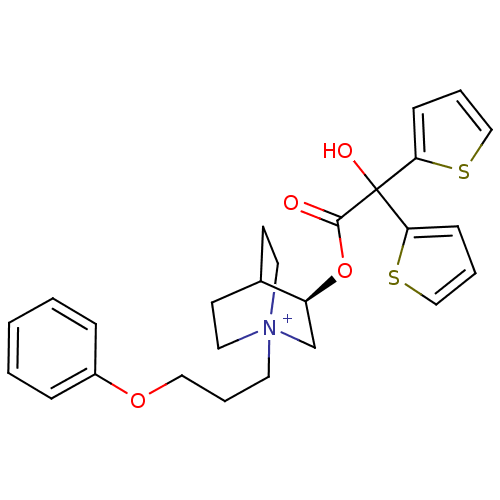 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296331 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296329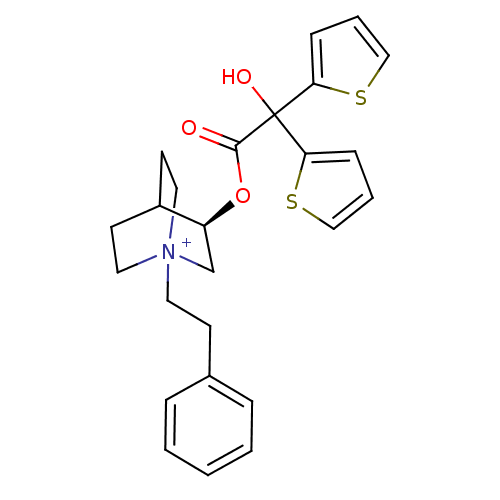 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296329 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296329 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296331 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296336 ((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50457489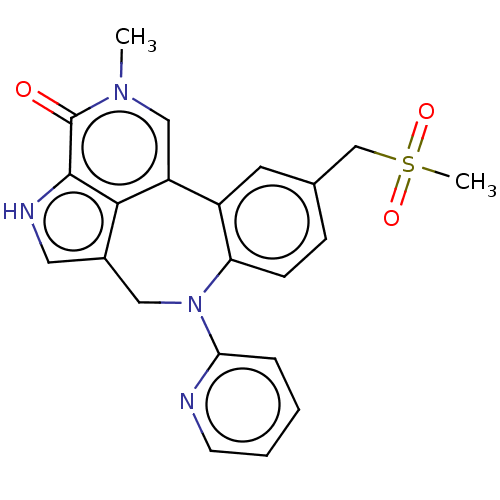 (CHEMBL4208129) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to BRD2 bromodomain 1 to 2 (G73 to A560 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by b... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM439590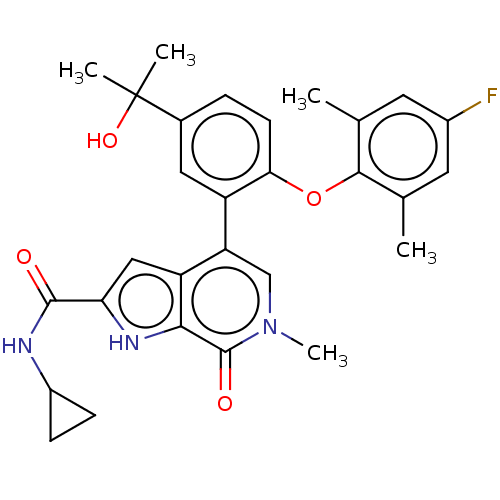 (US10633379, Example 121) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511849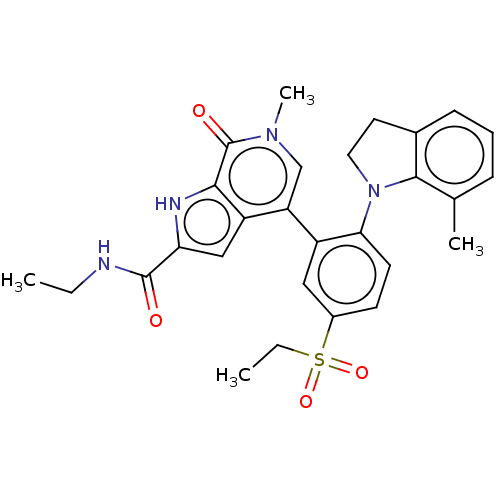 (CHEMBL4465299) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511850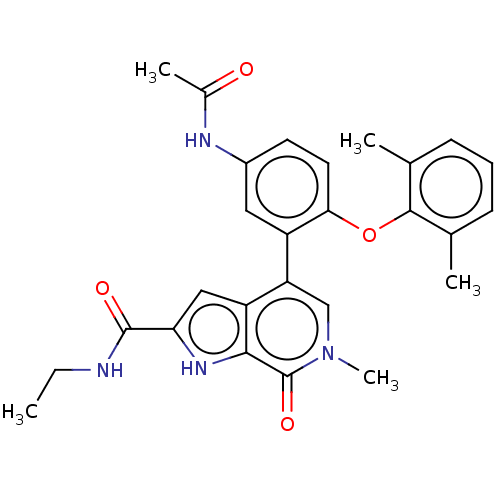 (CHEMBL4435166) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511875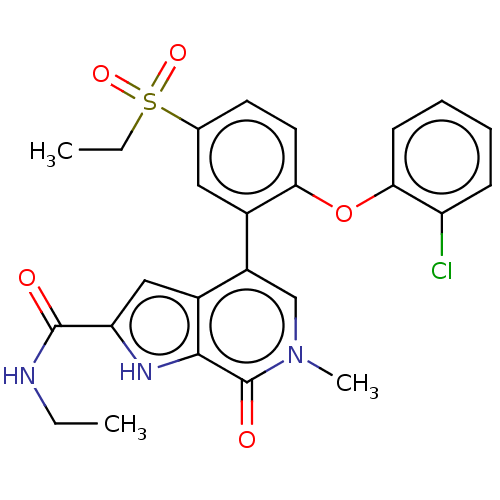 (CHEMBL4548794) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511864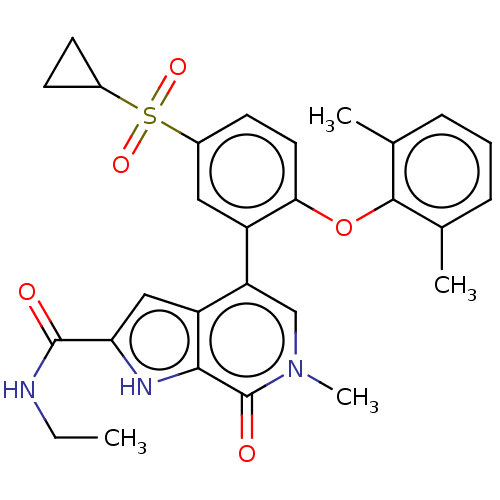 (CHEMBL4564879) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM439461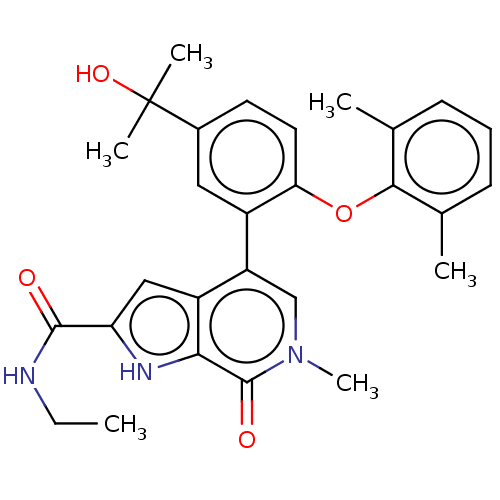 (US10633379, Example 3 | US10633379, Example 68) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50457489 (CHEMBL4208129) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to His-tagged BRD4 bromodomain 1 to 2 (K57 to K550 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50457489 (CHEMBL4208129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to BRDT bromodomain 1 to 2 (N21to P380 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by br... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511855 (CHEMBL4454597) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511870 (CHEMBL4461291) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50457496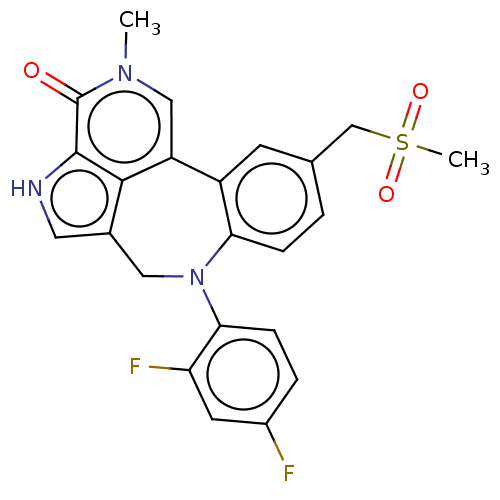 (CHEMBL4217457) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to BRD4 bromodomain 2 (E352 to M457 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by bromo... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511869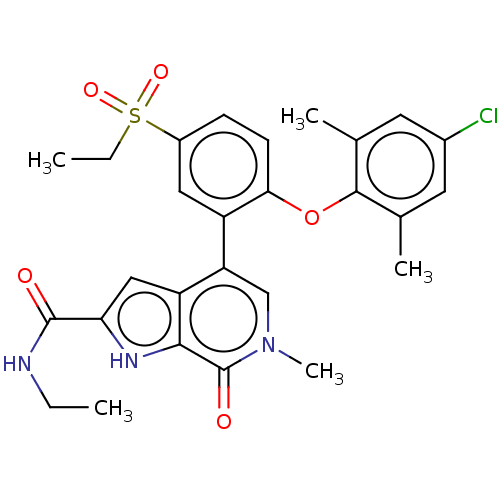 (CHEMBL4548127) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511859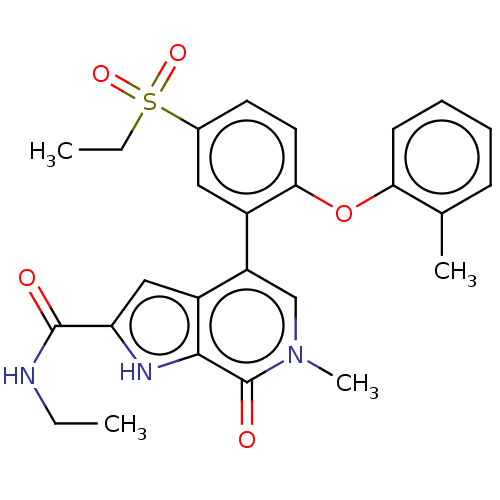 (CHEMBL4470856) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511861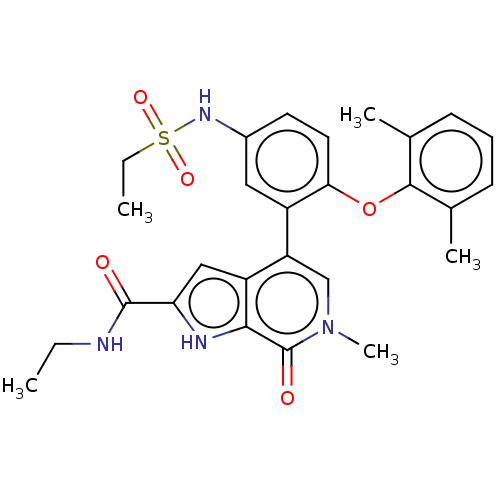 (CHEMBL4529861) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50378083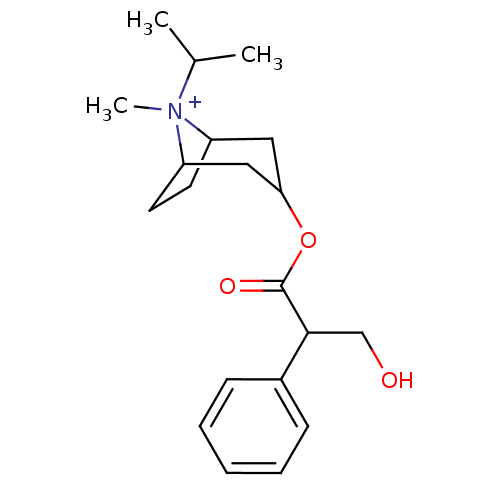 (Atrovent HFA | IPRATROPIUM BROMIDE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50457500 (CHEMBL4212498) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to His-tagged BRD4 bromodomain 1 to 2 (K57 to K550 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50457496 (CHEMBL4217457) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to His-tagged BRD4 bromodomain 1 to 2 (K57 to K550 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511868 (CHEMBL4560574) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511880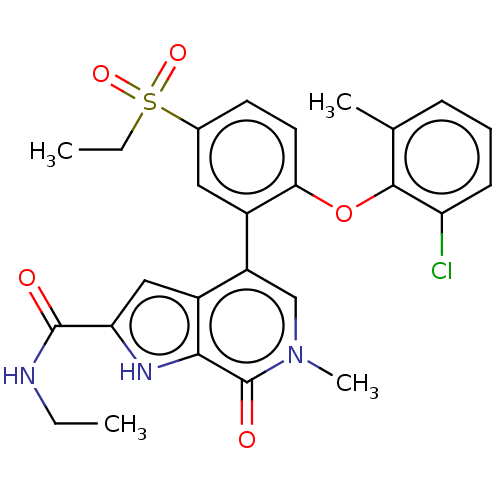 (CHEMBL4469571) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511871 (CHEMBL4454614) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511865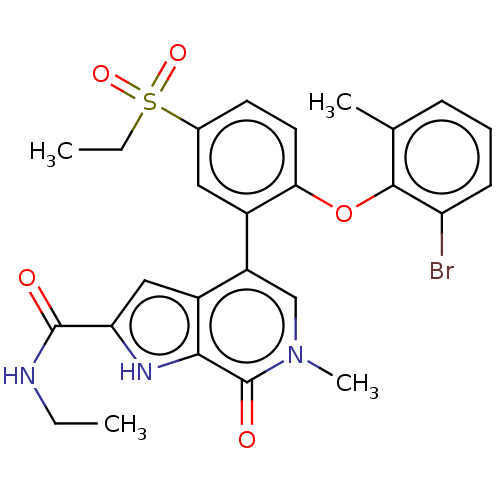 (CHEMBL4434761) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM439497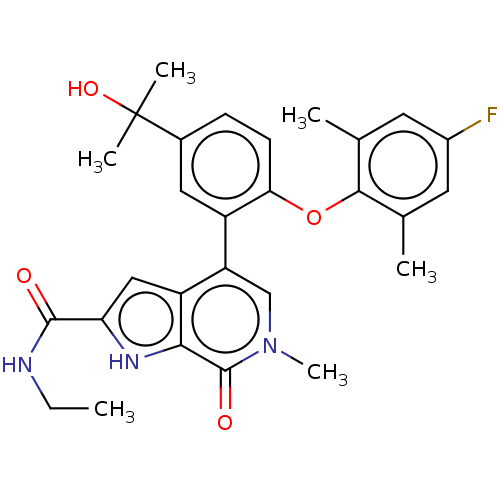 (US10633379, Example 35 | US10633379, Example 82) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of BRDT BD2 (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM439589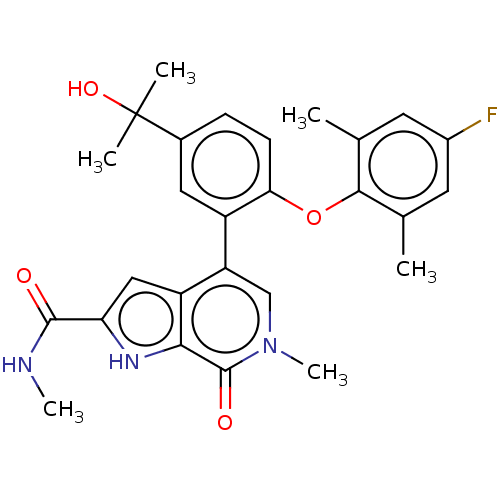 (US10633379, Example 120) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM220447 (US10633379, Compound X | US9296741, 36) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to BRD2 BD1 to BD2 (G73 to A560 residues) (unknown origin) | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50378083 (Atrovent HFA | IPRATROPIUM BROMIDE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 1.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511856 (CHEMBL4466931) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM439592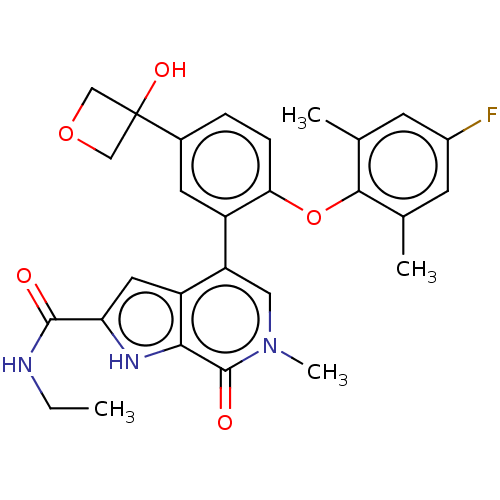 (US10633379, Example 123) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511876 (CHEMBL4462804) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511882 (CHEMBL4539875) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511866 (CHEMBL4461289) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511872 (CHEMBL4443563) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511854 (CHEMBL4471851) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM439494 (US10633379, Example 32) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511853 (CHEMBL4483338) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50457489 (CHEMBL4208129) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to BRD4 bromodomain 1 (K57 to E168 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by bromod... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50457495 (CHEMBL4215988) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to BRD4 bromodomain 1 (K57 to E168 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by bromod... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM439599 (US10633379, Example 129) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3725 total ) | Next | Last >> |