 Found 2093 hits with Last Name = 'cameron' and Initial = 'm'
Found 2093 hits with Last Name = 'cameron' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50531908
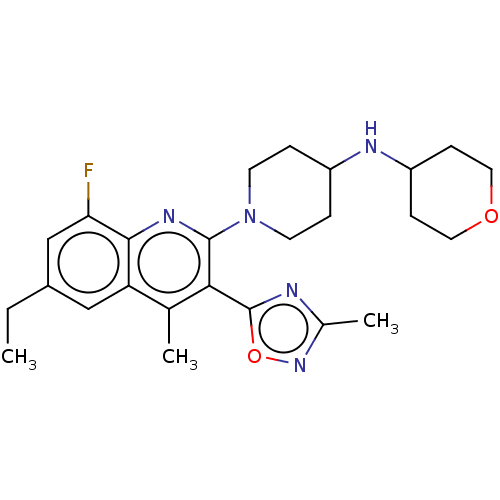
(Btrx-335140 | CYM-53093 | US10676469, Compound 142...)Show SMILES CCc1cc(F)c2nc(N3CCC(CC3)NC3CCOCC3)c(-c3nc(C)no3)c(C)c2c1 Show InChI InChI=1S/C25H32FN5O2/c1-4-17-13-20-15(2)22(25-27-16(3)30-33-25)24(29-23(20)21(26)14-17)31-9-5-18(6-10-31)28-19-7-11-32-12-8-19/h13-14,18-19,28H,4-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human KOR |
J Med Chem 62: 1761-1780 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01679
BindingDB Entry DOI: 10.7270/Q2CV4N6Q |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50609010
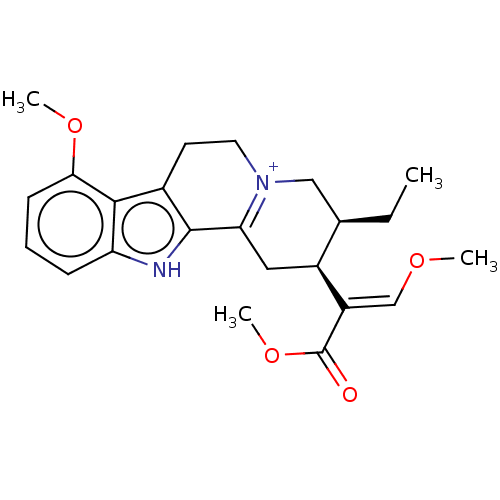
(CHEMBL5276728)Show SMILES CC[C@@H]1C[N+]2=C(C[C@@H]1\C(=C/OC)C(=O)OC)c1[nH]c3cccc(OC)c3c1CC2 |r,c:4| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50609010

(CHEMBL5276728)Show SMILES CC[C@@H]1C[N+]2=C(C[C@@H]1\C(=C/OC)C(=O)OC)c1[nH]c3cccc(OC)c3c1CC2 |r,c:4| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50519927
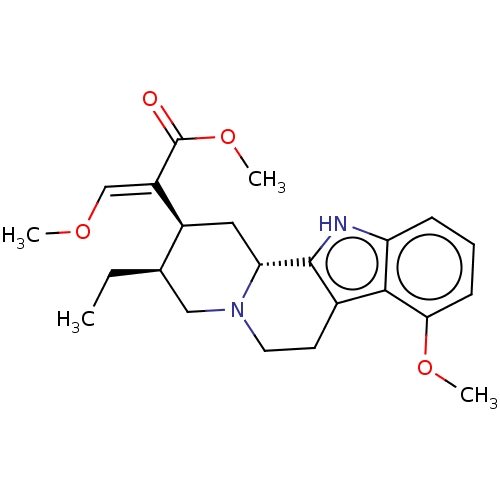
(CHEMBL4546925)Show SMILES [H][C@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C23H30N2O4/c1-5-14-12-25-10-9-15-21-18(7-6-8-20(21)28-3)24-22(15)19(25)11-16(14)17(13-27-2)23(26)29-4/h6-8,13-14,16,19,24H,5,9-12H2,1-4H3/b17-13+/t14-,16+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50609010

(CHEMBL5276728)Show SMILES CC[C@@H]1C[N+]2=C(C[C@@H]1\C(=C/OC)C(=O)OC)c1[nH]c3cccc(OC)c3c1CC2 |r,c:4| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50474151

(CHEMBL292521)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O)c12)C(=C/OC)\C(=O)OC Show InChI InChI=1S/C22H28N2O4/c1-4-13-11-24-9-8-14-20-17(6-5-7-19(20)25)23-21(14)18(24)10-15(13)16(12-27-2)22(26)28-3/h5-7,12-13,15,18,23,25H,4,8-11H2,1-3H3/b16-12+/t13-,15+,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50531908

(Btrx-335140 | CYM-53093 | US10676469, Compound 142...)Show SMILES CCc1cc(F)c2nc(N3CCC(CC3)NC3CCOCC3)c(-c3nc(C)no3)c(C)c2c1 Show InChI InChI=1S/C25H32FN5O2/c1-4-17-13-20-15(2)22(25-27-16(3)30-33-25)24(29-23(20)21(26)14-17)31-9-5-18(6-10-31)28-19-7-11-32-12-8-19/h13-14,18-19,28H,4-12H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human MOR |
J Med Chem 62: 1761-1780 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01679
BindingDB Entry DOI: 10.7270/Q2CV4N6Q |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50566316

(CHEMBL4859858)Show SMILES [H][C@@]12C[C@@H]([C@@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 578 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50519927

(CHEMBL4546925)Show SMILES [H][C@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C23H30N2O4/c1-5-14-12-25-10-9-15-21-18(7-6-8-20(21)28-3)24-22(15)19(25)11-16(14)17(13-27-2)23(26)29-4/h6-8,13-14,16,19,24H,5,9-12H2,1-4H3/b17-13+/t14-,16+,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 649 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50566317
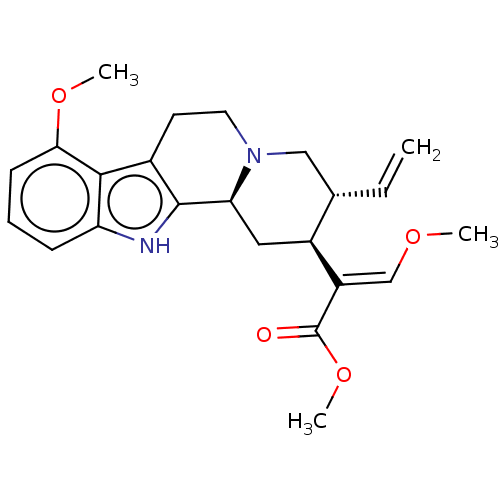
(CHEMBL4848517)Show SMILES [H][C@@]12C[C@@H]([C@H](CN1CCc1c2[nH]c2cccc(OC)c12)C=C)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 666 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50557257

(CHEMBL4789047)Show SMILES Cc1ccc(CN(CC(O)COc2cccc3[nH]ccc23)C(C)(C)C)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to beta1 adrenoceptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01450
BindingDB Entry DOI: 10.7270/Q2MS3XF8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50566317

(CHEMBL4848517)Show SMILES [H][C@@]12C[C@@H]([C@H](CN1CCc1c2[nH]c2cccc(OC)c12)C=C)C(=C/OC)\C(=O)OC |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 883 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50519927

(CHEMBL4546925)Show SMILES [H][C@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C23H30N2O4/c1-5-14-12-25-10-9-15-21-18(7-6-8-20(21)28-3)24-22(15)19(25)11-16(14)17(13-27-2)23(26)29-4/h6-8,13-14,16,19,24H,5,9-12H2,1-4H3/b17-13+/t14-,16+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50474151

(CHEMBL292521)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O)c12)C(=C/OC)\C(=O)OC Show InChI InChI=1S/C22H28N2O4/c1-4-13-11-24-9-8-14-20-17(6-5-7-19(20)25)23-21(14)18(24)10-15(13)16(12-27-2)22(26)28-3/h5-7,12-13,15,18,23,25H,4,8-11H2,1-3H3/b16-12+/t13-,15+,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50609011
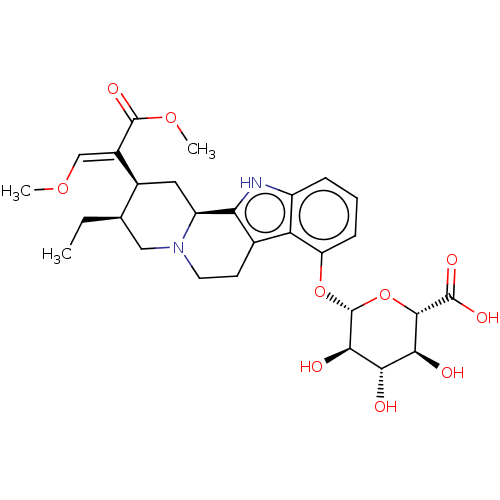
(CHEMBL5287921)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)c12)C(=C/OC)\C(=O)OC |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50557257

(CHEMBL4789047)Show SMILES Cc1ccc(CN(CC(O)COc2cccc3[nH]ccc23)C(C)(C)C)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to beta2 adrenoceptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01450
BindingDB Entry DOI: 10.7270/Q2MS3XF8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50609011

(CHEMBL5287921)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)c12)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50474151

(CHEMBL292521)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O)c12)C(=C/OC)\C(=O)OC Show InChI InChI=1S/C22H28N2O4/c1-4-13-11-24-9-8-14-20-17(6-5-7-19(20)25)23-21(14)18(24)10-15(13)16(12-27-2)22(26)28-3/h5-7,12-13,15,18,23,25H,4,8-11H2,1-3H3/b16-12+/t13-,15+,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50557257

(CHEMBL4789047)Show SMILES Cc1ccc(CN(CC(O)COc2cccc3[nH]ccc23)C(C)(C)C)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT1A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01450
BindingDB Entry DOI: 10.7270/Q2MS3XF8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50566316

(CHEMBL4859858)Show SMILES [H][C@@]12C[C@@H]([C@@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50609011

(CHEMBL5287921)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)c12)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50566317

(CHEMBL4848517)Show SMILES [H][C@@]12C[C@@H]([C@H](CN1CCc1c2[nH]c2cccc(OC)c12)C=C)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50609009
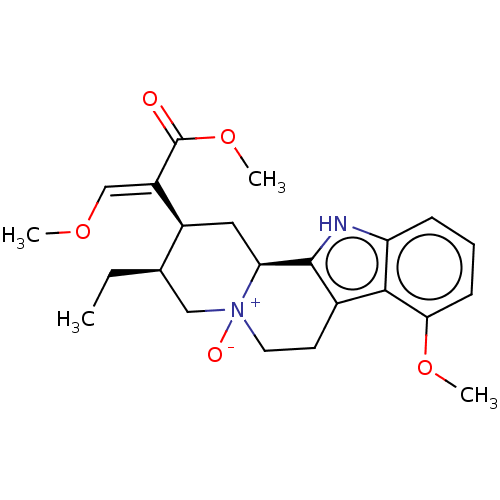
(CHEMBL56717)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)C[N+]1([O-])CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50609009

(CHEMBL56717)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)C[N+]1([O-])CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50609009

(CHEMBL56717)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)C[N+]1([O-])CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 7.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50566316

(CHEMBL4859858)Show SMILES [H][C@@]12C[C@@H]([C@@H](CC)CN1CCc1c2[nH]c2cccc(OC)c12)C(=C/OC)\C(=O)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | 7.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14 [G110A]
(Mus musculus (mouse)) | BDBM15240
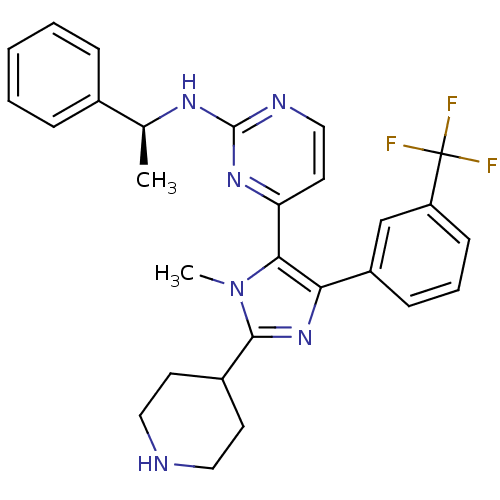
(4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...)Show SMILES C[C@H](Nc1nccc(n1)-c1c(nc(C2CCNCC2)n1C)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C28H29F3N6/c1-18(19-7-4-3-5-8-19)34-27-33-16-13-23(35-27)25-24(21-9-6-10-22(17-21)28(29,30)31)36-26(37(25)2)20-11-14-32-15-12-20/h3-10,13,16-18,20,32H,11-12,14-15H2,1-2H3,(H,33,34,35)/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with wild-type or mutant p38alpha enzymes, and substrates in the presence ATP/[gam... |
Nat Struct Biol 10: 764-9 (2003)
Article DOI: 10.1038/nsb949
BindingDB Entry DOI: 10.7270/Q2SX6BF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122386
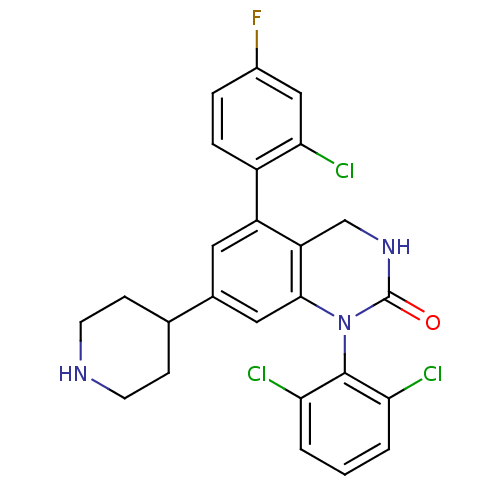
(5-(2-Chloro-4-fluoro-phenyl)-1-(2,6-dichloro-pheny...)Show SMILES Fc1ccc(c(Cl)c1)-c1cc(cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl)C1CCNCC1 Show InChI InChI=1S/C25H21Cl3FN3O/c26-20-2-1-3-21(27)24(20)32-23-11-15(14-6-8-30-9-7-14)10-18(19(23)13-31-25(32)33)17-5-4-16(29)12-22(17)28/h1-5,10-12,14,30H,6-9,13H2,(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122387

(5-(2-Chloro-4-fluoro-phenyl)-1-(2,6-dichloro-pheny...)Show SMILES Fc1ccc(c(Cl)c1)-c1cc(OC2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H21Cl3FN3O2/c26-20-2-1-3-21(27)24(20)32-23-12-16(34-15-6-8-30-9-7-15)11-18(19(23)13-31-25(32)33)17-5-4-14(29)10-22(17)28/h1-5,10-12,15,30H,6-9,13H2,(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122385
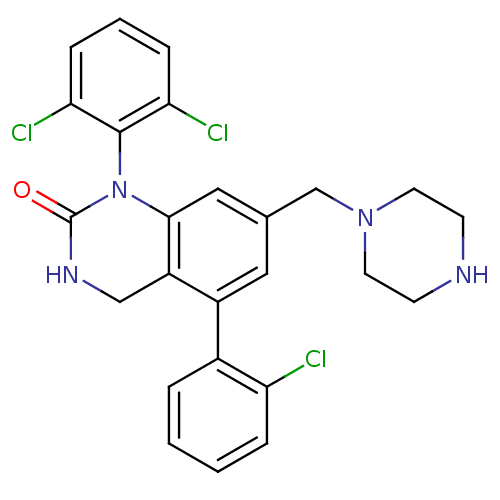
(5-(2-Chloro-phenyl)-1-(2,6-dichloro-phenyl)-7-pipe...)Show SMILES Clc1ccccc1-c1cc(CN2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H23Cl3N4O/c26-20-5-2-1-4-17(20)18-12-16(15-31-10-8-29-9-11-31)13-23-19(18)14-30-25(33)32(23)24-21(27)6-3-7-22(24)28/h1-7,12-13,29H,8-11,14-15H2,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122390
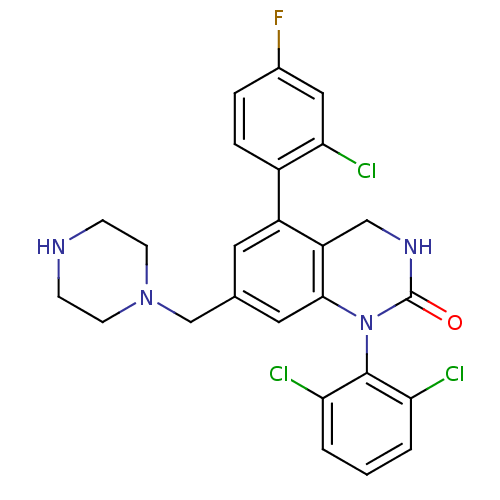
(5-(2-Chloro-4-fluoro-phenyl)-1-(2,6-dichloro-pheny...)Show SMILES Fc1ccc(c(Cl)c1)-c1cc(CN2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H22Cl3FN4O/c26-20-2-1-3-21(27)24(20)33-23-11-15(14-32-8-6-30-7-9-32)10-18(19(23)13-31-25(33)34)17-5-4-16(29)12-22(17)28/h1-5,10-12,30H,6-9,13-14H2,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147275

(US8957093, 97)Show SMILES CC[C@@H](NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1)c1ccccc1 |r| Show InChI InChI=1S/C34H32N2O3/c1-4-31(26-10-6-5-7-11-26)35-33(37)27-18-19-32-30(20-27)22(2)23(3)36(32)21-24-14-16-25(17-15-24)28-12-8-9-13-29(28)34(38)39/h5-20,31H,4,21H2,1-3H3,(H,35,37)(H,38,39)/t31-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PPAR-gamma-LBD (unknown origin) after 2 hrs by Lantha screen assay using fluormone Pan-PPAR green probe |
ACS Med Chem Lett 6: 998-1003 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00218
BindingDB Entry DOI: 10.7270/Q29026SZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM15240

(4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...)Show SMILES C[C@H](Nc1nccc(n1)-c1c(nc(C2CCNCC2)n1C)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C28H29F3N6/c1-18(19-7-4-3-5-8-19)34-27-33-16-13-23(35-27)25-24(21-9-6-10-22(17-21)28(29,30)31)36-26(37(25)2)20-11-14-32-15-12-20/h3-10,13,16-18,20,32H,11-12,14-15H2,1-2H3,(H,33,34,35)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with wild-type or mutant p38alpha enzymes, and substrates in the presence ATP/[gam... |
Nat Struct Biol 10: 764-9 (2003)
Article DOI: 10.1038/nsb949
BindingDB Entry DOI: 10.7270/Q2SX6BF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14 [G110D]
(Mus musculus (mouse)) | BDBM15240

(4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...)Show SMILES C[C@H](Nc1nccc(n1)-c1c(nc(C2CCNCC2)n1C)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C28H29F3N6/c1-18(19-7-4-3-5-8-19)34-27-33-16-13-23(35-27)25-24(21-9-6-10-22(17-21)28(29,30)31)36-26(37(25)2)20-11-14-32-15-12-20/h3-10,13,16-18,20,32H,11-12,14-15H2,1-2H3,(H,33,34,35)/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with wild-type or mutant p38alpha enzymes, and substrates in the presence ATP/[gam... |
Nat Struct Biol 10: 764-9 (2003)
Article DOI: 10.1038/nsb949
BindingDB Entry DOI: 10.7270/Q2SX6BF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM17060
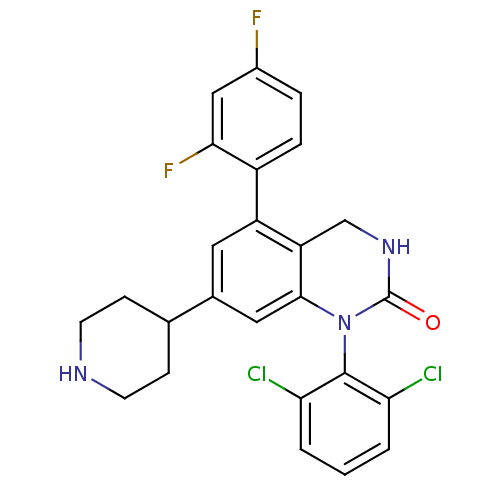
(1-(2,6-dichlorophenyl)-5-(2,4-difluorophenyl)-7-(p...)Show SMILES Fc1ccc(c(F)c1)-c1cc(cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl)C1CCNCC1 Show InChI InChI=1S/C25H21Cl2F2N3O/c26-20-2-1-3-21(27)24(20)32-23-11-15(14-6-8-30-9-7-14)10-18(19(23)13-31-25(32)33)17-5-4-16(28)12-22(17)29/h1-5,10-12,14,30H,6-9,13H2,(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122384
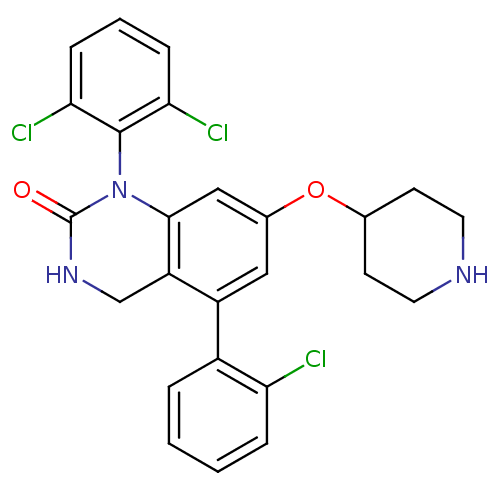
(5-(2-Chloro-phenyl)-1-(2,6-dichloro-phenyl)-7-(pip...)Show SMILES Clc1ccccc1-c1cc(OC2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H22Cl3N3O2/c26-20-5-2-1-4-17(20)18-12-16(33-15-8-10-29-11-9-15)13-23-19(18)14-30-25(32)31(23)24-21(27)6-3-7-22(24)28/h1-7,12-13,15,29H,8-11,14H2,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122379

(7-(1-tert-Butyl-piperidin-4-yl)-5-(2-chloro-4-fluo...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc2N(C(=O)NCc2c(c1)-c1ccc(F)cc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C29H29Cl3FN3O/c1-29(2,3)35-11-9-17(10-12-35)18-13-21(20-8-7-19(33)15-25(20)32)22-16-34-28(37)36(26(22)14-18)27-23(30)5-4-6-24(27)31/h4-8,13-15,17H,9-12,16H2,1-3H3,(H,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50531921
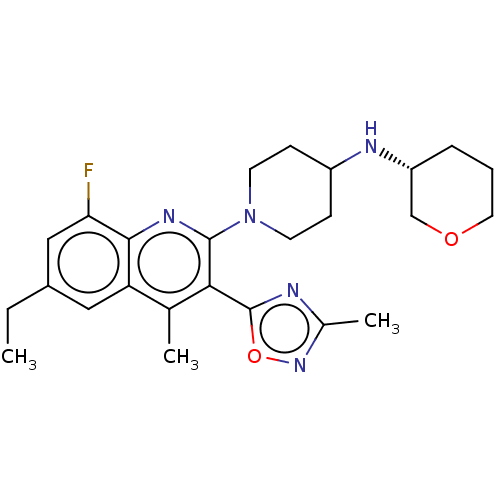
(CHEMBL4544914 | US10676469, Compound 148 | US11124...)Show SMILES CCc1cc(F)c2nc(N3CCC(CC3)N[C@@H]3CCCOC3)c(-c3nc(C)no3)c(C)c2c1 |r| Show InChI InChI=1S/C25H32FN5O2/c1-4-17-12-20-15(2)22(25-27-16(3)30-33-25)24(29-23(20)21(26)13-17)31-9-7-18(8-10-31)28-19-6-5-11-32-14-19/h12-13,18-19,28H,4-11,14H2,1-3H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... |
J Med Chem 62: 1761-1780 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01679
BindingDB Entry DOI: 10.7270/Q2CV4N6Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122382

(5-(2-Chloro-4-fluoro-phenyl)-7-[1-(1-cyclopropyl-e...)Show SMILES CC(C1CC1)N1CCC(CC1)c1cc2N(C(=O)NCc2c(c1)-c1ccc(F)cc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C30H29Cl3FN3O/c1-17(18-5-6-18)36-11-9-19(10-12-36)20-13-23(22-8-7-21(34)15-27(22)33)24-16-35-30(38)37(28(24)14-20)29-25(31)3-2-4-26(29)32/h2-4,7-8,13-15,17-19H,5-6,9-12,16H2,1H3,(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50306508
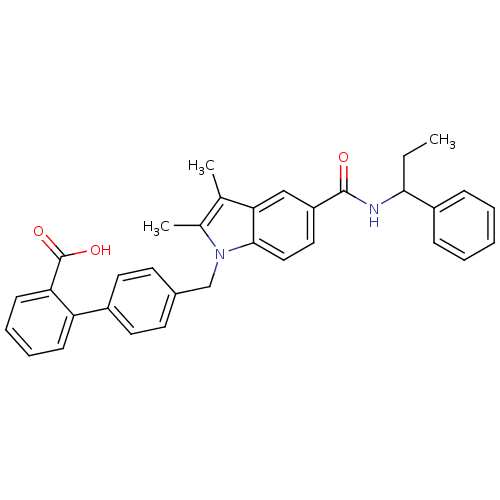
(4'-((2,3-dimethyl-5-(1-phenylpropylcarbamoyl)-1H-i...)Show SMILES CCC(NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1)c1ccccc1 Show InChI InChI=1S/C34H32N2O3/c1-4-31(26-10-6-5-7-11-26)35-33(37)27-18-19-32-30(20-27)22(2)23(3)36(32)21-24-14-16-25(17-15-24)28-12-8-9-13-29(28)34(38)39/h5-20,31H,4,21H2,1-3H3,(H,35,37)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PPAR-gamma-LBD (unknown origin) after 2 hrs by Lantha screen assay using fluormone Pan-PPAR green probe |
ACS Med Chem Lett 6: 998-1003 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00218
BindingDB Entry DOI: 10.7270/Q29026SZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50311713
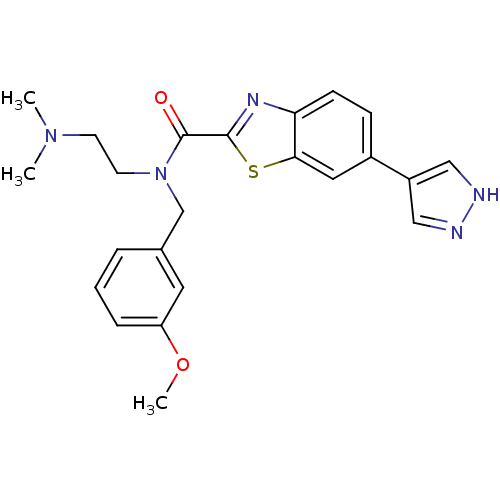
(CHEMBL1080279 | N-(2-(dimethylamino)ethyl)-N-(3-me...)Show SMILES COc1cccc(CN(CCN(C)C)C(=O)c2nc3ccc(cc3s2)-c2cn[nH]c2)c1 Show InChI InChI=1S/C23H25N5O2S/c1-27(2)9-10-28(15-16-5-4-6-19(11-16)30-3)23(29)22-26-20-8-7-17(12-21(20)31-22)18-13-24-25-14-18/h4-8,11-14H,9-10,15H2,1-3H3,(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute and Department of Molecular Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 19: 6686-90 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.115
BindingDB Entry DOI: 10.7270/Q2VX0GN0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122377

(5-(2-Chloro-4-fluoro-phenyl)-7-(1-cyclobutyl-piper...)Show SMILES Fc1ccc(c(Cl)c1)-c1cc(cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl)C1CCN(CC1)C1CCC1 Show InChI InChI=1S/C29H27Cl3FN3O/c30-24-5-2-6-25(31)28(24)36-27-14-18(17-9-11-35(12-10-17)20-3-1-4-20)13-22(23(27)16-34-29(36)37)21-8-7-19(33)15-26(21)32/h2,5-8,13-15,17,20H,1,3-4,9-12,16H2,(H,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122395

(5-(2-Chloro-4-fluoro-phenyl)-7-(1-cyclopropylmethy...)Show SMILES Fc1ccc(c(Cl)c1)-c1cc(cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl)C1CCN(CC2CC2)CC1 Show InChI InChI=1S/C29H27Cl3FN3O/c30-24-2-1-3-25(31)28(24)36-27-13-19(18-8-10-35(11-9-18)16-17-4-5-17)12-22(23(27)15-34-29(36)37)21-7-6-20(33)14-26(21)32/h1-3,6-7,12-14,17-18H,4-5,8-11,15-16H2,(H,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122405

(5-(2-Chloro-4-fluoro-phenyl)-1-(2,6-dichloro-pheny...)Show SMILES CN1CCC(CC1)c1cc2N(C(=O)NCc2c(c1)-c1ccc(F)cc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C26H23Cl3FN3O/c1-32-9-7-15(8-10-32)16-11-19(18-6-5-17(30)13-23(18)29)20-14-31-26(34)33(24(20)12-16)25-21(27)3-2-4-22(25)28/h2-6,11-13,15H,7-10,14H2,1H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122391

(5-(2-Chloro-phenyl)-1-(2,6-dichloro-phenyl)-7-(pip...)Show SMILES Clc1ccccc1-c1cc(NC2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H23Cl3N4O/c26-20-5-2-1-4-17(20)18-12-16(31-15-8-10-29-11-9-15)13-23-19(18)14-30-25(33)32(23)24-21(27)6-3-7-22(24)28/h1-7,12-13,15,29,31H,8-11,14H2,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50531915
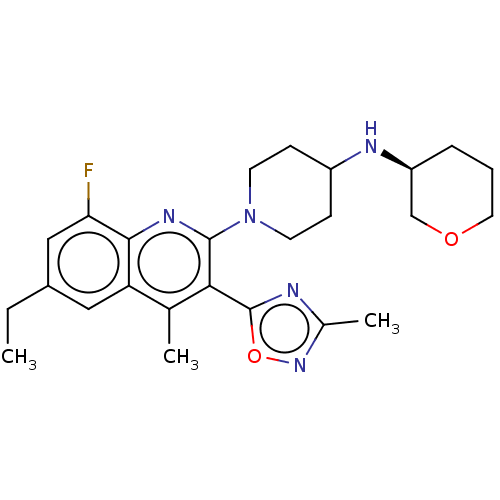
(CHEMBL4440683 | US10676469, Compound 144 | US11124...)Show SMILES CCc1cc(F)c2nc(N3CCC(CC3)N[C@H]3CCCOC3)c(-c3nc(C)no3)c(C)c2c1 |r| Show InChI InChI=1S/C25H32FN5O2/c1-4-17-12-20-15(2)22(25-27-16(3)30-33-25)24(29-23(20)21(26)13-17)31-9-7-18(8-10-31)28-19-6-5-11-32-14-19/h12-13,18-19,28H,4-11,14H2,1-3H3/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... |
J Med Chem 62: 1761-1780 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01679
BindingDB Entry DOI: 10.7270/Q2CV4N6Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147214
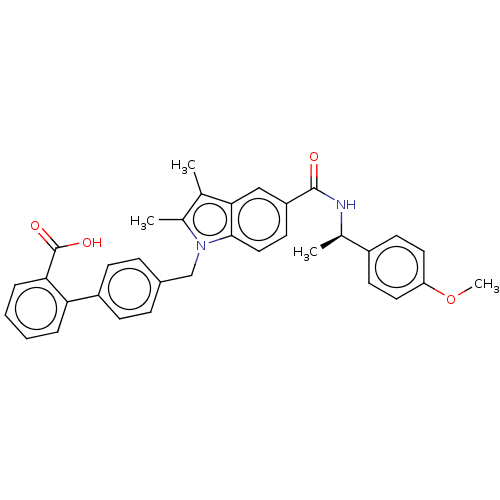
(US8957093, 23)Show SMILES COc1ccc(cc1)[C@@H](C)NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1 |r| Show InChI InChI=1S/C34H32N2O4/c1-21-23(3)36(20-24-9-11-26(12-10-24)29-7-5-6-8-30(29)34(38)39)32-18-15-27(19-31(21)32)33(37)35-22(2)25-13-16-28(40-4)17-14-25/h5-19,22H,20H2,1-4H3,(H,35,37)(H,38,39)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PPAR-gamma-LBD (unknown origin) after 2 hrs by Lantha screen assay using fluormone Pan-PPAR green probe |
ACS Med Chem Lett 6: 998-1003 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00218
BindingDB Entry DOI: 10.7270/Q29026SZ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50531910

(CHEMBL4576339)Show SMILES CCc1ccc2nc(N3CCC(CC3)N[C@H]3CCCC[C@@H]3O)c(cc2c1)-c1nc(C)no1 |r| Show InChI InChI=1S/C25H33N5O2/c1-3-17-8-9-21-18(14-17)15-20(25-26-16(2)29-32-25)24(28-21)30-12-10-19(11-13-30)27-22-6-4-5-7-23(22)31/h8-9,14-15,19,22-23,27,31H,3-7,10-13H2,1-2H3/t22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... |
J Med Chem 62: 1761-1780 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01679
BindingDB Entry DOI: 10.7270/Q2CV4N6Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122383
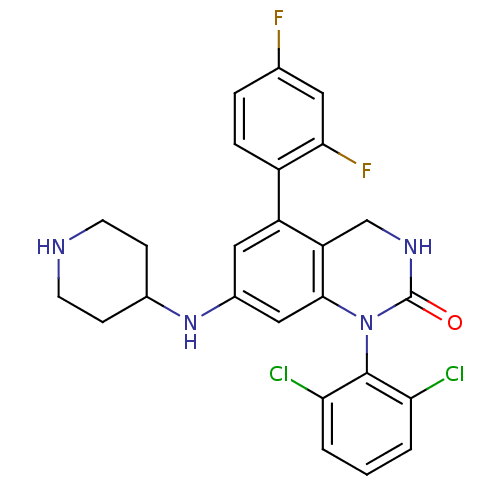
(1-(2,6-Dichloro-phenyl)-5-(2,4-difluoro-phenyl)-7-...)Show SMILES Fc1ccc(c(F)c1)-c1cc(NC2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H22Cl2F2N4O/c26-20-2-1-3-21(27)24(20)33-23-12-16(32-15-6-8-30-9-7-15)11-18(19(23)13-31-25(33)34)17-5-4-14(28)10-22(17)29/h1-5,10-12,15,30,32H,6-9,13H2,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122389
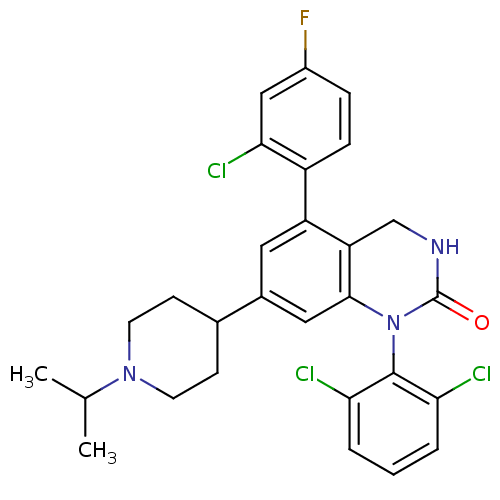
(5-(2-Chloro-4-fluoro-phenyl)-1-(2,6-dichloro-pheny...)Show SMILES CC(C)N1CCC(CC1)c1cc2N(C(=O)NCc2c(c1)-c1ccc(F)cc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C28H27Cl3FN3O/c1-16(2)34-10-8-17(9-11-34)18-12-21(20-7-6-19(32)14-25(20)31)22-15-33-28(36)35(26(22)13-18)27-23(29)4-3-5-24(27)30/h3-7,12-14,16-17H,8-11,15H2,1-2H3,(H,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data