

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277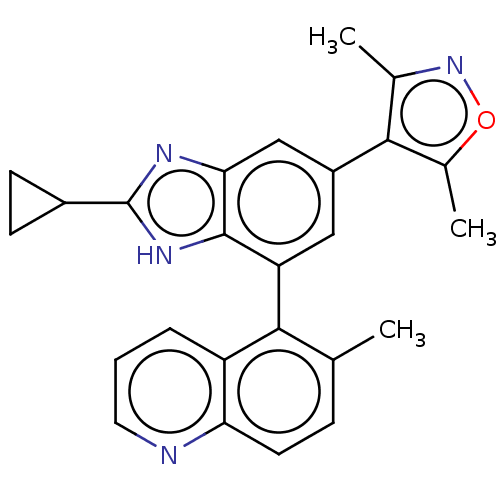 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain2 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Central University Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor | Bioorg Med Chem Lett 19: 1022-5 (2009) Article DOI: 10.1016/j.bmcl.2008.11.029 BindingDB Entry DOI: 10.7270/Q2Z89C8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50243745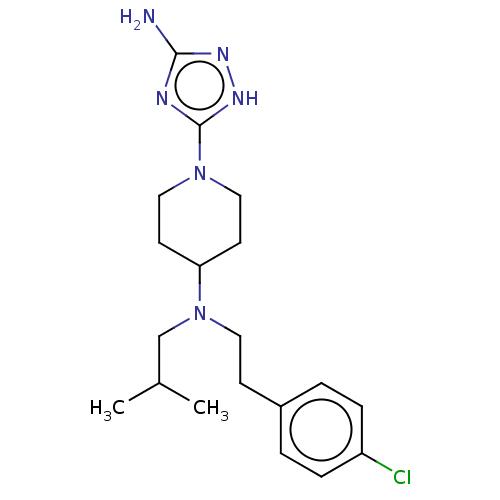 (CHEMBL4076989) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of mouse recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243745 (CHEMBL4076989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of tetra-acetylated Histone H4 peptide binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50324701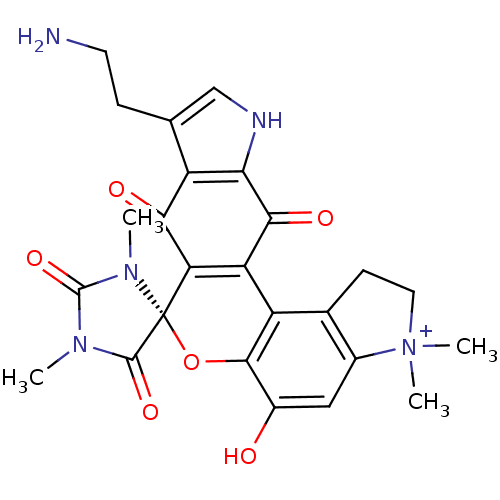 (CHEMBL1221472 | Exiguamine A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase | Nat Chem Biol 4: 535-7 (2008) Article DOI: 10.1038/nchembio.107 BindingDB Entry DOI: 10.7270/Q2P55NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21975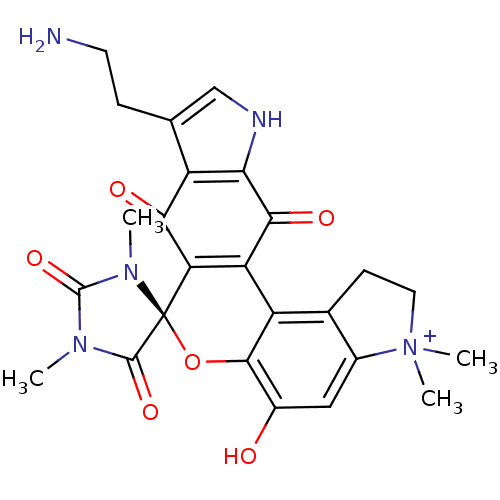 ((4S)-16'-(2-aminoethyl)-9'-hydroxy-1,3,6',6'-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 41 | -43.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50380682 (CHEMBL2017291 | I-BET151 (16)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of tetra-acetylated Histone H4 peptide binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50324700 (CHEMBL1221412 | Exiguamine B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase | Nat Chem Biol 4: 535-7 (2008) Article DOI: 10.1038/nchembio.107 BindingDB Entry DOI: 10.7270/Q2P55NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50324699 (CHEMBL1221473 | Seco-exiguamine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase | Nat Chem Biol 4: 535-7 (2008) Article DOI: 10.1038/nchembio.107 BindingDB Entry DOI: 10.7270/Q2P55NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain2 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of tetra-acetylated Histone H4 peptide binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21979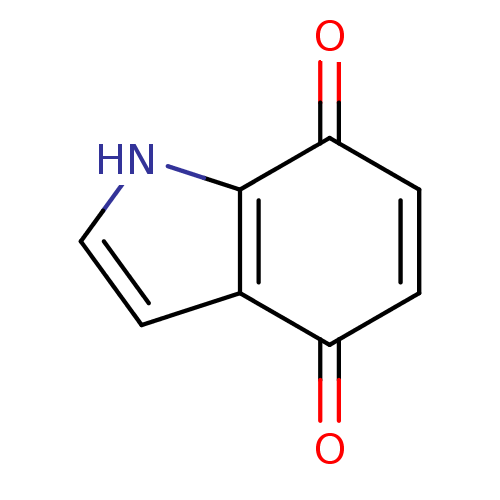 (4,7-dihydro-1H-indole-4,7-dione | Indolequinone, 1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 190 | -39.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21982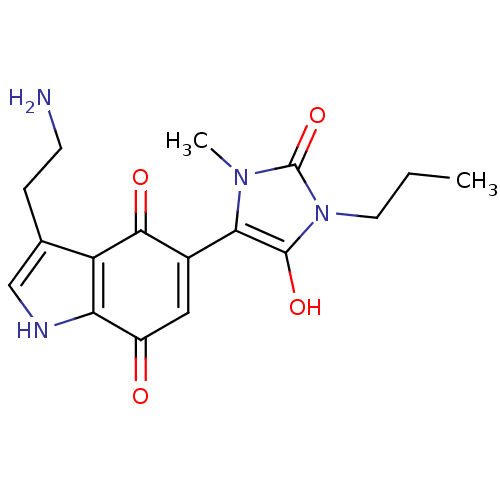 (3-(2-aminoethyl)-5-(3-methyl-2,5-dioxo-1-propylimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 200 | -39.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21981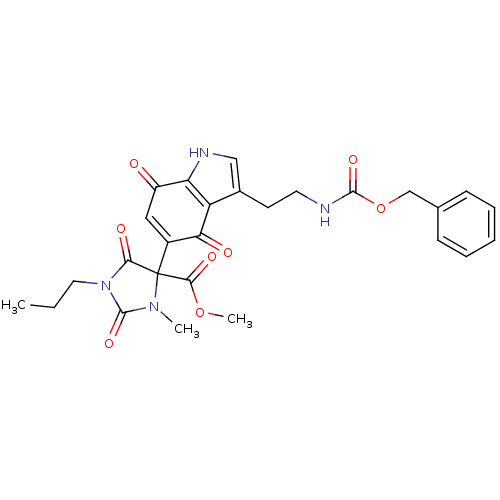 (Tryptamine quinone, 21 | methyl 4-[3-(2-{[(benzylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 260 | -39.1 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21984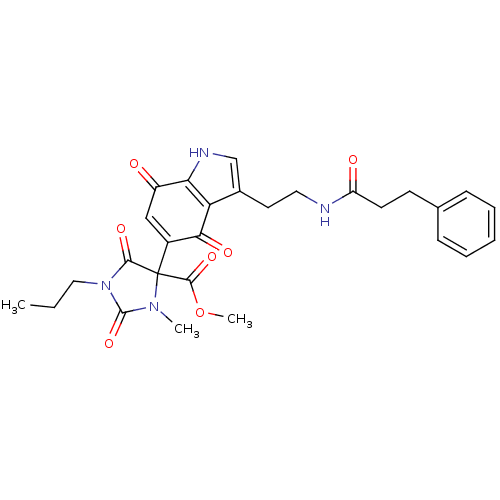 (Tryptamine quinone, 25 | methyl 4-{4,7-dioxo-3-[2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 260 | -39.1 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50243745 (CHEMBL4076989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged chitotriosidase expressed in CHO-K1 cells using 4-methylumbelliferyl-beta-D-N,N',N\... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50380682 (CHEMBL2017291 | I-BET151 (16)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 385 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain2 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21980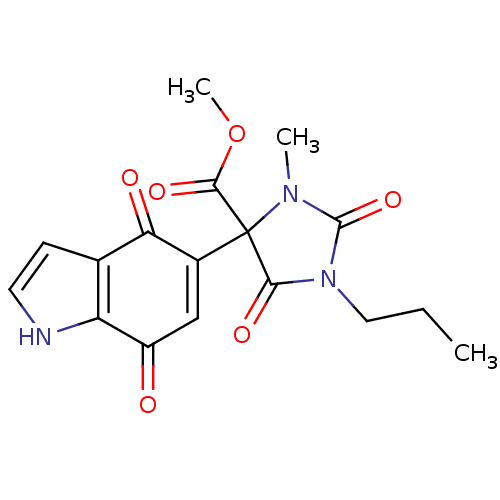 (Indolequinone, 20 | methyl 4-(4,7-dioxo-4,7-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 420 | -37.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21976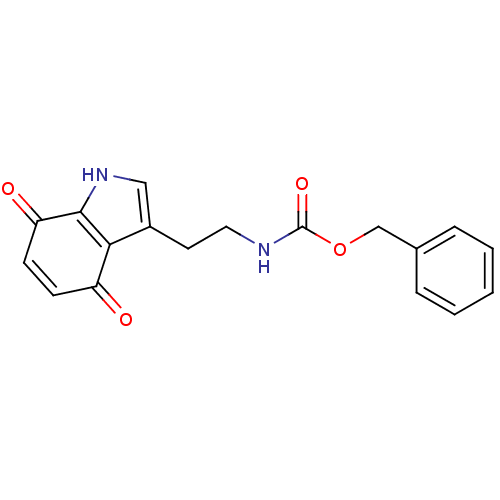 (Tryptamine quinone, 9 | benzyl N-[2-(4,7-dioxo-4,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.49E+3 | -34.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Mus musculus) | BDBM50243745 (CHEMBL4076989) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of mouse recombinant full length C-terminal His-tagged chitotriosidase expressed in CHO-K1 cells using 4-methylumbelliferyl-beta-D-N,N',N\... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21977 (Tryptamine quinone, 13 | benzyl N-{2-[5-(1,3-dioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.09E+4 | -29.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21973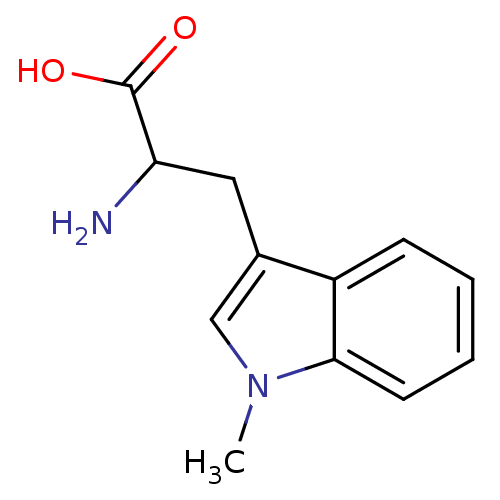 (1-Methyltryptophan, 1 | 2-amino-3-(1-methyl-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO using L-tryptophan as substrate | Bioorg Med Chem Lett 24: 3403-6 (2014) Article DOI: 10.1016/j.bmcl.2014.05.084 BindingDB Entry DOI: 10.7270/Q23R0VH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21973 (1-Methyltryptophan, 1 | 2-amino-3-(1-methyl-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.20E+4 | -25.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin reductase 2 (Human) | BDBM616841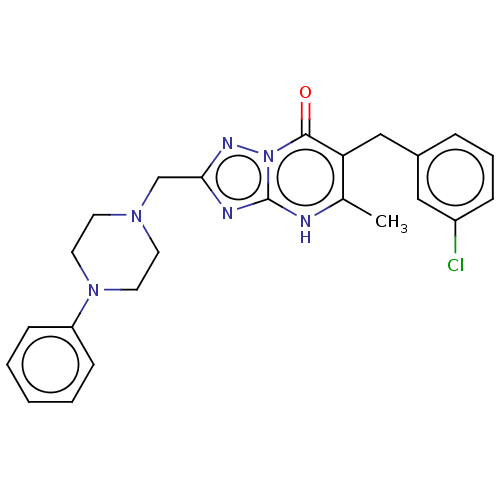 (6-(3-chlorobenzyl)-5-methyl-2-((4-phenylpiperazin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27P93JH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin reductase 2 (Human) | BDBM616837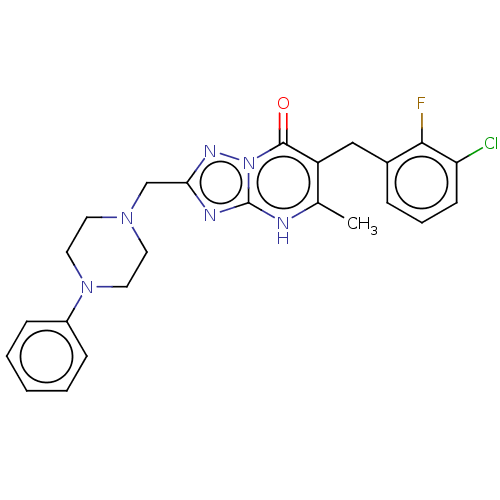 (6-(3-chloro-2-fluorobenzyl)-5-methyl-2-((4-phenylp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27P93JH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin reductase 2 (Human) | BDBM616833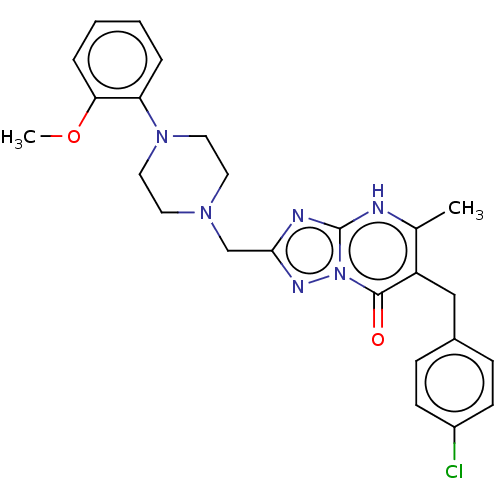 (6-(4-chlorobenzyl)-2-((4-(2-methoxyphenyl)piperazi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27P93JH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50241089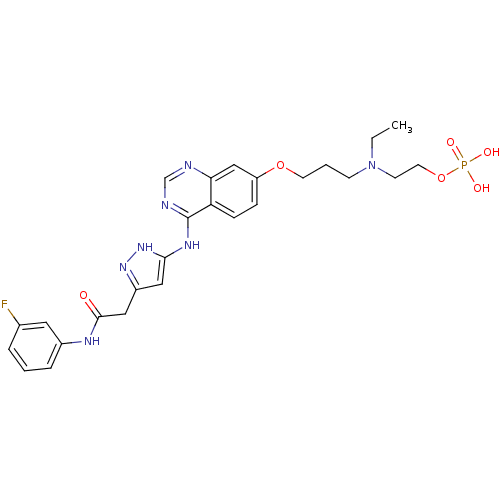 (2-(ethyl(3-(4-(5-(2-(3-fluorophenylamino)-2-oxoeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant GST-tagged N-terminal truncated human Aurora A (123 to 401 residues) expressed in Sf9 insect cell using tetra-LRRASLG pepti... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01806 BindingDB Entry DOI: 10.7270/Q2Q2444S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin reductase 2 (Human) | BDBM616851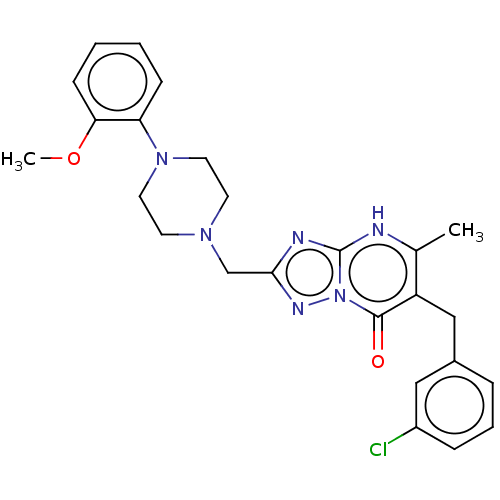 (6-(3-chlorobenzyl)-2-((4-(2-methoxyphenyl)piperazi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27P93JH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50533742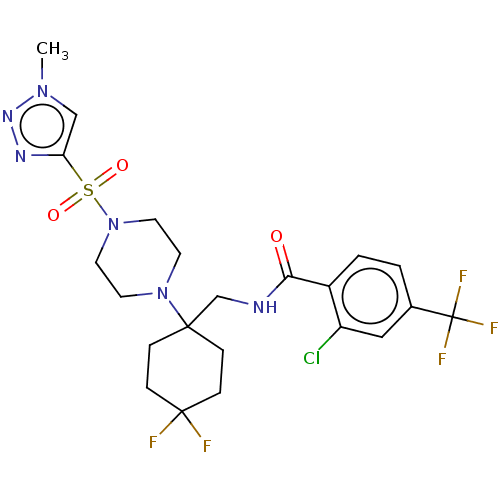 (CHEMBL4593786) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.671 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Inhibition of glycine transporter-1B in human JAR cells assessed as reduction in [14C]glycine uptake preincubated for 10 mins followed by [14C]glycin... | J Med Chem 59: 8473-94 (2016) Article DOI: 10.1021/acs.jmedchem.6b00914 BindingDB Entry DOI: 10.7270/Q23R0XC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50533732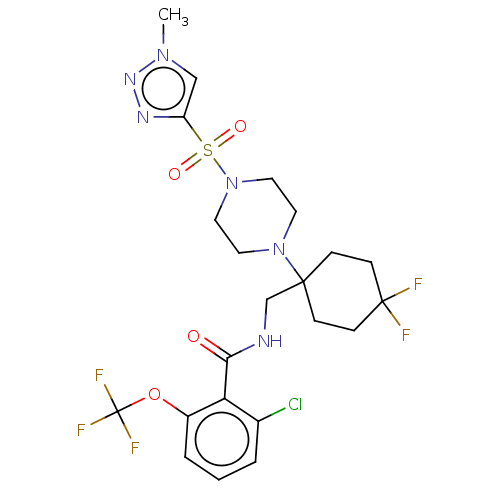 (CHEMBL4526580) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.726 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Inhibition of glycine transporter-1B in human JAR cells assessed as reduction in [14C]glycine uptake preincubated for 10 mins followed by [14C]glycin... | J Med Chem 59: 8473-94 (2016) Article DOI: 10.1021/acs.jmedchem.6b00914 BindingDB Entry DOI: 10.7270/Q23R0XC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50533748 (CHEMBL4468117) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.733 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Inhibition of glycine transporter-1B in human JAR cells assessed as reduction in [14C]glycine uptake preincubated for 10 mins followed by [14C]glycin... | J Med Chem 59: 8473-94 (2016) Article DOI: 10.1021/acs.jmedchem.6b00914 BindingDB Entry DOI: 10.7270/Q23R0XC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1B (Homo sapiens (Human)) | BDBM50262079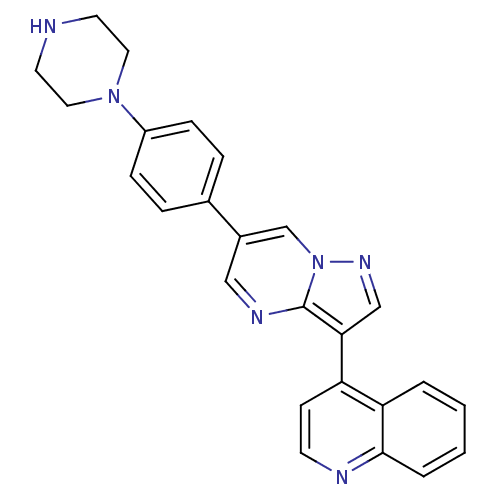 (4-(6-(4-(piperazin-1-yl)phenyl)pyrazolo[1,5-a]pyri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human ALK6 using casein as substrate in presence of 10 uM ATP by radiometric kinase assay | J Med Chem 60: 1495-1508 (2017) Article DOI: 10.1021/acs.jmedchem.6b01679 BindingDB Entry DOI: 10.7270/Q2TQ63S4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 3 (Homo sapiens (Human)) | BDBM50413752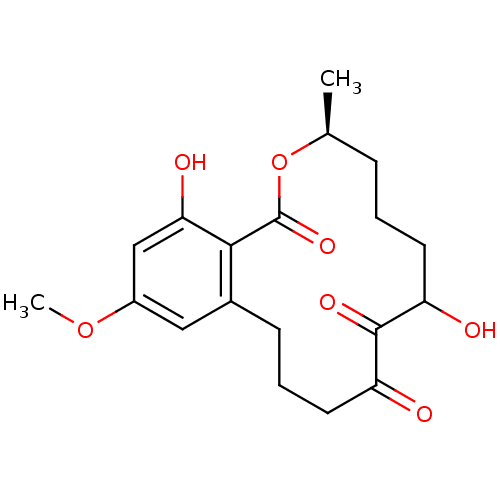 (CHEMBL2012519 | L-783277) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human FLT4 using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-33P]-ATP by scintillation counting method | J Med Chem 60: 1495-1508 (2017) Article DOI: 10.1021/acs.jmedchem.6b01679 BindingDB Entry DOI: 10.7270/Q2TQ63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50533727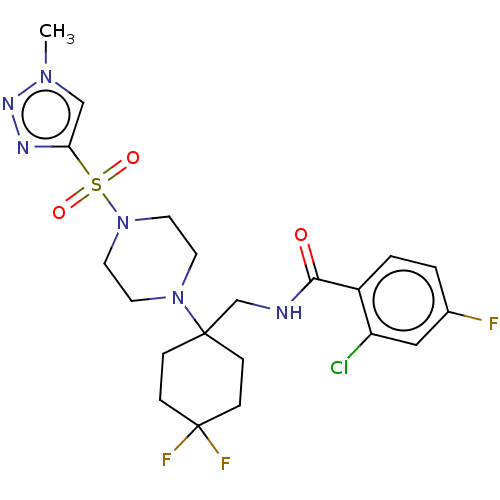 (CHEMBL4456392) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Inhibition of glycine transporter-1B in human JAR cells assessed as reduction in [14C]glycine uptake preincubated for 10 mins followed by [14C]glycin... | J Med Chem 59: 8473-94 (2016) Article DOI: 10.1021/acs.jmedchem.6b00914 BindingDB Entry DOI: 10.7270/Q23R0XC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50253806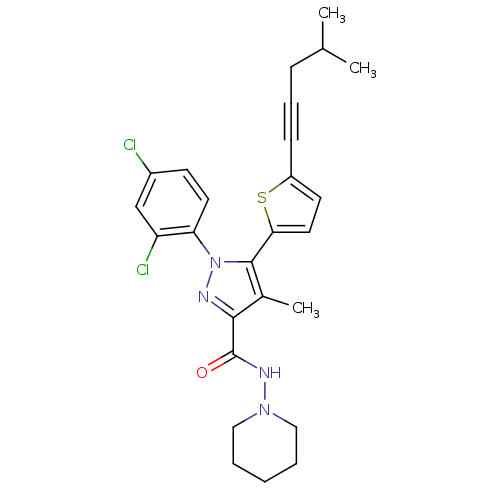 (1-(2,4-Dichlorophenyl)-4-methyl-5-(5-(4-methylpent...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cells | J Med Chem 51: 5397-412 (2008) Article DOI: 10.1021/jm800066v BindingDB Entry DOI: 10.7270/Q24M94BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50253844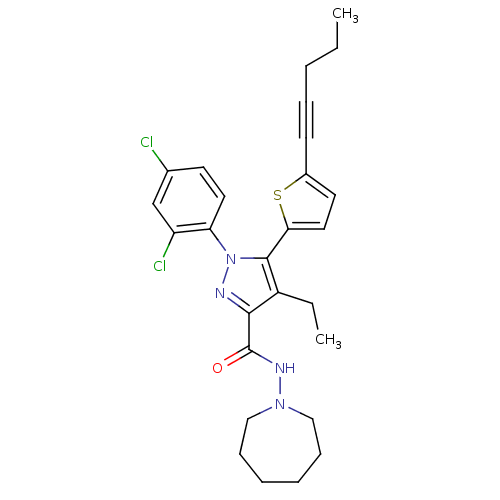 (CHEMBL460987 | N-(Azepan-1-yl)-1-(2,4-dichlorophen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cells | J Med Chem 51: 5397-412 (2008) Article DOI: 10.1021/jm800066v BindingDB Entry DOI: 10.7270/Q24M94BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50253843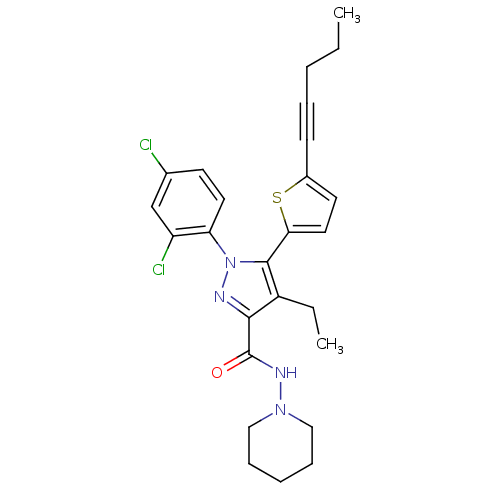 (1-(2,4-Dichlorophenyl)-4-ethyl-5-(5-(pent-1-ynyl)t...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cells | J Med Chem 51: 5397-412 (2008) Article DOI: 10.1021/jm800066v BindingDB Entry DOI: 10.7270/Q24M94BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50253807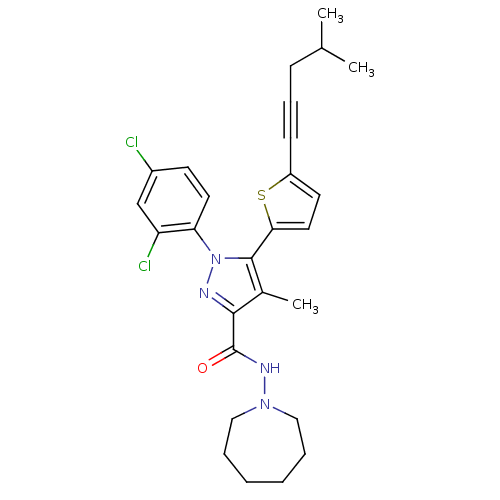 (CHEMBL462686 | N-(Azepan-1-yl)-1-(2,4-dichlorophen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cells | J Med Chem 51: 5397-412 (2008) Article DOI: 10.1021/jm800066v BindingDB Entry DOI: 10.7270/Q24M94BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50533730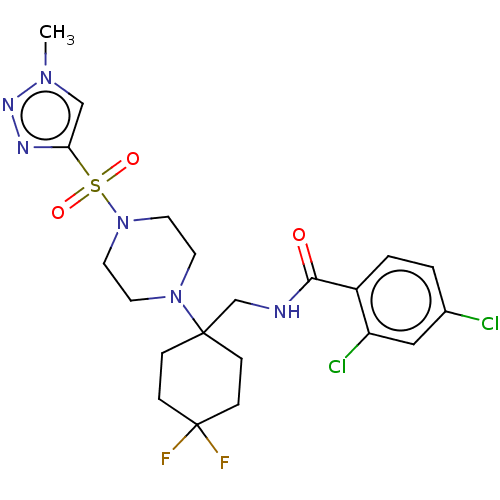 (CHEMBL4582587) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Inhibition of glycine transporter-1B in human JAR cells assessed as reduction in [14C]glycine uptake preincubated for 10 mins followed by [14C]glycin... | J Med Chem 59: 8473-94 (2016) Article DOI: 10.1021/acs.jmedchem.6b00914 BindingDB Entry DOI: 10.7270/Q23R0XC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50253805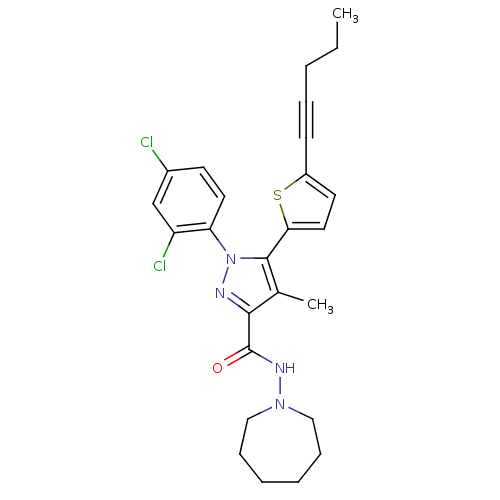 (CHEMBL460132 | N-(Azepan-1-yl)-1-(2,4-dichlorophen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cells | J Med Chem 51: 5397-412 (2008) Article DOI: 10.1021/jm800066v BindingDB Entry DOI: 10.7270/Q24M94BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50253758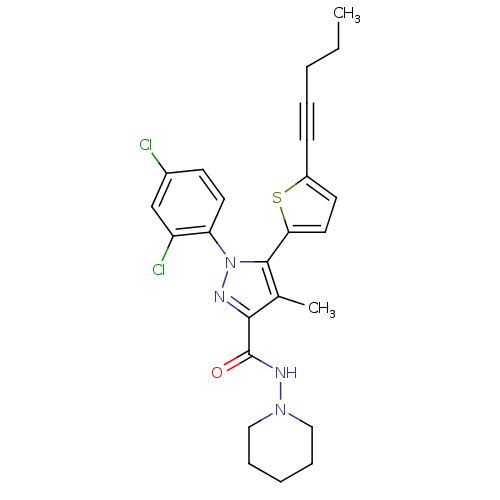 (1-(2,4-Dichlorophenyl)-4-methyl-5-(5-(pent-1-ynyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cells | J Med Chem 51: 5397-412 (2008) Article DOI: 10.1021/jm800066v BindingDB Entry DOI: 10.7270/Q24M94BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50533749 (CHEMBL4516089) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Inhibition of glycine transporter-1B in human JAR cells assessed as reduction in [14C]glycine uptake preincubated for 10 mins followed by [14C]glycin... | J Med Chem 59: 8473-94 (2016) Article DOI: 10.1021/acs.jmedchem.6b00914 BindingDB Entry DOI: 10.7270/Q23R0XC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50253918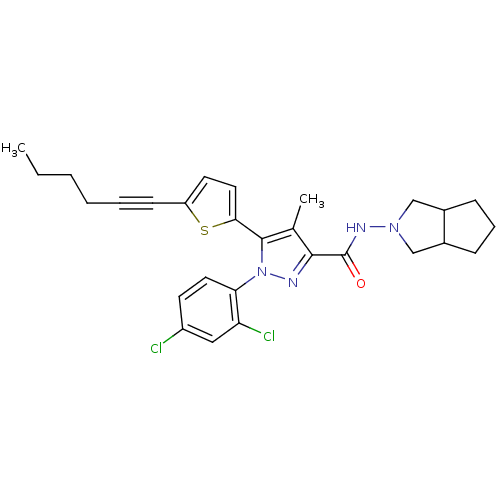 (1-(2,4-Dichlorophenyl)-5-(5-(hex-1-ynyl)thiophen-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 51: 5397-412 (2008) Article DOI: 10.1021/jm800066v BindingDB Entry DOI: 10.7270/Q24M94BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50253917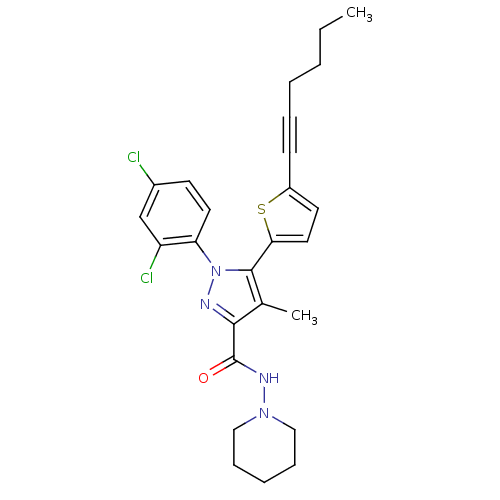 (1-(2,4-Dichlorophenyl)-5-(5-(hex-1-ynyl)thiophen-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 51: 5397-412 (2008) Article DOI: 10.1021/jm800066v BindingDB Entry DOI: 10.7270/Q24M94BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50533725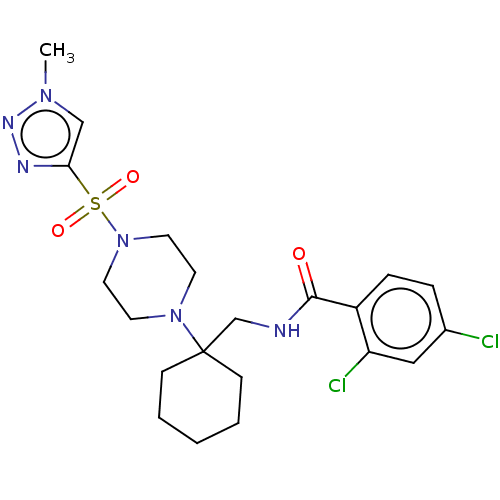 (CHEMBL4579165) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Inhibition of glycine transporter-1B in human JAR cells assessed as reduction in [14C]glycine uptake preincubated for 10 mins followed by [14C]glycin... | J Med Chem 59: 8473-94 (2016) Article DOI: 10.1021/acs.jmedchem.6b00914 BindingDB Entry DOI: 10.7270/Q23R0XC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50533723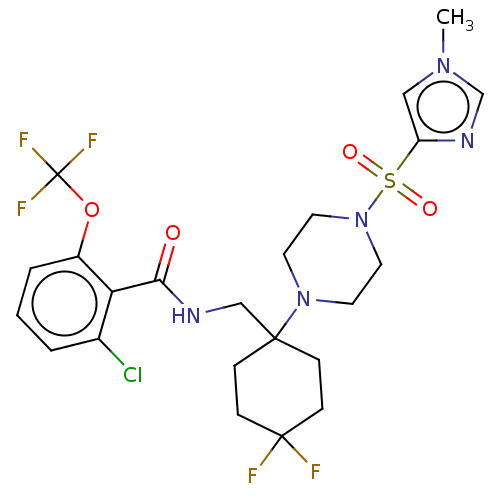 (CHEMBL4572086) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Inhibition of glycine transporter-1B in human JAR cells assessed as reduction in [14C]glycine uptake preincubated for 10 mins followed by [14C]glycin... | J Med Chem 59: 8473-94 (2016) Article DOI: 10.1021/acs.jmedchem.6b00914 BindingDB Entry DOI: 10.7270/Q23R0XC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50413752 (CHEMBL2012519 | L-783277) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human FLT3 using EAIYAAPFAKKK peptide as substrate in presence of [gamma-33P]-ATP by scintillation counting method | J Med Chem 60: 1495-1508 (2017) Article DOI: 10.1021/acs.jmedchem.6b01679 BindingDB Entry DOI: 10.7270/Q2TQ63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1744 total ) | Next | Last >> |