 Found 503 hits with Last Name = 'ipek' and Initial = 'm'
Found 503 hits with Last Name = 'ipek' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Collagenase 3
(Homo sapiens (Human)) | BDBM50356214

(CHEMBL1910471)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2cccc(c2)C#N)cc1)C(O)=O |r| Show InChI InChI=1S/C25H24N2O5S/c1-17(2)24(25(28)29)27-33(30,31)23-12-8-21(9-13-23)20-6-10-22(11-7-20)32-16-19-5-3-4-18(14-19)15-26/h3-14,17,24,27H,16H2,1-2H3,(H,28,29)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356215

(CHEMBL1910473)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2ccc(cc2)C#N)cc1)C(O)=O |r| Show InChI InChI=1S/C25H24N2O5S/c1-17(2)24(25(28)29)27-33(30,31)23-13-9-21(10-14-23)20-7-11-22(12-8-20)32-16-19-5-3-18(15-26)4-6-19/h3-14,17,24,27H,16H2,1-2H3,(H,28,29)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356209
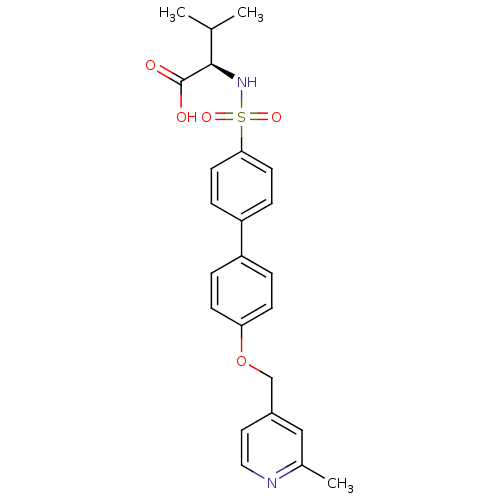
(CHEMBL1910466)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2ccnc(C)c2)cc1)C(O)=O |r| Show InChI InChI=1S/C24H26N2O5S/c1-16(2)23(24(27)28)26-32(29,30)22-10-6-20(7-11-22)19-4-8-21(9-5-19)31-15-18-12-13-25-17(3)14-18/h4-14,16,23,26H,15H2,1-3H3,(H,27,28)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356210
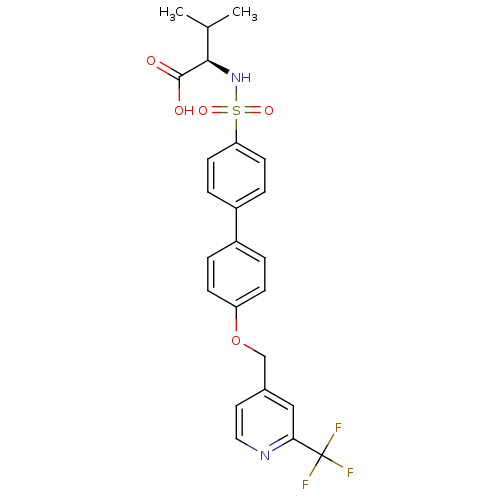
(CHEMBL1910467)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2ccnc(c2)C(F)(F)F)cc1)C(O)=O |r| Show InChI InChI=1S/C24H23F3N2O5S/c1-15(2)22(23(30)31)29-35(32,33)20-9-5-18(6-10-20)17-3-7-19(8-4-17)34-14-16-11-12-28-21(13-16)24(25,26)27/h3-13,15,22,29H,14H2,1-2H3,(H,30,31)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50259007
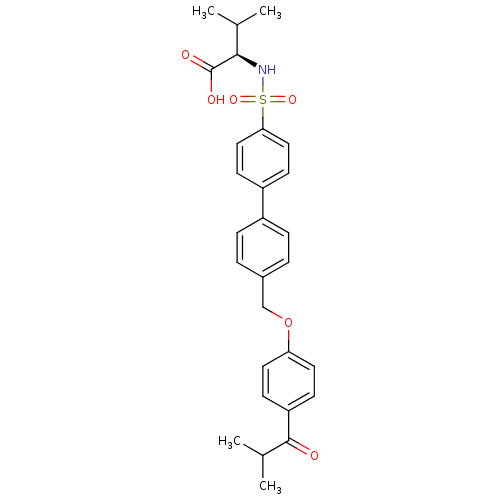
((R)-2-(4'-((4-isobutyrylphenoxy)methyl)biphenyl-4-...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc(cc2)C(=O)C(C)C)cc1)C(O)=O |r| Show InChI InChI=1S/C28H31NO6S/c1-18(2)26(28(31)32)29-36(33,34)25-15-11-22(12-16-25)21-7-5-20(6-8-21)17-35-24-13-9-23(10-14-24)27(30)19(3)4/h5-16,18-19,26,29H,17H2,1-4H3,(H,31,32)/t26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356208

(CHEMBL1910465)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2ccncc2)cc1)C(O)=O |r| Show InChI InChI=1S/C23H24N2O5S/c1-16(2)22(23(26)27)25-31(28,29)21-9-5-19(6-10-21)18-3-7-20(8-4-18)30-15-17-11-13-24-14-12-17/h3-14,16,22,25H,15H2,1-2H3,(H,26,27)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50259007

((R)-2-(4'-((4-isobutyrylphenoxy)methyl)biphenyl-4-...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc(cc2)C(=O)C(C)C)cc1)C(O)=O |r| Show InChI InChI=1S/C28H31NO6S/c1-18(2)26(28(31)32)29-36(33,34)25-15-11-22(12-16-25)21-7-5-20(6-8-21)17-35-24-13-9-23(10-14-24)27(30)19(3)4/h5-16,18-19,26,29H,17H2,1-4H3,(H,31,32)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356218
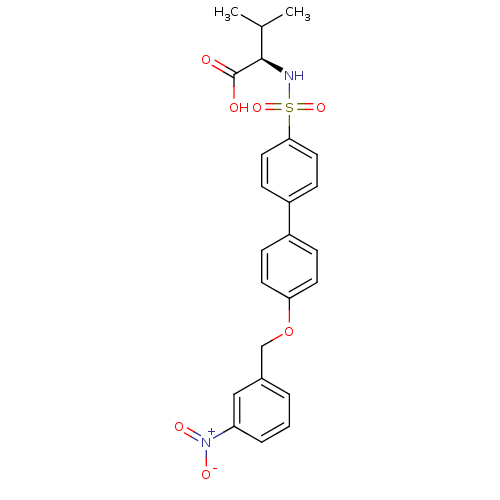
(CHEMBL1910476)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2cccc(c2)[N+]([O-])=O)cc1)C(O)=O |r| Show InChI InChI=1S/C24H24N2O7S/c1-16(2)23(24(27)28)25-34(31,32)22-12-8-19(9-13-22)18-6-10-21(11-7-18)33-15-17-4-3-5-20(14-17)26(29)30/h3-14,16,23,25H,15H2,1-2H3,(H,27,28)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356206
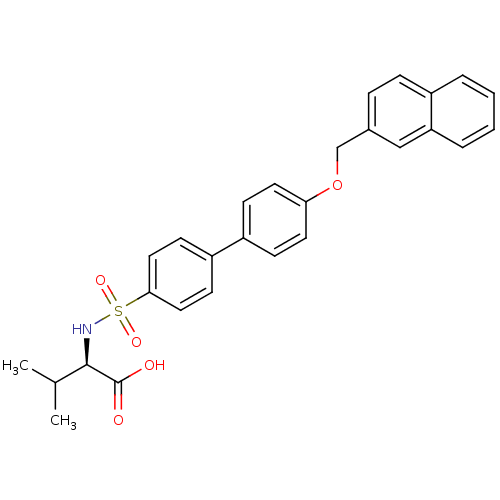
(CHEMBL1910459)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2ccc3ccccc3c2)cc1)C(O)=O |r| Show InChI InChI=1S/C28H27NO5S/c1-19(2)27(28(30)31)29-35(32,33)26-15-11-23(12-16-26)22-9-13-25(14-10-22)34-18-20-7-8-21-5-3-4-6-24(21)17-20/h3-17,19,27,29H,18H2,1-2H3,(H,30,31)/t27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356222

(CHEMBL1910648)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2cccc(Cl)c2)cc1)C(O)=O |r| Show InChI InChI=1S/C24H24ClNO5S/c1-16(2)23(24(27)28)26-32(29,30)22-12-8-19(9-13-22)18-6-10-21(11-7-18)31-15-17-4-3-5-20(25)14-17/h3-14,16,23,26H,15H2,1-2H3,(H,27,28)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50426487
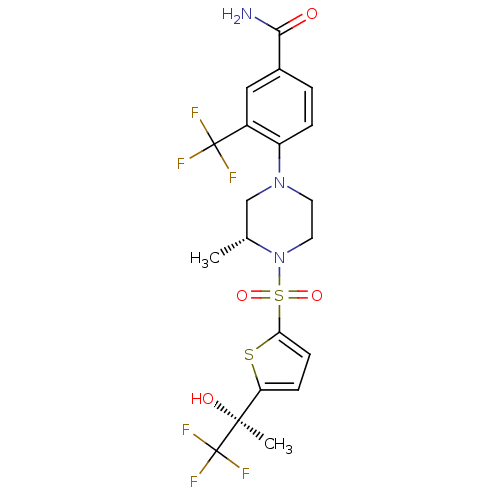
(CHEMBL2322708)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(s1)[C@](C)(O)C(F)(F)F)c1ccc(cc1C(F)(F)F)C(N)=O |r| Show InChI InChI=1S/C20H21F6N3O4S2/c1-11-10-28(14-4-3-12(17(27)30)9-13(14)19(21,22)23)7-8-29(11)35(32,33)16-6-5-15(34-16)18(2,31)20(24,25)26/h3-6,9,11,31H,7-8,10H2,1-2H3,(H2,27,30)/t11-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells using cortisol as substrate incubated for 30 mins prior to substrate addition measured after 1... |
ACS Med Chem Lett 4: 118-23 (2013)
Article DOI: 10.1021/ml300352x
BindingDB Entry DOI: 10.7270/Q25B03TM |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356216
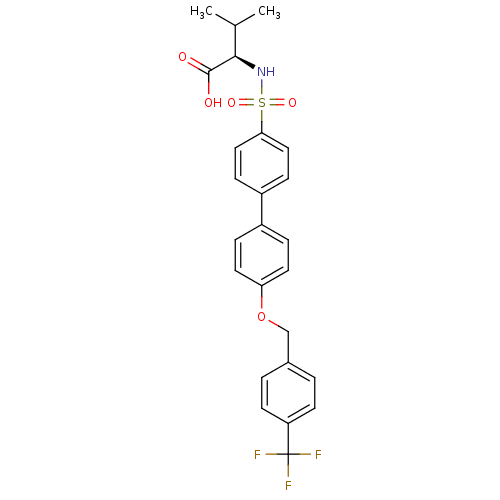
(CHEMBL1910474)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2ccc(cc2)C(F)(F)F)cc1)C(O)=O |r| Show InChI InChI=1S/C25H24F3NO5S/c1-16(2)23(24(30)31)29-35(32,33)22-13-7-19(8-14-22)18-5-11-21(12-6-18)34-15-17-3-9-20(10-4-17)25(26,27)28/h3-14,16,23,29H,15H2,1-2H3,(H,30,31)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356221
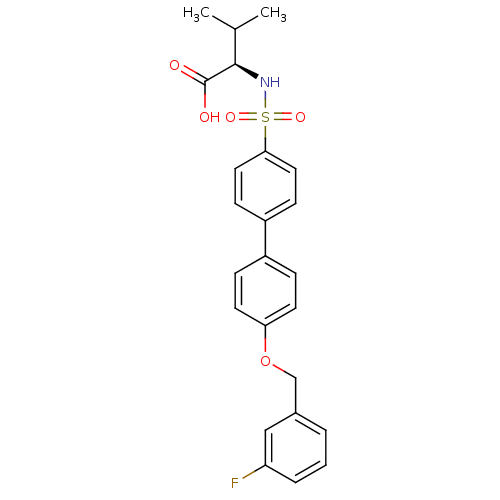
(CHEMBL1910647)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2cccc(F)c2)cc1)C(O)=O |r| Show InChI InChI=1S/C24H24FNO5S/c1-16(2)23(24(27)28)26-32(29,30)22-12-8-19(9-13-22)18-6-10-21(11-7-18)31-15-17-4-3-5-20(25)14-17/h3-14,16,23,26H,15H2,1-2H3,(H,27,28)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50259009
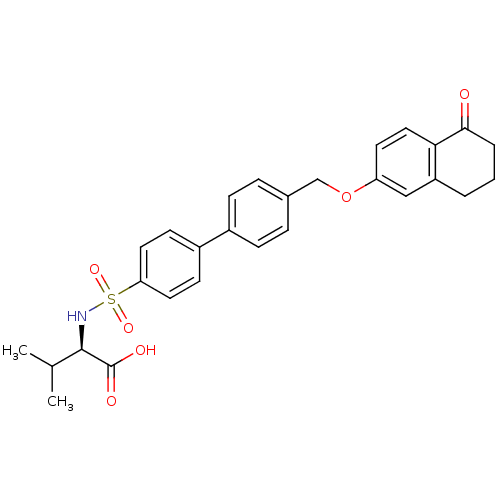
((R)-3-methyl-2-(4'-((5-oxo-5,6,7,8-tetrahydronapht...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc3C(=O)CCCc3c2)cc1)C(O)=O |r| Show InChI InChI=1S/C28H29NO6S/c1-18(2)27(28(31)32)29-36(33,34)24-13-10-21(11-14-24)20-8-6-19(7-9-20)17-35-23-12-15-25-22(16-23)4-3-5-26(25)30/h6-16,18,27,29H,3-5,17H2,1-2H3,(H,31,32)/t27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356219
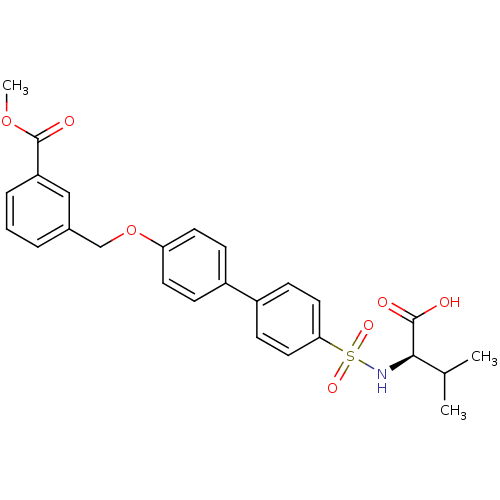
(CHEMBL1910649)Show SMILES COC(=O)c1cccc(COc2ccc(cc2)-c2ccc(cc2)S(=O)(=O)N[C@H](C(C)C)C(O)=O)c1 |r| Show InChI InChI=1S/C26H27NO7S/c1-17(2)24(25(28)29)27-35(31,32)23-13-9-20(10-14-23)19-7-11-22(12-8-19)34-16-18-5-4-6-21(15-18)26(30)33-3/h4-15,17,24,27H,16H2,1-3H3,(H,28,29)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356220

(CHEMBL1910650)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2cccc(c2)C(O)=O)cc1)C(O)=O |r| Show InChI InChI=1S/C25H25NO7S/c1-16(2)23(25(29)30)26-34(31,32)22-12-8-19(9-13-22)18-6-10-21(11-7-18)33-15-17-4-3-5-20(14-17)24(27)28/h3-14,16,23,26H,15H2,1-2H3,(H,27,28)(H,29,30)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356203
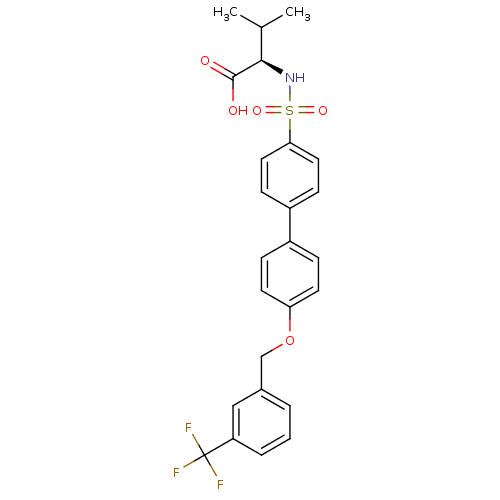
(CHEMBL1910472)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2cccc(c2)C(F)(F)F)cc1)C(O)=O |r| Show InChI InChI=1S/C25H24F3NO5S/c1-16(2)23(24(30)31)29-35(32,33)22-12-8-19(9-13-22)18-6-10-21(11-7-18)34-15-17-4-3-5-20(14-17)25(26,27)28/h3-14,16,23,29H,15H2,1-2H3,(H,30,31)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50258875

((R)-2-(4'-((4-(4-fluorobenzoyl)phenoxy)methyl)biph...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc(cc2)C(=O)c2ccc(F)cc2)cc1)C(O)=O |r| Show InChI InChI=1S/C31H28FNO6S/c1-20(2)29(31(35)36)33-40(37,38)28-17-11-23(12-18-28)22-5-3-21(4-6-22)19-39-27-15-9-25(10-16-27)30(34)24-7-13-26(32)14-8-24/h3-18,20,29,33H,19H2,1-2H3,(H,35,36)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM24047

((2R)-3-methyl-2-[(4-{4-[(3-methyl-1-benzofuran-2-y...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2oc3ccccc3c2C)cc1)C(O)=O |r| Show InChI InChI=1S/C27H27NO6S/c1-17(2)26(27(29)30)28-35(31,32)22-14-10-20(11-15-22)19-8-12-21(13-9-19)33-16-25-18(3)23-6-4-5-7-24(23)34-25/h4-15,17,26,28H,16H2,1-3H3,(H,29,30)/t26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM24047

((2R)-3-methyl-2-[(4-{4-[(3-methyl-1-benzofuran-2-y...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2oc3ccccc3c2C)cc1)C(O)=O |r| Show InChI InChI=1S/C27H27NO6S/c1-17(2)26(27(29)30)28-35(31,32)22-14-10-20(11-15-22)19-8-12-21(13-9-19)33-16-25-18(3)23-6-4-5-7-24(23)34-25/h4-15,17,26,28H,16H2,1-3H3,(H,29,30)/t26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Wyeth Research
| Assay Description
A continuous assay was used in which the substrate is a synthetic peptide containing a fluorescent group (7-methoxycoumarin), which is quenched by en... |
Bioorg Med Chem Lett 16: 311-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.001
BindingDB Entry DOI: 10.7270/Q2CJ8BS1 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356213
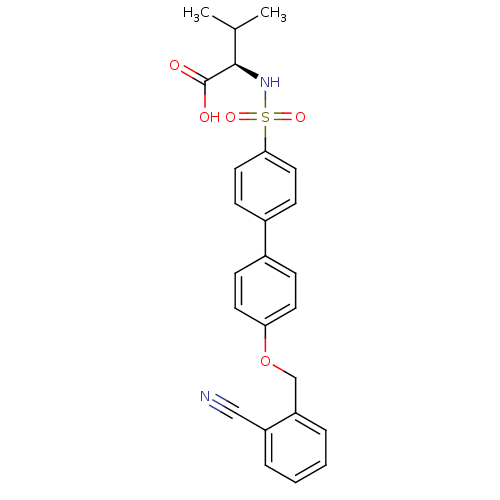
(CHEMBL1910470)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2ccccc2C#N)cc1)C(O)=O |r| Show InChI InChI=1S/C25H24N2O5S/c1-17(2)24(25(28)29)27-33(30,31)23-13-9-19(10-14-23)18-7-11-22(12-8-18)32-16-21-6-4-3-5-20(21)15-26/h3-14,17,24,27H,16H2,1-2H3,(H,28,29)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356207
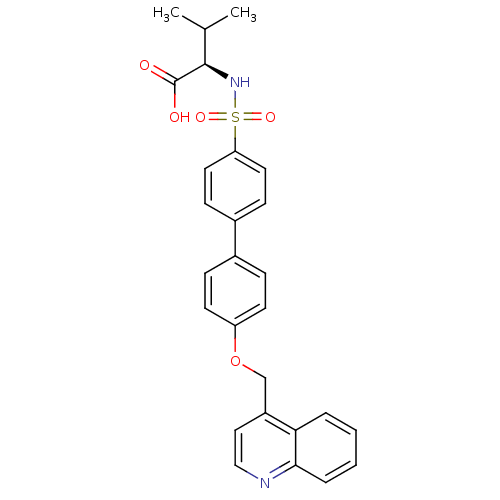
(CHEMBL1910460)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2ccnc3ccccc23)cc1)C(O)=O |r| Show InChI InChI=1S/C27H26N2O5S/c1-18(2)26(27(30)31)29-35(32,33)23-13-9-20(10-14-23)19-7-11-22(12-8-19)34-17-21-15-16-28-25-6-4-3-5-24(21)25/h3-16,18,26,29H,17H2,1-2H3,(H,30,31)/t26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50258917

((R)-2-(4'-((4-(cyclohexanecarbonyl)phenoxy)methyl)...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc(cc2)C(=O)C2CCCCC2)cc1)C(O)=O |r| Show InChI InChI=1S/C31H35NO6S/c1-21(2)29(31(34)35)32-39(36,37)28-18-14-24(15-19-28)23-10-8-22(9-11-23)20-38-27-16-12-26(13-17-27)30(33)25-6-4-3-5-7-25/h8-19,21,25,29,32H,3-7,20H2,1-2H3,(H,34,35)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356204
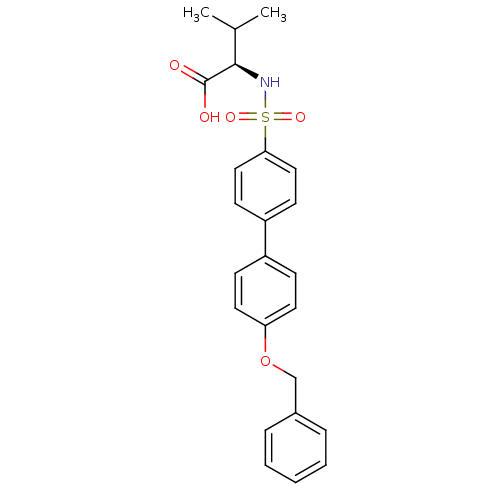
(CHEMBL1910453)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2ccccc2)cc1)C(O)=O |r| Show InChI InChI=1S/C24H25NO5S/c1-17(2)23(24(26)27)25-31(28,29)22-14-10-20(11-15-22)19-8-12-21(13-9-19)30-16-18-6-4-3-5-7-18/h3-15,17,23,25H,16H2,1-2H3,(H,26,27)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50258875

((R)-2-(4'-((4-(4-fluorobenzoyl)phenoxy)methyl)biph...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc(cc2)C(=O)c2ccc(F)cc2)cc1)C(O)=O |r| Show InChI InChI=1S/C31H28FNO6S/c1-20(2)29(31(35)36)33-40(37,38)28-17-11-23(12-18-28)22-5-3-21(4-6-22)19-39-27-15-9-25(10-16-27)30(34)24-7-13-26(32)14-8-24/h3-18,20,29,33H,19H2,1-2H3,(H,35,36)/t29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50259009

((R)-3-methyl-2-(4'-((5-oxo-5,6,7,8-tetrahydronapht...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(COc2ccc3C(=O)CCCc3c2)cc1)C(O)=O |r| Show InChI InChI=1S/C28H29NO6S/c1-18(2)27(28(31)32)29-36(33,34)24-13-10-21(11-14-24)20-8-6-19(7-9-20)17-35-23-12-15-25-22(16-23)4-3-5-26(25)30/h6-16,18,27,29H,3-5,17H2,1-2H3,(H,31,32)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 19: 2487-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.056
BindingDB Entry DOI: 10.7270/Q29886WB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356205

(CHEMBL1910458)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2cccc3ccccc23)cc1)C(O)=O |r| Show InChI InChI=1S/C28H27NO5S/c1-19(2)27(28(30)31)29-35(32,33)25-16-12-21(13-17-25)20-10-14-24(15-11-20)34-18-23-8-5-7-22-6-3-4-9-26(22)23/h3-17,19,27,29H,18H2,1-2H3,(H,30,31)/t27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50356242
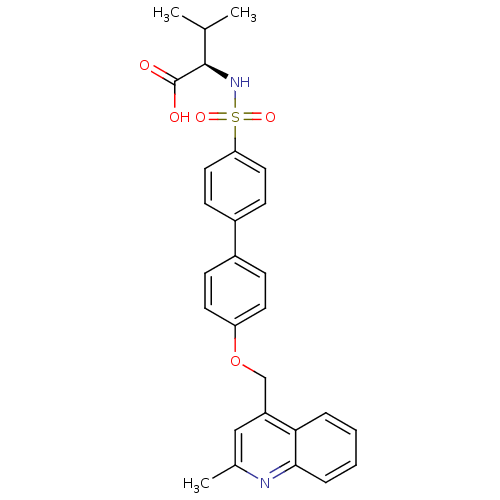
(CHEMBL1910461)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(O)=O |r| Show InChI InChI=1S/C28H28N2O5S/c1-18(2)27(28(31)32)30-36(33,34)24-14-10-21(11-15-24)20-8-12-23(13-9-20)35-17-22-16-19(3)29-26-7-5-4-6-25(22)26/h4-16,18,27,30H,17H2,1-3H3,(H,31,32)/t27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 21: 6800-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.036
BindingDB Entry DOI: 10.7270/Q2BV7H23 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50426487

(CHEMBL2322708)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(s1)[C@](C)(O)C(F)(F)F)c1ccc(cc1C(F)(F)F)C(N)=O |r| Show InChI InChI=1S/C20H21F6N3O4S2/c1-11-10-28(14-4-3-12(17(27)30)9-13(14)19(21,22)23)7-8-29(11)35(32,33)16-6-5-15(34-16)18(2,31)20(24,25)26/h3-6,9,11,31H,7-8,10H2,1-2H3,(H2,27,30)/t11-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO cells using cortisol as substrate incubated for 30 mins prior to substrate addition measured after 1... |
ACS Med Chem Lett 4: 118-23 (2013)
Article DOI: 10.1021/ml300352x
BindingDB Entry DOI: 10.7270/Q25B03TM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50426488
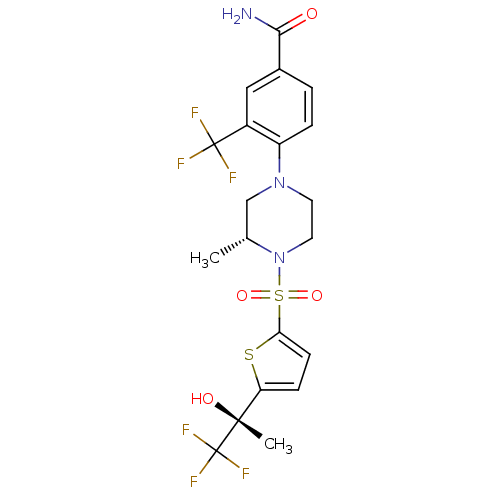
(CHEMBL2322707)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(s1)[C@@](C)(O)C(F)(F)F)c1ccc(cc1C(F)(F)F)C(N)=O |r| Show InChI InChI=1S/C20H21F6N3O4S2/c1-11-10-28(14-4-3-12(17(27)30)9-13(14)19(21,22)23)7-8-29(11)35(32,33)16-6-5-15(34-16)18(2,31)20(24,25)26/h3-6,9,11,31H,7-8,10H2,1-2H3,(H2,27,30)/t11-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO cells using cortisol as substrate incubated for 30 mins prior to substrate addition measured after 1... |
ACS Med Chem Lett 4: 118-23 (2013)
Article DOI: 10.1021/ml300352x
BindingDB Entry DOI: 10.7270/Q25B03TM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50426489
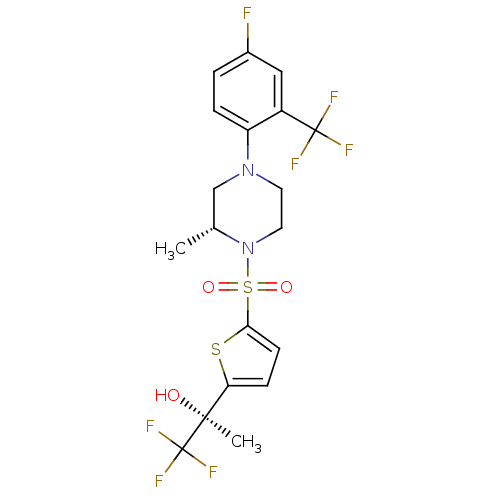
(CHEMBL2322706)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(s1)[C@](C)(O)C(F)(F)F)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C19H19F7N2O3S2/c1-11-10-27(14-4-3-12(20)9-13(14)18(21,22)23)7-8-28(11)33(30,31)16-6-5-15(32-16)17(2,29)19(24,25)26/h3-6,9,11,29H,7-8,10H2,1-2H3/t11-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO cells using cortisol as substrate incubated for 30 mins prior to substrate addition measured after 1... |
ACS Med Chem Lett 4: 118-23 (2013)
Article DOI: 10.1021/ml300352x
BindingDB Entry DOI: 10.7270/Q25B03TM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50426475
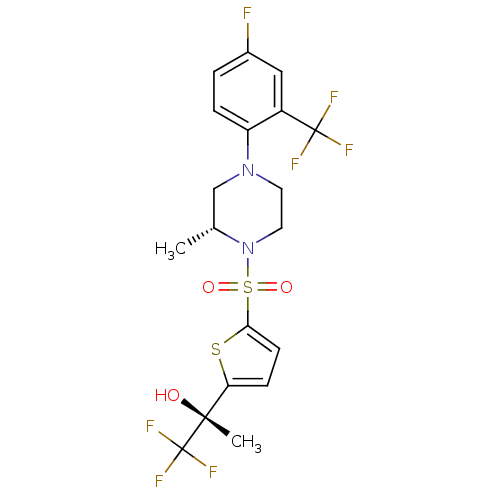
(CHEMBL2322705)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(s1)[C@@](C)(O)C(F)(F)F)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C19H19F7N2O3S2/c1-11-10-27(14-4-3-12(20)9-13(14)18(21,22)23)7-8-28(11)33(30,31)16-6-5-15(32-16)17(2,29)19(24,25)26/h3-6,9,11,29H,7-8,10H2,1-2H3/t11-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO cells using cortisol as substrate incubated for 30 mins prior to substrate addition measured after 1... |
ACS Med Chem Lett 4: 118-23 (2013)
Article DOI: 10.1021/ml300352x
BindingDB Entry DOI: 10.7270/Q25B03TM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50426475

(CHEMBL2322705)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(s1)[C@@](C)(O)C(F)(F)F)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C19H19F7N2O3S2/c1-11-10-27(14-4-3-12(20)9-13(14)18(21,22)23)7-8-28(11)33(30,31)16-6-5-15(32-16)17(2,29)19(24,25)26/h3-6,9,11,29H,7-8,10H2,1-2H3/t11-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO cells using cortisol as substrate incubated for 30 mins prior to substrate addition measured after 1... |
ACS Med Chem Lett 4: 118-23 (2013)
Article DOI: 10.1021/ml300352x
BindingDB Entry DOI: 10.7270/Q25B03TM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50426479
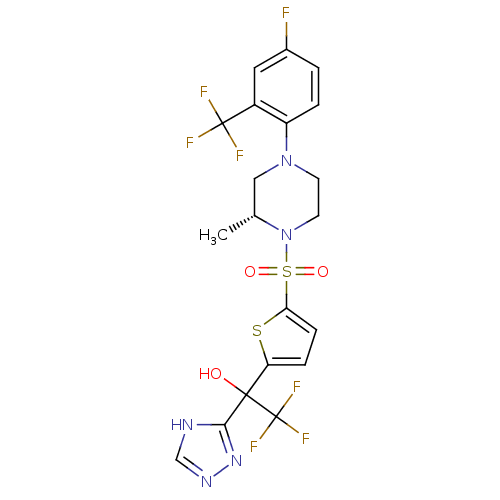
(CHEMBL2323023)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(s1)C(O)(c1nnc[nH]1)C(F)(F)F)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C20H18F7N5O3S2/c1-11-9-31(14-3-2-12(21)8-13(14)19(22,23)24)6-7-32(11)37(34,35)16-5-4-15(36-16)18(33,20(25,26)27)17-28-10-29-30-17/h2-5,8,10-11,33H,6-7,9H2,1H3,(H,28,29,30)/t11-,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells using cortisol as substrate incubated for 30 mins prior to substrate addition measured after 1... |
ACS Med Chem Lett 4: 118-23 (2013)
Article DOI: 10.1021/ml300352x
BindingDB Entry DOI: 10.7270/Q25B03TM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50426478
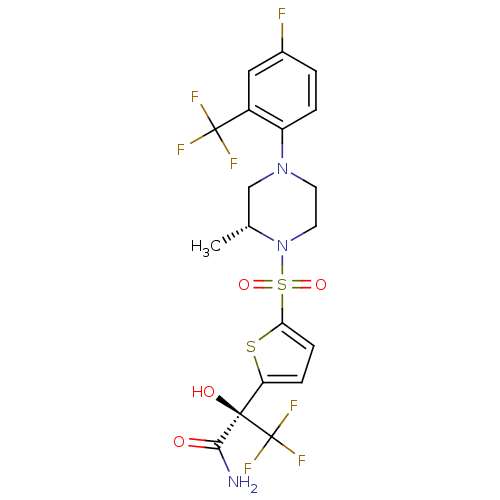
(CHEMBL2323021)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(s1)[C@@](O)(C(N)=O)C(F)(F)F)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C19H18F7N3O4S2/c1-10-9-28(13-3-2-11(20)8-12(13)18(21,22)23)6-7-29(10)35(32,33)15-5-4-14(34-15)17(31,16(27)30)19(24,25)26/h2-5,8,10,31H,6-7,9H2,1H3,(H2,27,30)/t10-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells using cortisol as substrate incubated for 30 mins prior to substrate addition measured after 1... |
ACS Med Chem Lett 4: 118-23 (2013)
Article DOI: 10.1021/ml300352x
BindingDB Entry DOI: 10.7270/Q25B03TM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50426488

(CHEMBL2322707)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(s1)[C@@](C)(O)C(F)(F)F)c1ccc(cc1C(F)(F)F)C(N)=O |r| Show InChI InChI=1S/C20H21F6N3O4S2/c1-11-10-28(14-4-3-12(17(27)30)9-13(14)19(21,22)23)7-8-29(11)35(32,33)16-6-5-15(34-16)18(2,31)20(24,25)26/h3-6,9,11,31H,7-8,10H2,1-2H3,(H2,27,30)/t11-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells using cortisol as substrate incubated for 30 mins prior to substrate addition measured after 1... |
ACS Med Chem Lett 4: 118-23 (2013)
Article DOI: 10.1021/ml300352x
BindingDB Entry DOI: 10.7270/Q25B03TM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50426489

(CHEMBL2322706)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(s1)[C@](C)(O)C(F)(F)F)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C19H19F7N2O3S2/c1-11-10-27(14-4-3-12(20)9-13(14)18(21,22)23)7-8-28(11)33(30,31)16-6-5-15(32-16)17(2,29)19(24,25)26/h3-6,9,11,29H,7-8,10H2,1-2H3/t11-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells using cortisol as substrate incubated for 30 mins prior to substrate addition measured after 1... |
ACS Med Chem Lett 4: 118-23 (2013)
Article DOI: 10.1021/ml300352x
BindingDB Entry DOI: 10.7270/Q25B03TM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50295399
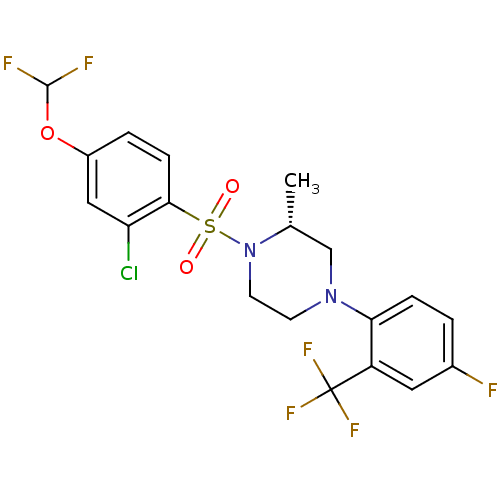
((2R)-1-(2-Chloro-4-(difluoromethoxy)phenylsulfonyl...)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(OC(F)F)cc1Cl)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C19H17ClF6N2O3S/c1-11-10-27(16-4-2-12(21)8-14(16)19(24,25)26)6-7-28(11)32(29,30)17-5-3-13(9-15(17)20)31-18(22)23/h2-5,8-9,11,18H,6-7,10H2,1H3/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11betaHSD1 expressed in CHO cells |
J Med Chem 52: 5449-61 (2009)
Article DOI: 10.1021/jm900639u
BindingDB Entry DOI: 10.7270/Q2JM29NS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50295399

((2R)-1-(2-Chloro-4-(difluoromethoxy)phenylsulfonyl...)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(OC(F)F)cc1Cl)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C19H17ClF6N2O3S/c1-11-10-27(16-4-2-12(21)8-14(16)19(24,25)26)6-7-28(11)32(29,30)17-5-3-13(9-15(17)20)31-18(22)23/h2-5,8-9,11,18H,6-7,10H2,1H3/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11betaHSD1 expressed in CHO cells in presence of 10 % (v/v) human serum |
J Med Chem 52: 5449-61 (2009)
Article DOI: 10.1021/jm900639u
BindingDB Entry DOI: 10.7270/Q2JM29NS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50295401
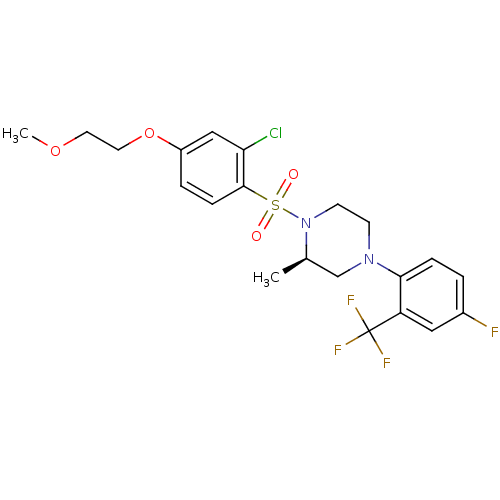
((2R)-1-(2-Chloro-4-(2-methoxyethoxy)phenylsulfonyl...)Show SMILES COCCOc1ccc(c(Cl)c1)S(=O)(=O)N1CCN(C[C@H]1C)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C21H23ClF4N2O4S/c1-14-13-27(19-5-3-15(23)11-17(19)21(24,25)26)7-8-28(14)33(29,30)20-6-4-16(12-18(20)22)32-10-9-31-2/h3-6,11-12,14H,7-10,13H2,1-2H3/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11betaHSD1 expressed in CHO cells |
J Med Chem 52: 5449-61 (2009)
Article DOI: 10.1021/jm900639u
BindingDB Entry DOI: 10.7270/Q2JM29NS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50295402
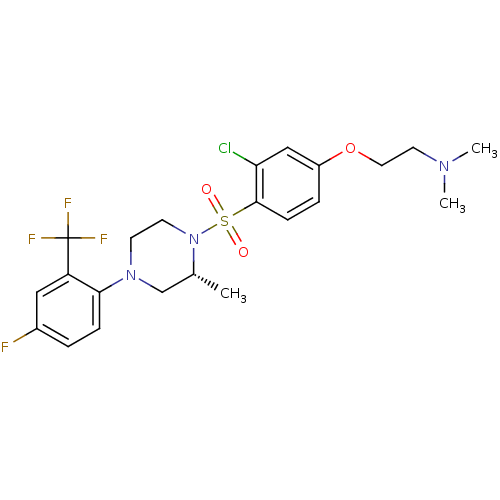
((2R)-2-(3-Chloro-4-(4-(4-fluoro-2-(trifluoromethyl...)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(OCCN(C)C)cc1Cl)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C22H26ClF4N3O3S/c1-15-14-29(20-6-4-16(24)12-18(20)22(25,26)27)8-9-30(15)34(31,32)21-7-5-17(13-19(21)23)33-11-10-28(2)3/h4-7,12-13,15H,8-11,14H2,1-3H3/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11betaHSD1 expressed in CHO cells |
J Med Chem 52: 5449-61 (2009)
Article DOI: 10.1021/jm900639u
BindingDB Entry DOI: 10.7270/Q2JM29NS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50295402

((2R)-2-(3-Chloro-4-(4-(4-fluoro-2-(trifluoromethyl...)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(OCCN(C)C)cc1Cl)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C22H26ClF4N3O3S/c1-15-14-29(20-6-4-16(24)12-18(20)22(25,26)27)8-9-30(15)34(31,32)21-7-5-17(13-19(21)23)33-11-10-28(2)3/h4-7,12-13,15H,8-11,14H2,1-3H3/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11betaHSD1 expressed in CHO cells in presence of 10 % (v/v) human serum |
J Med Chem 52: 5449-61 (2009)
Article DOI: 10.1021/jm900639u
BindingDB Entry DOI: 10.7270/Q2JM29NS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50295403
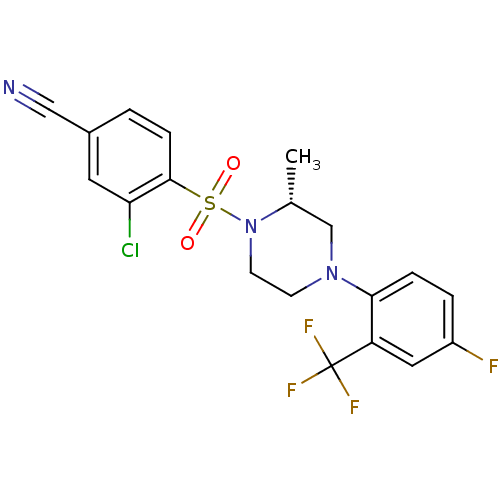
((2R)-3-Chloro-4-(4-(4-fluoro-2-(trifluoromethyl)ph...)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(cc1Cl)C#N)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C19H16ClF4N3O2S/c1-12-11-26(17-4-3-14(21)9-15(17)19(22,23)24)6-7-27(12)30(28,29)18-5-2-13(10-25)8-16(18)20/h2-5,8-9,12H,6-7,11H2,1H3/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11betaHSD1 expressed in CHO cells |
J Med Chem 52: 5449-61 (2009)
Article DOI: 10.1021/jm900639u
BindingDB Entry DOI: 10.7270/Q2JM29NS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50295370
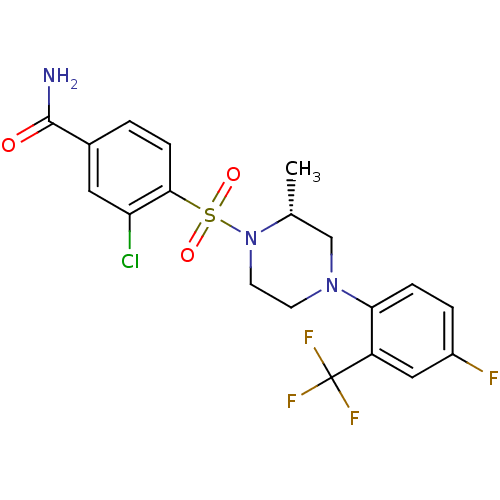
((2R)-3-Chloro-4-({(2R)-4-[4-fluoro-2-(trifluoromet...)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(cc1Cl)C(N)=O)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C19H18ClF4N3O3S/c1-11-10-26(16-4-3-13(21)9-14(16)19(22,23)24)6-7-27(11)31(29,30)17-5-2-12(18(25)28)8-15(17)20/h2-5,8-9,11H,6-7,10H2,1H3,(H2,25,28)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 expressed in CHO cells |
J Med Chem 52: 5449-61 (2009)
Article DOI: 10.1021/jm900639u
BindingDB Entry DOI: 10.7270/Q2JM29NS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50295370

((2R)-3-Chloro-4-({(2R)-4-[4-fluoro-2-(trifluoromet...)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(cc1Cl)C(N)=O)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C19H18ClF4N3O3S/c1-11-10-26(16-4-3-13(21)9-14(16)19(22,23)24)6-7-27(11)31(29,30)17-5-2-12(18(25)28)8-15(17)20/h2-5,8-9,11H,6-7,10H2,1H3,(H2,25,28)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11betaHSD1 expressed in CHO cells |
J Med Chem 52: 5449-61 (2009)
Article DOI: 10.1021/jm900639u
BindingDB Entry DOI: 10.7270/Q2JM29NS |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50426479

(CHEMBL2323023)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(s1)C(O)(c1nnc[nH]1)C(F)(F)F)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C20H18F7N5O3S2/c1-11-9-31(14-3-2-12(21)8-13(14)19(22,23)24)6-7-32(11)37(34,35)16-5-4-15(36-16)18(33,20(25,26)27)17-28-10-29-30-17/h2-5,8,10-11,33H,6-7,9H2,1H3,(H,28,29,30)/t11-,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO cells using cortisol as substrate incubated for 30 mins prior to substrate addition measured after 1... |
ACS Med Chem Lett 4: 118-23 (2013)
Article DOI: 10.1021/ml300352x
BindingDB Entry DOI: 10.7270/Q25B03TM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50426480
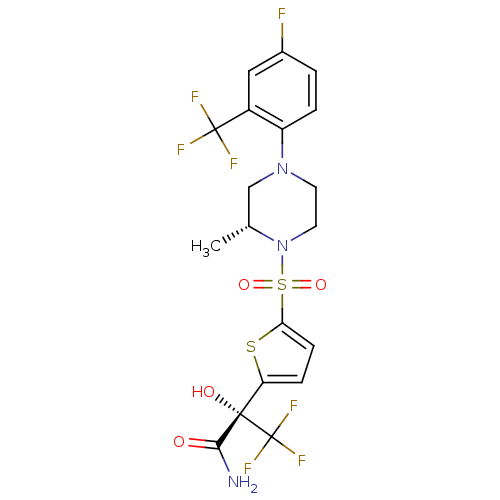
(CHEMBL2323022)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(s1)[C@](O)(C(N)=O)C(F)(F)F)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C19H18F7N3O4S2/c1-10-9-28(13-3-2-11(20)8-12(13)18(21,22)23)6-7-29(10)35(32,33)15-5-4-14(34-15)17(31,16(27)30)19(24,25)26/h2-5,8,10,31H,6-7,9H2,1H3,(H2,27,30)/t10-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO cells using cortisol as substrate incubated for 30 mins prior to substrate addition measured after 1... |
ACS Med Chem Lett 4: 118-23 (2013)
Article DOI: 10.1021/ml300352x
BindingDB Entry DOI: 10.7270/Q25B03TM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50426478

(CHEMBL2323021)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ccc(s1)[C@@](O)(C(N)=O)C(F)(F)F)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C19H18F7N3O4S2/c1-10-9-28(13-3-2-11(20)8-12(13)18(21,22)23)6-7-29(10)35(32,33)15-5-4-14(34-15)17(31,16(27)30)19(24,25)26/h2-5,8,10,31H,6-7,9H2,1H3,(H2,27,30)/t10-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO cells using cortisol as substrate incubated for 30 mins prior to substrate addition measured after 1... |
ACS Med Chem Lett 4: 118-23 (2013)
Article DOI: 10.1021/ml300352x
BindingDB Entry DOI: 10.7270/Q25B03TM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50426482
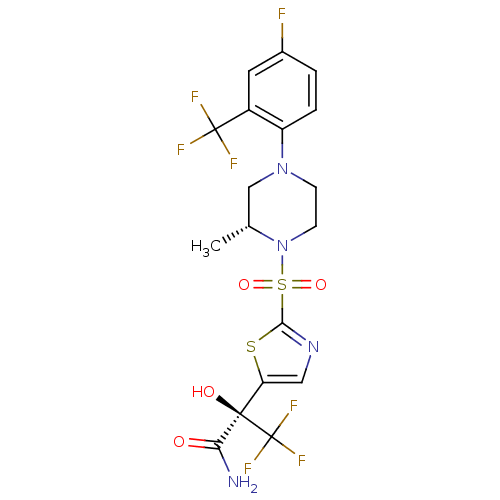
(CHEMBL2323019)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1ncc(s1)[C@@](O)(C(N)=O)C(F)(F)F)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C18H17F7N4O4S2/c1-9-8-28(12-3-2-10(19)6-11(12)17(20,21)22)4-5-29(9)35(32,33)15-27-7-13(34-15)16(31,14(26)30)18(23,24)25/h2-3,6-7,9,31H,4-5,8H2,1H3,(H2,26,30)/t9-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO cells using cortisol as substrate incubated for 30 mins prior to substrate addition measured after 1... |
ACS Med Chem Lett 4: 118-23 (2013)
Article DOI: 10.1021/ml300352x
BindingDB Entry DOI: 10.7270/Q25B03TM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50426483
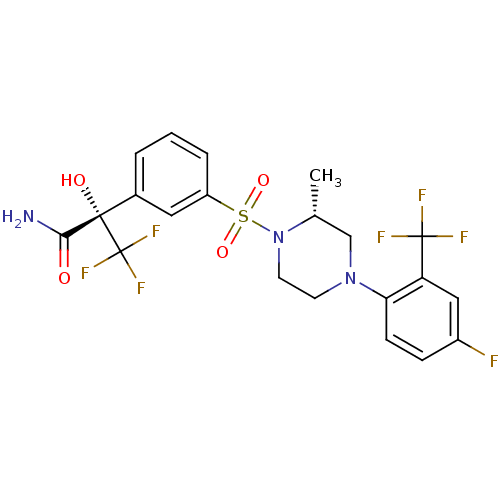
(CHEMBL2323018)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1cccc(c1)[C@](O)(C(N)=O)C(F)(F)F)c1ccc(F)cc1C(F)(F)F |r| Show InChI InChI=1S/C21H20F7N3O4S/c1-12-11-30(17-6-5-14(22)10-16(17)20(23,24)25)7-8-31(12)36(34,35)15-4-2-3-13(9-15)19(33,18(29)32)21(26,27)28/h2-6,9-10,12,33H,7-8,11H2,1H3,(H2,29,32)/t12-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO cells using cortisol as substrate incubated for 30 mins prior to substrate addition measured after 1... |
ACS Med Chem Lett 4: 118-23 (2013)
Article DOI: 10.1021/ml300352x
BindingDB Entry DOI: 10.7270/Q25B03TM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data