 Found 809 hits with Last Name = 'jain' and Initial = 'm'
Found 809 hits with Last Name = 'jain' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50178975
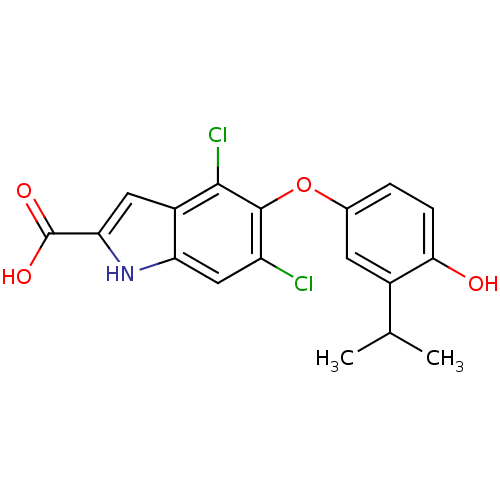
(4,6-dichloro-5-(4-hydroxy-3-isopropylphenoxy)-1H-i...)Show SMILES CC(C)c1cc(Oc2c(Cl)cc3[nH]c(cc3c2Cl)C(O)=O)ccc1O Show InChI InChI=1S/C18H15Cl2NO4/c1-8(2)10-5-9(3-4-15(10)22)25-17-12(19)7-13-11(16(17)20)6-14(21-13)18(23)24/h3-8,21-22H,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity to TRbeta1 |
J Med Chem 55: 5649-75 (2012)
Article DOI: 10.1021/jm2004706
BindingDB Entry DOI: 10.7270/Q2DZ09FJ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18869
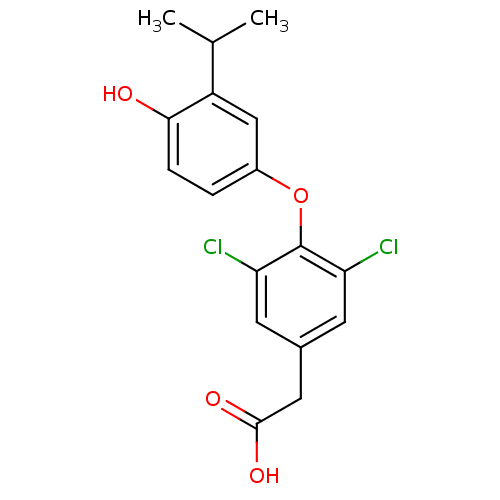
(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity to TRbeta1 |
J Med Chem 55: 5649-75 (2012)
Article DOI: 10.1021/jm2004706
BindingDB Entry DOI: 10.7270/Q2DZ09FJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of F10a assessed as S-2765 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of F10a assessed as S-2765 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18869

(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 7.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity to TRalpha1 |
J Med Chem 55: 5649-75 (2012)
Article DOI: 10.1021/jm2004706
BindingDB Entry DOI: 10.7270/Q2DZ09FJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase WNK1
(Homo sapiens (Human)) | BDBM50258546
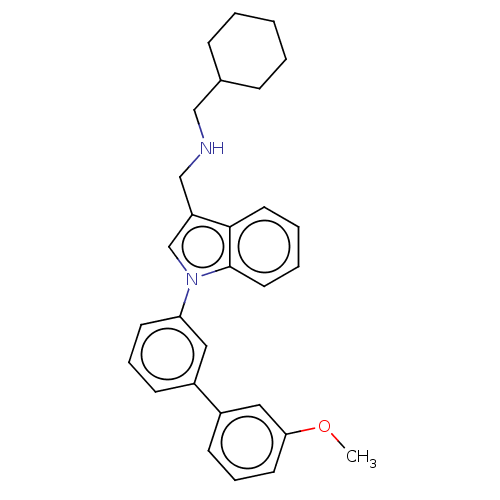
(CHEMBL4087727)Show SMILES COc1cccc(c1)-c1cccc(c1)-n1cc(CNCC2CCCCC2)c2ccccc12 Show InChI InChI=1S/C29H32N2O/c1-32-27-14-8-12-24(18-27)23-11-7-13-26(17-23)31-21-25(28-15-5-6-16-29(28)31)20-30-19-22-9-3-2-4-10-22/h5-8,11-18,21-22,30H,2-4,9-10,19-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc. , Cambridge, Massachusetts 02139-4133, United States.
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of recombinant human N-terminal GST-tagged WNK1 (1 to 491 residues) expressed in baculovirus expression system using fluor... |
J Med Chem 60: 7099-7107 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00708
BindingDB Entry DOI: 10.7270/Q29W0HXP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in HEK293 cells |
J Med Chem 50: 5951-66 (2007)
Article DOI: 10.1021/jm061490u
BindingDB Entry DOI: 10.7270/Q2Q81CTK |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM403460

(US10335399, Example 2-5 | US10806724, Example 2-5)Show InChI InChI=1S/C17H16F2N2O2/c1-17(2)6-5-11-12(10-4-3-9(18)7-13(10)19)8-14(15(20)22)21-16(11)23-17/h3-4,7-8H,5-6H2,1-2H3,(H2,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM403460

(US10335399, Example 2-5 | US10806724, Example 2-5)Show InChI InChI=1S/C17H16F2N2O2/c1-17(2)6-5-11-12(10-4-3-9(18)7-13(10)19)8-14(15(20)22)21-16(11)23-17/h3-4,7-8H,5-6H2,1-2H3,(H2,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50584759

(CHEMBL5089623)Show SMILES COc1ccc(c(F)c1)-c1cc(nc2OC(C)(C)CCc12)C(N)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50584759

(CHEMBL5089623)Show SMILES COc1ccc(c(F)c1)-c1cc(nc2OC(C)(C)CCc12)C(N)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50226179
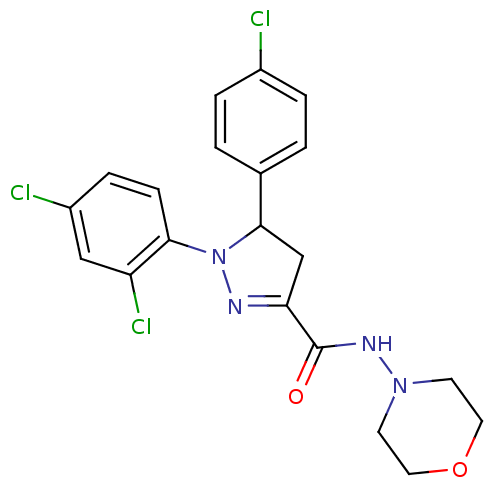
((+)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-...)Show SMILES Clc1ccc(cc1)C1CC(=NN1c1ccc(Cl)cc1Cl)C(=O)NN1CCOCC1 |c:10| Show InChI InChI=1S/C20H19Cl3N4O2/c21-14-3-1-13(2-4-14)19-12-17(20(28)25-26-7-9-29-10-8-26)24-27(19)18-6-5-15(22)11-16(18)23/h1-6,11,19H,7-10,12H2,(H,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in HEK293 cells |
J Med Chem 50: 5951-66 (2007)
Article DOI: 10.1021/jm061490u
BindingDB Entry DOI: 10.7270/Q2Q81CTK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50226179

((+)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-...)Show SMILES Clc1ccc(cc1)C1CC(=NN1c1ccc(Cl)cc1Cl)C(=O)NN1CCOCC1 |c:10| Show InChI InChI=1S/C20H19Cl3N4O2/c21-14-3-1-13(2-4-14)19-12-17(20(28)25-26-7-9-29-10-8-26)24-27(19)18-6-5-15(22)11-16(18)23/h1-6,11,19H,7-10,12H2,(H,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in HEK293 cells |
J Med Chem 50: 5951-66 (2007)
Article DOI: 10.1021/jm061490u
BindingDB Entry DOI: 10.7270/Q2Q81CTK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50226179

((+)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-...)Show SMILES Clc1ccc(cc1)C1CC(=NN1c1ccc(Cl)cc1Cl)C(=O)NN1CCOCC1 |c:10| Show InChI InChI=1S/C20H19Cl3N4O2/c21-14-3-1-13(2-4-14)19-12-17(20(28)25-26-7-9-29-10-8-26)24-27(19)18-6-5-15(22)11-16(18)23/h1-6,11,19H,7-10,12H2,(H,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 475 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in HEK293 cells |
J Med Chem 50: 5951-66 (2007)
Article DOI: 10.1021/jm061490u
BindingDB Entry DOI: 10.7270/Q2Q81CTK |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50401076
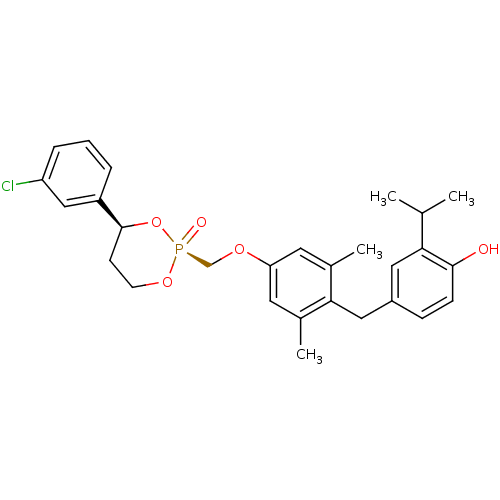
(CHEMBL457748)Show SMILES CC(C)c1cc(Cc2c(C)cc(OC[P@@]3(=O)OCC[C@H](O3)c3cccc(Cl)c3)cc2C)ccc1O |r| Show InChI InChI=1S/C28H32ClO5P/c1-18(2)25-14-21(8-9-27(25)30)15-26-19(3)12-24(13-20(26)4)32-17-35(31)33-11-10-28(34-35)22-6-5-7-23(29)16-22/h5-9,12-14,16,18,28,30H,10-11,15,17H2,1-4H3/t28-,35+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity to TRbeta1 |
J Med Chem 55: 5649-75 (2012)
Article DOI: 10.1021/jm2004706
BindingDB Entry DOI: 10.7270/Q2DZ09FJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN552122 from human recombinant CB2 receptor expressed in CHOK1 cells |
J Med Chem 50: 5951-66 (2007)
Article DOI: 10.1021/jm061490u
BindingDB Entry DOI: 10.7270/Q2Q81CTK |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50105934

(2-(6-Amino-purin-7-ylmethoxy)-ethanol | CHEMBL1260...)Show InChI InChI=1S/C8H11N5O2/c9-7-6-8(11-3-10-7)12-4-13(6)5-15-2-1-14/h3-4,14H,1-2,5H2,(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against adenosine deaminase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50401076

(CHEMBL457748)Show SMILES CC(C)c1cc(Cc2c(C)cc(OC[P@@]3(=O)OCC[C@H](O3)c3cccc(Cl)c3)cc2C)ccc1O |r| Show InChI InChI=1S/C28H32ClO5P/c1-18(2)25-14-21(8-9-27(25)30)15-26-19(3)12-24(13-20(26)4)32-17-35(31)33-11-10-28(34-35)22-6-5-7-23(29)16-22/h5-9,12-14,16,18,28,30H,10-11,15,17H2,1-4H3/t28-,35+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity to TRalpha1 |
J Med Chem 55: 5649-75 (2012)
Article DOI: 10.1021/jm2004706
BindingDB Entry DOI: 10.7270/Q2DZ09FJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of thrombin assessed as S-2238 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C assessed as S-2366 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C assessed as S-2366 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of tPA assessed as S-2288 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of tPA assessed as S-2288 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of plasmin assessed as S-2302 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50392592

(CHEMBL2153381)Show SMILES CCN(CC)CC(=O)NS(=C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H27Cl2N5O4S/c1-4-33(5-2)16-24(34)32-38(3,37)20-10-6-17(7-11-20)25(35)30-22-12-8-18(27)14-21(22)26(36)31-23-13-9-19(28)15-29-23/h6-15H,3-5,16H2,1-2H3,(H,30,35)(H,29,31,36)(H,32,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of thrombin assessed as S-2238 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50392589

(CHEMBL2153377)Show SMILES CCN=CC(=O)N=S(C)(=O)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C24H23Cl2N5O4S/c1-3-27-14-22(32)31-36(2,35)18-8-4-15(5-9-18)23(33)29-20-10-6-16(25)12-19(20)24(34)30-21-11-7-17(26)13-28-21/h4-14,36H,3H2,1-2H3,(H,29,33)(H,28,30,34)(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of plasmin assessed as S-2302 substrate hydrolysis by microplate reader analysis |
Eur J Med Chem 58: 136-52 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.005
BindingDB Entry DOI: 10.7270/Q2571D4Q |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50226179

((+)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-...)Show SMILES Clc1ccc(cc1)C1CC(=NN1c1ccc(Cl)cc1Cl)C(=O)NN1CCOCC1 |c:10| Show InChI InChI=1S/C20H19Cl3N4O2/c21-14-3-1-13(2-4-14)19-12-17(20(28)25-26-7-9-29-10-8-26)24-27(19)18-6-5-15(22)11-16(18)23/h1-6,11,19H,7-10,12H2,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN552122 from human recombinant CB2 receptor expressed in CHOK1 cells |
J Med Chem 50: 5951-66 (2007)
Article DOI: 10.1021/jm061490u
BindingDB Entry DOI: 10.7270/Q2Q81CTK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50226179

((+)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-...)Show SMILES Clc1ccc(cc1)C1CC(=NN1c1ccc(Cl)cc1Cl)C(=O)NN1CCOCC1 |c:10| Show InChI InChI=1S/C20H19Cl3N4O2/c21-14-3-1-13(2-4-14)19-12-17(20(28)25-26-7-9-29-10-8-26)24-27(19)18-6-5-15(22)11-16(18)23/h1-6,11,19H,7-10,12H2,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN552122 from human recombinant CB2 receptor expressed in CHOK1 cells |
J Med Chem 50: 5951-66 (2007)
Article DOI: 10.1021/jm061490u
BindingDB Entry DOI: 10.7270/Q2Q81CTK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50226179

((+)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-...)Show SMILES Clc1ccc(cc1)C1CC(=NN1c1ccc(Cl)cc1Cl)C(=O)NN1CCOCC1 |c:10| Show InChI InChI=1S/C20H19Cl3N4O2/c21-14-3-1-13(2-4-14)19-12-17(20(28)25-26-7-9-29-10-8-26)24-27(19)18-6-5-15(22)11-16(18)23/h1-6,11,19H,7-10,12H2,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN552122 from human recombinant CB2 receptor expressed in CHOK1 cells |
J Med Chem 50: 5951-66 (2007)
Article DOI: 10.1021/jm061490u
BindingDB Entry DOI: 10.7270/Q2Q81CTK |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50369958
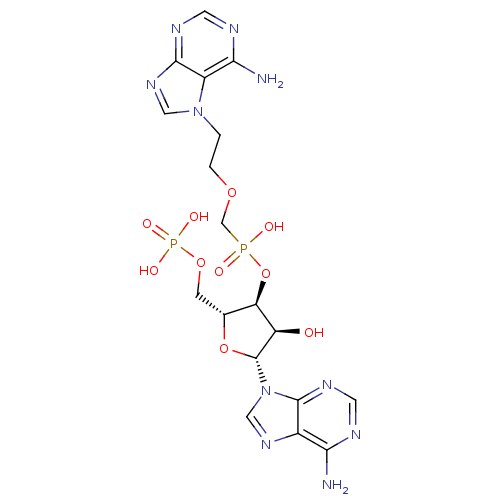
(CHEMBL1790862)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](OP(O)(=O)COCCn2cnc3ncnc(N)c23)[C@H]1O Show InChI InChI=1S/C18H24N10O10P2/c19-14-10-17(24-5-21-14)28(7-25-10)18-12(29)13(9(37-18)3-36-40(32,33)34)38-39(30,31)8-35-2-1-27-6-26-16-11(27)15(20)22-4-23-16/h4-7,9,12-13,18,29H,1-3,8H2,(H,30,31)(H2,19,21,24)(H2,20,22,23)(H2,32,33,34)/t9-,12-,13-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against adenosine deaminase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50029650

(2-(6-Amino-purin-9-ylmethoxy)-ethanol | CHEMBL3775...)Show InChI InChI=1S/C8H11N5O2/c9-7-6-8(11-3-10-7)13(4-12-6)5-15-2-1-14/h3-4,14H,1-2,5H2,(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against adenosine deaminase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Phosphoribosyl pyrophosphate synthase-associated protein 2
(Homo sapiens (Human)) | BDBM50369958

(CHEMBL1790862)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](OP(O)(=O)COCCn2cnc3ncnc(N)c23)[C@H]1O Show InChI InChI=1S/C18H24N10O10P2/c19-14-10-17(24-5-21-14)28(7-25-10)18-12(29)13(9(37-18)3-36-40(32,33)34)38-39(30,31)8-35-2-1-27-6-26-16-11(27)15(20)22-4-23-16/h4-7,9,12-13,18,29H,1-3,8H2,(H,30,31)(H2,19,21,24)(H2,20,22,23)(H2,32,33,34)/t9-,12-,13-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against PRPP synthetase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50001103
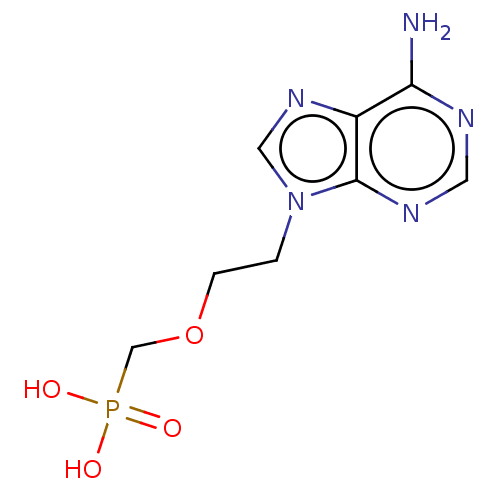
((2-(6-amino-9H-purin-9-yl)ethoxy)methylphosphonic ...)Show InChI InChI=1S/C8H12N5O4P/c9-7-6-8(11-3-10-7)13(4-12-6)1-2-17-5-18(14,15)16/h3-4H,1-2,5H2,(H2,9,10,11)(H2,14,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against adenosine deaminase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50105935

(CHEMBL121723 | [2-(6-Amino-purin-9-yl)-ethoxymethy...)Show SMILES COC1=C(OC)\C(OC1=O)=C\COP(O)(=O)COCCn1cnc2c(N)ncnc12 |c:2| Show InChI InChI=1S/C16H20N5O8P/c1-25-12-10(29-16(22)13(12)26-2)3-5-28-30(23,24)9-27-6-4-21-8-20-11-14(17)18-7-19-15(11)21/h3,7-8H,4-6,9H2,1-2H3,(H,23,24)(H2,17,18,19)/b10-3- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against adenosine deaminase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50369957
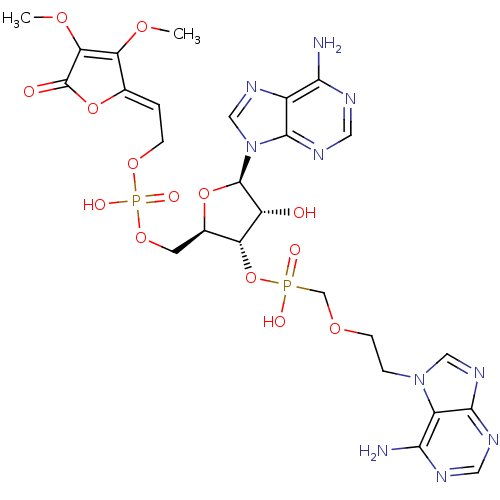
(CHEMBL1790864)Show SMILES COC1=C(OC)\C(OC1=O)=C\COP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(=O)COCCn1cnc2ncnc(N)c12)n1cnc2c(N)ncnc12 |c:2| Show InChI InChI=1S/C26H32N10O14P2/c1-43-19-13(49-26(38)20(19)44-2)3-5-46-52(41,42)47-7-14-18(17(37)25(48-14)36-11-33-15-21(27)29-9-32-24(15)36)50-51(39,40)12-45-6-4-35-10-34-23-16(35)22(28)30-8-31-23/h3,8-11,14,17-18,25,37H,4-7,12H2,1-2H3,(H,39,40)(H,41,42)(H2,27,29,32)(H2,28,30,31)/b13-3-/t14-,17-,18-,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against adenosine deaminase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50105931
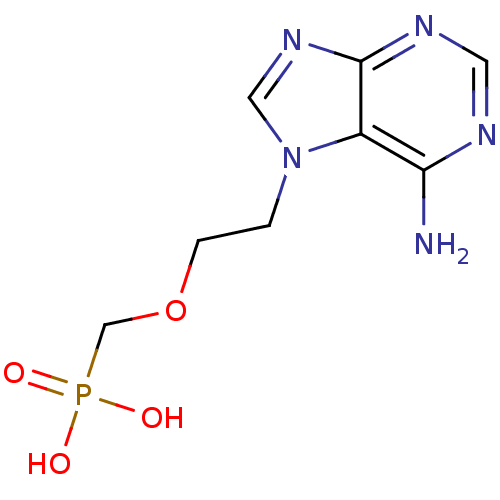
(CHEMBL123655 | [2-(6-Amino-purin-7-yl)-ethoxymethy...)Show InChI InChI=1S/C8H12N5O4P/c9-7-6-8(11-3-10-7)12-4-13(6)1-2-17-5-18(14,15)16/h3-4H,1-2,5H2,(H2,9,10,11)(H2,14,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against adenosine deaminase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Phosphoribosyl pyrophosphate synthase-associated protein 2
(Homo sapiens (Human)) | BDBM50001103

((2-(6-amino-9H-purin-9-yl)ethoxy)methylphosphonic ...)Show InChI InChI=1S/C8H12N5O4P/c9-7-6-8(11-3-10-7)13(4-12-6)1-2-17-5-18(14,15)16/h3-4H,1-2,5H2,(H2,9,10,11)(H2,14,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against PRPP synthetase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Phosphoribosyl pyrophosphate synthase-associated protein 2
(Homo sapiens (Human)) | BDBM50105931

(CHEMBL123655 | [2-(6-Amino-purin-7-yl)-ethoxymethy...)Show InChI InChI=1S/C8H12N5O4P/c9-7-6-8(11-3-10-7)12-4-13(6)1-2-17-5-18(14,15)16/h3-4H,1-2,5H2,(H2,9,10,11)(H2,14,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.70E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against PRPP synthetase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385806
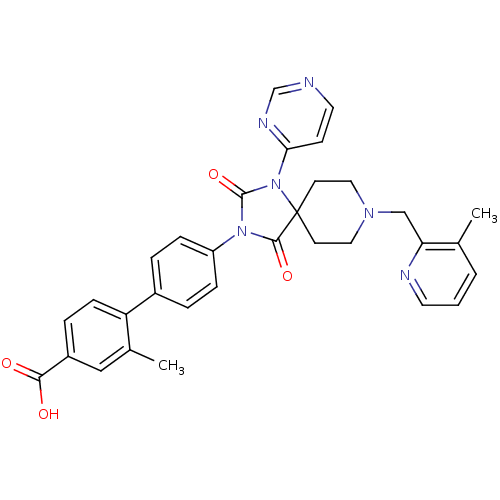
(CHEMBL2041193)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1ccncn1 Show InChI InChI=1S/C32H30N6O4/c1-21-4-3-14-34-27(21)19-36-16-12-32(13-17-36)30(41)37(31(42)38(32)28-11-15-33-20-35-28)25-8-5-23(6-9-25)26-10-7-24(29(39)40)18-22(26)2/h3-11,14-15,18,20H,12-13,16-17,19H2,1-2H3,(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50385806

(CHEMBL2041193)Show SMILES Cc1cccnc1CN1CCC2(CC1)N(C(=O)N(C2=O)c1ccc(cc1)-c1ccc(cc1C)C(O)=O)c1ccncn1 Show InChI InChI=1S/C32H30N6O4/c1-21-4-3-14-34-27(21)19-36-16-12-32(13-17-36)30(41)37(31(42)38(32)28-11-15-33-20-35-28)25-8-5-23(6-9-25)26-10-7-24(29(39)40)18-22(26)2/h3-11,14-15,18,20H,12-13,16-17,19H2,1-2H3,(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD1 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50323737
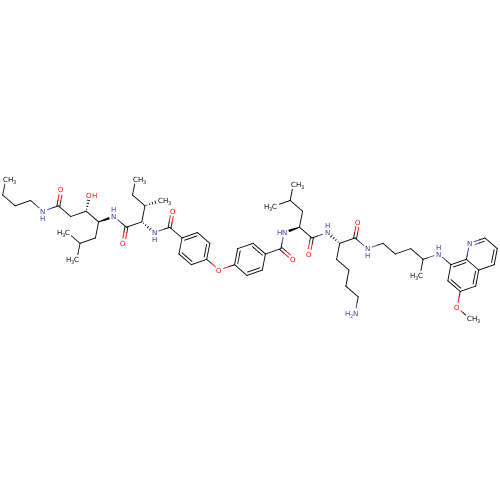
(CHEMBL1213687 | N-((2S)-1-((2S)-6-amino-1-(4-(6-me...)Show SMILES CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)[C@@H](C)CC |r| Show InChI InChI=1S/C59H87N9O9/c1-10-12-29-61-52(70)36-51(69)48(32-37(3)4)66-59(75)53(39(7)11-2)68-56(72)42-22-26-45(27-23-42)77-44-24-20-41(21-25-44)55(71)67-50(33-38(5)6)58(74)65-47(19-13-14-28-60)57(73)63-31-15-17-40(8)64-49-35-46(76-9)34-43-18-16-30-62-54(43)49/h16,18,20-27,30,34-35,37-40,47-48,50-51,53,64,69H,10-15,17,19,28-29,31-33,36,60H2,1-9H3,(H,61,70)(H,63,73)(H,65,74)(H,66,75)(H,67,71)(H,68,72)/t39-,40?,47-,48-,50-,51-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Eur J Med Chem 45: 3245-64 (2010)
Article DOI: 10.1016/j.ejmech.2010.04.011
BindingDB Entry DOI: 10.7270/Q26D5T5P |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50316499

(CHEMBL4167582)Show SMILES OC(=O)CCNC(=O)c1cc(O)c(cn1)C(=O)NC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C23H21N3O5/c27-19-13-18(23(31)24-12-11-20(28)29)25-14-17(19)22(30)26-21(15-7-3-1-4-8-15)16-9-5-2-6-10-16/h1-10,13-14,21H,11-12H2,(H,24,31)(H,25,27)(H,26,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50316852

(CHEMBL4171446)Show SMILES Oc1nc(ncc1NC(=O)Cc1ccc(cc1)-c1ccccc1)-n1cccn1 Show InChI InChI=1S/C21H17N5O2/c27-19(13-15-7-9-17(10-8-15)16-5-2-1-3-6-16)24-18-14-22-21(25-20(18)28)26-12-4-11-23-26/h1-12,14H,13H2,(H,24,27)(H,22,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50316816
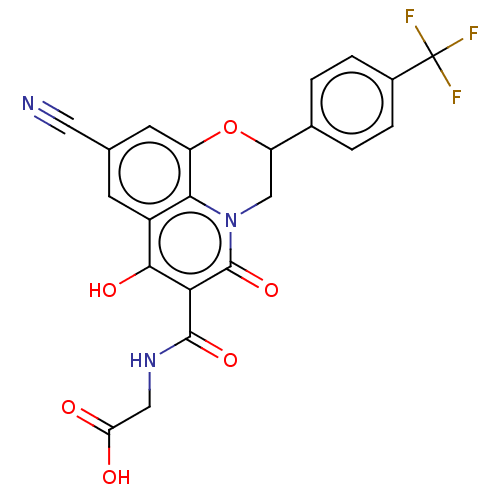
(CHEMBL4161223)Show SMILES OC(=O)CNC(=O)c1c(O)c2cc(cc3OC(Cn(c23)c1=O)c1ccc(cc1)C(F)(F)F)C#N Show InChI InChI=1S/C22H14F3N3O6/c23-22(24,25)12-3-1-11(2-4-12)15-9-28-18-13(5-10(7-26)6-14(18)34-15)19(31)17(21(28)33)20(32)27-8-16(29)30/h1-6,15,31H,8-9H2,(H,27,32)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50316816

(CHEMBL4161223)Show SMILES OC(=O)CNC(=O)c1c(O)c2cc(cc3OC(Cn(c23)c1=O)c1ccc(cc1)C(F)(F)F)C#N Show InChI InChI=1S/C22H14F3N3O6/c23-22(24,25)12-3-1-11(2-4-12)15-9-28-18-13(5-10(7-26)6-14(18)34-15)19(31)17(21(28)33)20(32)27-8-16(29)30/h1-6,15,31H,8-9H2,(H,27,32)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN3
(Homo sapiens (Human)) | BDBM50317545
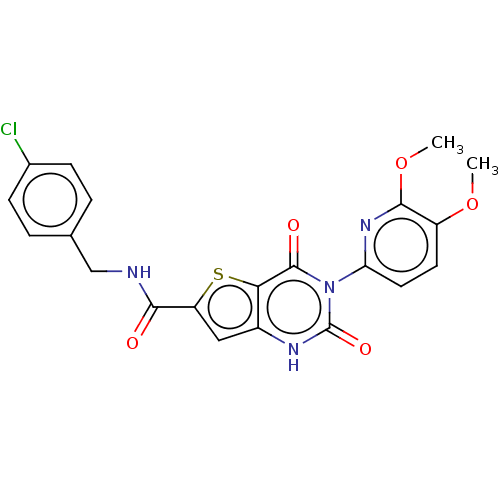
(CHEMBL4173371)Show SMILES COc1ccc(nc1OC)-n1c(=O)[nH]c2cc(sc2c1=O)C(=O)NCc1ccc(Cl)cc1 Show InChI InChI=1S/C21H17ClN4O5S/c1-30-14-7-8-16(25-19(14)31-2)26-20(28)17-13(24-21(26)29)9-15(32-17)18(27)23-10-11-3-5-12(22)6-4-11/h3-9H,10H2,1-2H3,(H,23,27)(H,24,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD3 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50321025

(CHEMBL4170335)Show SMILES OC(=O)CNC(=O)c1c(O)c2cc(cc3NC(Cn(c23)c1=O)c1ccc(cc1)C(F)(F)F)C#N Show InChI InChI=1S/C22H15F3N4O5/c23-22(24,25)12-3-1-11(2-4-12)15-9-29-18-13(5-10(7-26)6-14(18)28-15)19(32)17(21(29)34)20(33)27-8-16(30)31/h1-6,15,28,32H,8-9H2,(H,27,33)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadila Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of FLAG- tagged full length HIF-PHD2 (unknown origin) expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQ... |
J Med Chem 61: 6964-6982 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01686
BindingDB Entry DOI: 10.7270/Q2H70JC7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM234969

(US9556148, 40 | n-(cyanomethyl)-4-(5-methyl-2-((4-...)Show SMILES Cc1cnc(Nc2ccc(cc2)N2CCC(CC2)N2CCOCC2)nc1-c1ccc(cc1)C(=O)NCC#N Show InChI InChI=1S/C29H33N7O2/c1-21-20-32-29(34-27(21)22-2-4-23(5-3-22)28(37)31-13-12-30)33-24-6-8-25(9-7-24)35-14-10-26(11-15-35)36-16-18-38-19-17-36/h2-9,20,26H,10-11,13-19H2,1H3,(H,31,37)(H,32,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128728
BindingDB Entry DOI: 10.7270/Q2474FX8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase WNK1 [166-489]
(Homo sapiens (Human)) | BDBM203827
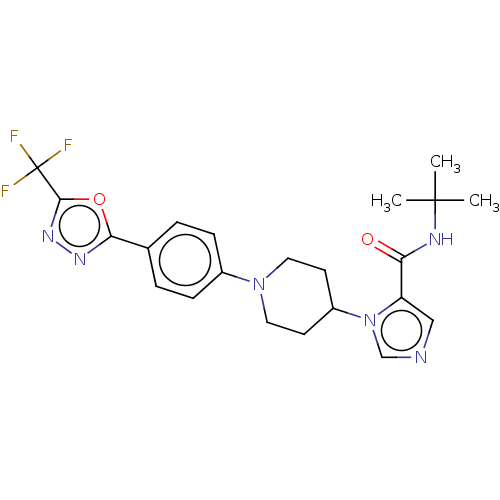
(N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...)Show SMILES CC(C)(C)NC(=O)c1cncn1C1CCN(CC1)c1ccc(cc1)-c1nnc(o1)C(F)(F)F Show InChI InChI=1S/C22H25F3N6O2/c1-21(2,3)27-18(32)17-12-26-13-31(17)16-8-10-30(11-9-16)15-6-4-14(5-7-15)19-28-29-20(33-19)22(23,24)25/h4-7,12-13,16H,8-11H2,1-3H3,(H,27,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes
| Assay Description
Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... |
Nat Chem Biol 12: 896-898 (2016)
Article DOI: 10.1038/nchembio.2168
BindingDB Entry DOI: 10.7270/Q2ZK5FH5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50600312
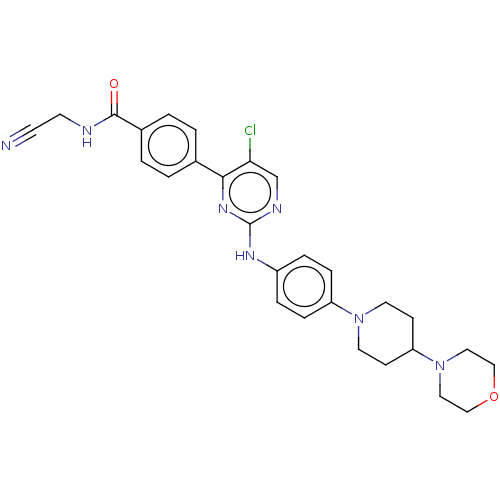
(CHEMBL5189266)Show SMILES Clc1cnc(Nc2ccc(cc2)N2CCC(CC2)N2CCOCC2)nc1-c1ccc(cc1)C(=O)NCC#N | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128728
BindingDB Entry DOI: 10.7270/Q2474FX8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data