 Found 150 hits with Last Name = 'mohr' and Initial = 'm'
Found 150 hits with Last Name = 'mohr' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50082930
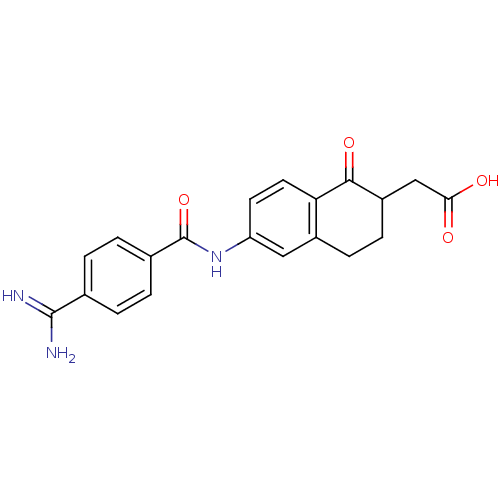
(CHEMBL117662 | [6-(4-Carbamimidoyl-benzoylamino)-1...)Show SMILES NC(=N)c1ccc(cc1)C(=O)Nc1ccc2C(=O)C(CC(O)=O)CCc2c1 Show InChI InChI=1S/C20H19N3O4/c21-19(22)11-1-3-12(4-2-11)20(27)23-15-7-8-16-13(9-15)5-6-14(18(16)26)10-17(24)25/h1-4,7-9,14H,5-6,10H2,(H3,21,22)(H,23,27)(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to purified human alpha IIb beta3 integrin (GP IIb-IIIa) |
J Med Chem 42: 4875-89 (1999)
BindingDB Entry DOI: 10.7270/Q2NC60DZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50082933
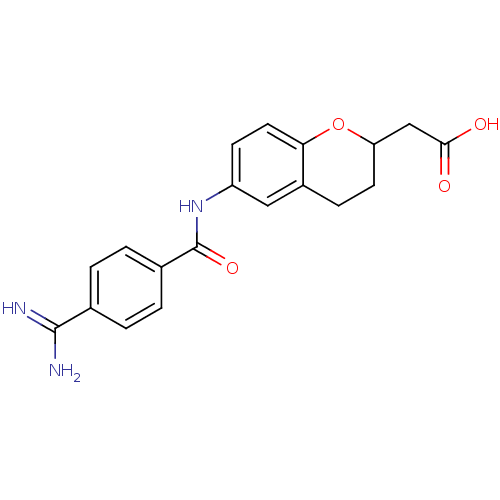
(CHEMBL323720 | [6-(4-Carbamimidoyl-benzoylamino)-c...)Show SMILES NC(=N)c1ccc(cc1)C(=O)Nc1ccc2OC(CC(O)=O)CCc2c1 Show InChI InChI=1S/C19H19N3O4/c20-18(21)11-1-3-12(4-2-11)19(25)22-14-6-8-16-13(9-14)5-7-15(26-16)10-17(23)24/h1-4,6,8-9,15H,5,7,10H2,(H3,20,21)(H,22,25)(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to purified human alpha IIb beta3 integrin (GP IIb-IIIa) |
J Med Chem 42: 4875-89 (1999)
BindingDB Entry DOI: 10.7270/Q2NC60DZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50058914
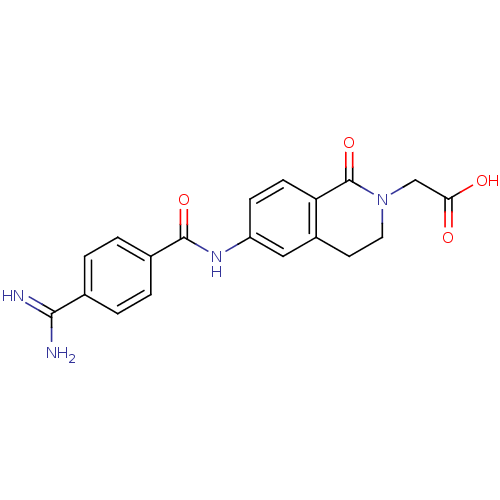
(CHEMBL303597 | [6-(4-Carbamimidoyl-benzoylamino)-1...)Show SMILES NC(=N)c1ccc(cc1)C(=O)Nc1ccc2C(=O)N(CC(O)=O)CCc2c1 Show InChI InChI=1S/C19H18N4O4/c20-17(21)11-1-3-12(4-2-11)18(26)22-14-5-6-15-13(9-14)7-8-23(19(15)27)10-16(24)25/h1-6,9H,7-8,10H2,(H3,20,21)(H,22,26)(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to purified human alpha IIb beta3 integrin (GP IIb-IIIa) |
J Med Chem 42: 4875-89 (1999)
BindingDB Entry DOI: 10.7270/Q2NC60DZ |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit alpha/beta/delta/gamma
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128281
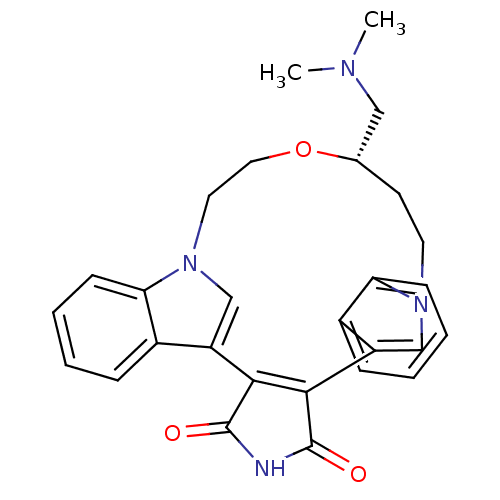
(18-dimethylaminomethyl-(18S)-17-oxa-4,14,21-triaza...)Show SMILES CN(C)C[C@@H]1CCn2cc(C3=C(C(=O)NC3=O)c3cn(CCO1)c1ccccc31)c1ccccc21 |t:10| Show InChI InChI=1S/C28H28N4O3/c1-30(2)15-18-11-12-31-16-21(19-7-3-5-9-23(19)31)25-26(28(34)29-27(25)33)22-17-32(13-14-35-18)24-10-6-4-8-20(22)24/h3-10,16-18H,11-15H2,1-2H3,(H,29,33,34)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128280
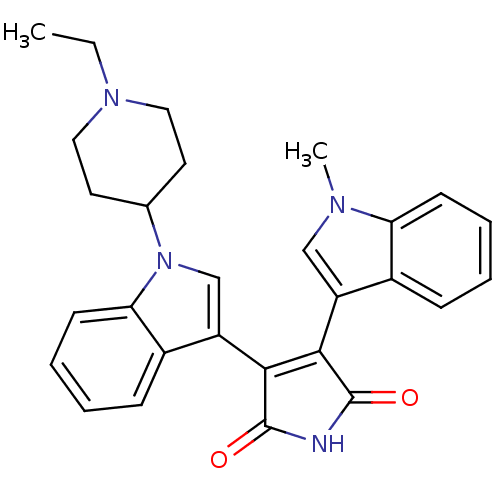
(3-[1-(1-Ethyl-piperidin-4-yl)-1H-indol-3-yl]-4-(1-...)Show SMILES CCN1CCC(CC1)n1cc(C2=C(C(=O)NC2=O)c2cn(C)c3ccccc23)c2ccccc12 |t:12| Show InChI InChI=1S/C28H28N4O2/c1-3-31-14-12-18(13-15-31)32-17-22(20-9-5-7-11-24(20)32)26-25(27(33)29-28(26)34)21-16-30(2)23-10-6-4-8-19(21)23/h4-11,16-18H,3,12-15H2,1-2H3,(H,29,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50082932
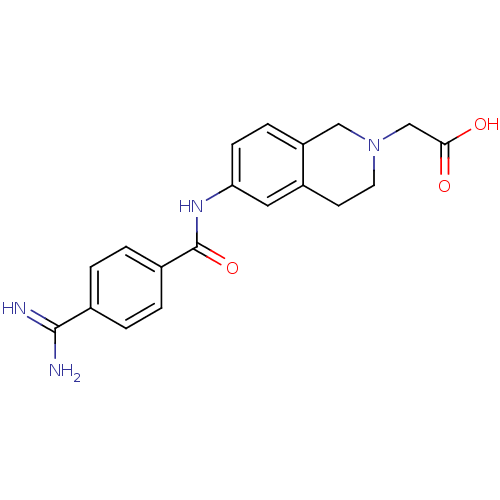
(CHEMBL147258 | [6-(4-Carbamimidoyl-benzoylamino)-1...)Show SMILES NC(=N)c1ccc(cc1)C(=O)Nc1ccc2CC(CC(O)=O)CCc2c1 Show InChI InChI=1S/C20H21N3O3/c21-19(22)13-3-5-14(6-4-13)20(26)23-17-8-7-15-9-12(10-18(24)25)1-2-16(15)11-17/h3-8,11-12H,1-2,9-10H2,(H3,21,22)(H,23,26)(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to purified human alpha IIb beta3 integrin (GP IIb-IIIa) |
J Med Chem 42: 4875-89 (1999)
BindingDB Entry DOI: 10.7270/Q2NC60DZ |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C eta |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15471

(4-[(1-{[2-oxo-2-(thiophen-3-yl)ethyl]amino}cyclope...)Show InChI InChI=1S/C19H22N2O3S/c20-18(23)14-3-5-16(6-4-14)24-13-19(8-1-2-9-19)21-11-17(22)15-7-10-25-12-15/h3-7,10,12,21H,1-2,8-9,11,13H2,(H2,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
Bioorg Med Chem Lett 17: 1765-8 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.074
BindingDB Entry DOI: 10.7270/Q247483F |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50082931
(CHEMBL148205 | [6-(4-Carbamimidoyl-benzoylamino)-3...)Show SMILES NC(=N)c1ccc(cc1)C(=O)Nc1ccc2CN(CC(O)=O)CCc2c1 Show InChI InChI=1S/C19H20N4O3/c20-18(21)12-1-3-13(4-2-12)19(26)22-16-6-5-15-10-23(11-17(24)25)8-7-14(15)9-16/h1-6,9H,7-8,10-11H2,(H3,20,21)(H,22,26)(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to purified human alpha IIb beta3 integrin (GP IIb-IIIa) |
J Med Chem 42: 4875-89 (1999)
BindingDB Entry DOI: 10.7270/Q2NC60DZ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128281

(18-dimethylaminomethyl-(18S)-17-oxa-4,14,21-triaza...)Show SMILES CN(C)C[C@@H]1CCn2cc(C3=C(C(=O)NC3=O)c3cn(CCO1)c1ccccc31)c1ccccc21 |t:10| Show InChI InChI=1S/C28H28N4O3/c1-30(2)15-18-11-12-31-16-21(19-7-3-5-9-23(19)31)25-26(28(34)29-27(25)33)22-17-32(13-14-35-18)24-10-6-4-8-20(22)24/h3-10,16-18H,11-15H2,1-2H3,(H,29,33,34)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128282
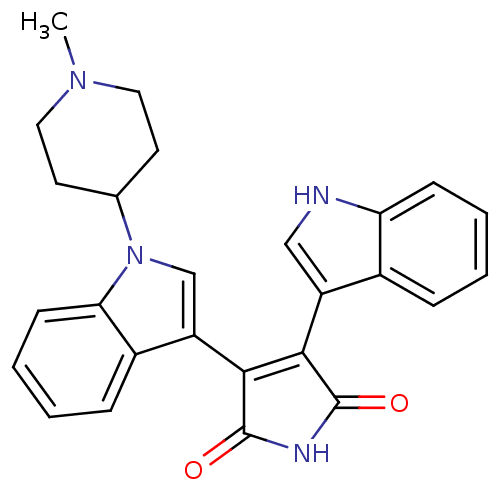
(3-(1H-Indol-3-yl)-4-[1-(1-methyl-piperidin-4-yl)-1...)Show SMILES CN1CCC(CC1)n1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc12 |t:11| Show InChI InChI=1S/C26H24N4O2/c1-29-12-10-16(11-13-29)30-15-20(18-7-3-5-9-22(18)30)24-23(25(31)28-26(24)32)19-14-27-21-8-4-2-6-17(19)21/h2-9,14-16,27H,10-13H2,1H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128290
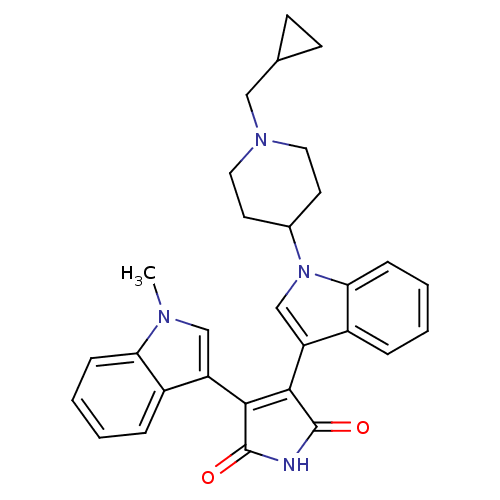
(3-[1-(1-Cyclopropylmethyl-piperidin-4-yl)-1H-indol...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(C3CCN(CC4CC4)CC3)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C30H30N4O2/c1-32-17-23(21-6-2-4-8-25(21)32)27-28(30(36)31-29(27)35)24-18-34(26-9-5-3-7-22(24)26)20-12-14-33(15-13-20)16-19-10-11-19/h2-9,17-20H,10-16H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128287
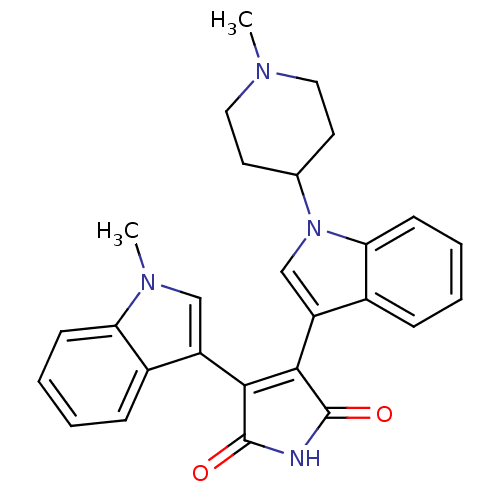
(3-(1-Methyl-1H-indol-3-yl)-4-[1-(1-methyl-piperidi...)Show SMILES CN1CCC(CC1)n1cc(C2=C(C(=O)NC2=O)c2cn(C)c3ccccc23)c2ccccc12 |t:11| Show InChI InChI=1S/C27H26N4O2/c1-29-13-11-17(12-14-29)31-16-21(19-8-4-6-10-23(19)31)25-24(26(32)28-27(25)33)20-15-30(2)22-9-5-3-7-18(20)22/h3-10,15-17H,11-14H2,1-2H3,(H,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C epsilon |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128286

(3-[1-(1-Isopropyl-piperidin-4-yl)-1H-indol-3-yl]-4...)Show SMILES CC(C)N1CCC(CC1)n1cc(C2=C(C(=O)NC2=O)c2cn(C)c3ccccc23)c2ccccc12 |t:13| Show InChI InChI=1S/C29H30N4O2/c1-18(2)32-14-12-19(13-15-32)33-17-23(21-9-5-7-11-25(21)33)27-26(28(34)30-29(27)35)22-16-31(3)24-10-6-4-8-20(22)24/h4-11,16-19H,12-15H2,1-3H3,(H,30,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128282

(3-(1H-Indol-3-yl)-4-[1-(1-methyl-piperidin-4-yl)-1...)Show SMILES CN1CCC(CC1)n1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc12 |t:11| Show InChI InChI=1S/C26H24N4O2/c1-29-12-10-16(11-13-29)30-15-20(18-7-3-5-9-22(18)30)24-23(25(31)28-26(24)32)19-14-27-21-8-4-2-6-17(19)21/h2-9,14-16,27H,10-13H2,1H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128280

(3-[1-(1-Ethyl-piperidin-4-yl)-1H-indol-3-yl]-4-(1-...)Show SMILES CCN1CCC(CC1)n1cc(C2=C(C(=O)NC2=O)c2cn(C)c3ccccc23)c2ccccc12 |t:12| Show InChI InChI=1S/C28H28N4O2/c1-3-31-14-12-18(13-15-31)32-17-22(20-9-5-7-11-24(20)32)26-25(27(33)29-28(26)34)21-16-30(2)23-10-6-4-8-19(21)23/h4-11,16-18H,3,12-15H2,1-2H3,(H,29,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128287

(3-(1-Methyl-1H-indol-3-yl)-4-[1-(1-methyl-piperidi...)Show SMILES CN1CCC(CC1)n1cc(C2=C(C(=O)NC2=O)c2cn(C)c3ccccc23)c2ccccc12 |t:11| Show InChI InChI=1S/C27H26N4O2/c1-29-13-11-17(12-14-29)31-16-21(19-8-4-6-10-23(19)31)25-24(26(32)28-27(25)33)20-15-30(2)22-9-5-3-7-18(20)22/h3-10,15-17H,11-14H2,1-2H3,(H,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128283
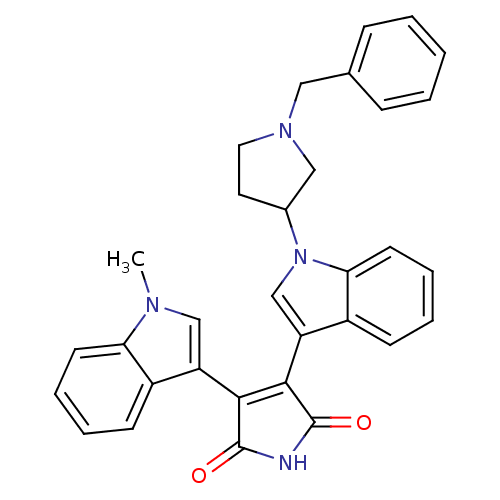
(3-[1-(1-Benzyl-pyrrolidin-3-yl)-1H-indol-3-yl]-4-(...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(C3CCN(Cc4ccccc4)C3)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C32H28N4O2/c1-34-19-25(23-11-5-7-13-27(23)34)29-30(32(38)33-31(29)37)26-20-36(28-14-8-6-12-24(26)28)22-15-16-35(18-22)17-21-9-3-2-4-10-21/h2-14,19-20,22H,15-18H2,1H3,(H,33,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128283

(3-[1-(1-Benzyl-pyrrolidin-3-yl)-1H-indol-3-yl]-4-(...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(C3CCN(Cc4ccccc4)C3)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C32H28N4O2/c1-34-19-25(23-11-5-7-13-27(23)34)29-30(32(38)33-31(29)37)26-20-36(28-14-8-6-12-24(26)28)22-15-16-35(18-22)17-21-9-3-2-4-10-21/h2-14,19-20,22H,15-18H2,1H3,(H,33,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128286

(3-[1-(1-Isopropyl-piperidin-4-yl)-1H-indol-3-yl]-4...)Show SMILES CC(C)N1CCC(CC1)n1cc(C2=C(C(=O)NC2=O)c2cn(C)c3ccccc23)c2ccccc12 |t:13| Show InChI InChI=1S/C29H30N4O2/c1-18(2)32-14-12-19(13-15-32)33-17-23(21-9-5-7-11-25(21)33)27-26(28(34)30-29(27)35)22-16-31(3)24-10-6-4-8-20(22)24/h4-11,16-19H,12-15H2,1-3H3,(H,30,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50082927
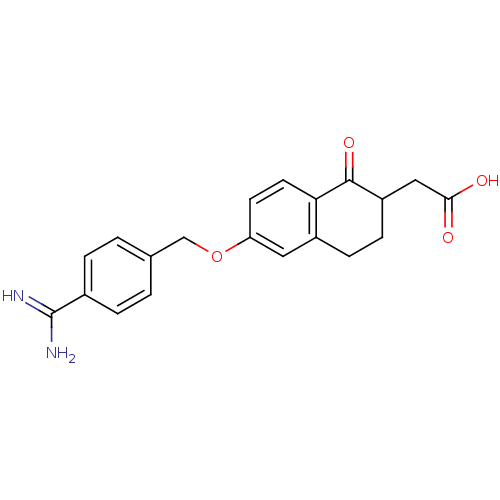
(CHEMBL344117 | [6-(4-Carbamimidoyl-benzyloxy)-1-ox...)Show SMILES NC(=N)c1ccc(COc2ccc3C(=O)C(CC(O)=O)CCc3c2)cc1 Show InChI InChI=1S/C20H20N2O4/c21-20(22)13-3-1-12(2-4-13)11-26-16-7-8-17-14(9-16)5-6-15(19(17)25)10-18(23)24/h1-4,7-9,15H,5-6,10-11H2,(H3,21,22)(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to purified human alpha IIb beta3 integrin (GP IIb-IIIa) |
J Med Chem 42: 4875-89 (1999)
BindingDB Entry DOI: 10.7270/Q2NC60DZ |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C delta |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128290

(3-[1-(1-Cyclopropylmethyl-piperidin-4-yl)-1H-indol...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(C3CCN(CC4CC4)CC3)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C30H30N4O2/c1-32-17-23(21-6-2-4-8-25(21)32)27-28(30(36)31-29(27)35)24-18-34(26-9-5-3-7-22(24)26)20-12-14-33(15-13-20)16-19-10-11-19/h2-9,17-20H,10-16H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128288
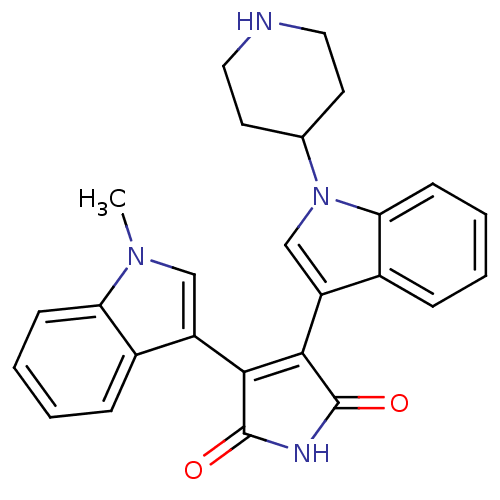
(3-(1-Methyl-1H-indol-3-yl)-4-(1-piperidin-4-yl-1H-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(C3CCNCC3)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C26H24N4O2/c1-29-14-19(17-6-2-4-8-21(17)29)23-24(26(32)28-25(23)31)20-15-30(16-10-12-27-13-11-16)22-9-5-3-7-18(20)22/h2-9,14-16,27H,10-13H2,1H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128288

(3-(1-Methyl-1H-indol-3-yl)-4-(1-piperidin-4-yl-1H-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(C3CCNCC3)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C26H24N4O2/c1-29-14-19(17-6-2-4-8-21(17)29)23-24(26(32)28-25(23)31)20-15-30(16-10-12-27-13-11-16)22-9-5-3-7-18(20)22/h2-9,14-16,27H,10-13H2,1H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128285
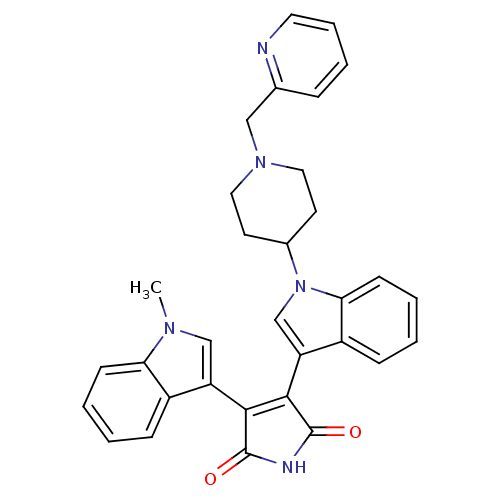
(3-(1-Methyl-1H-indol-3-yl)-4-[1-(1-pyridin-2-ylmet...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(C3CCN(Cc4ccccn4)CC3)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C32H29N5O2/c1-35-19-25(23-9-2-4-11-27(23)35)29-30(32(39)34-31(29)38)26-20-37(28-12-5-3-10-24(26)28)22-13-16-36(17-14-22)18-21-8-6-7-15-33-21/h2-12,15,19-20,22H,13-14,16-18H2,1H3,(H,34,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50082928
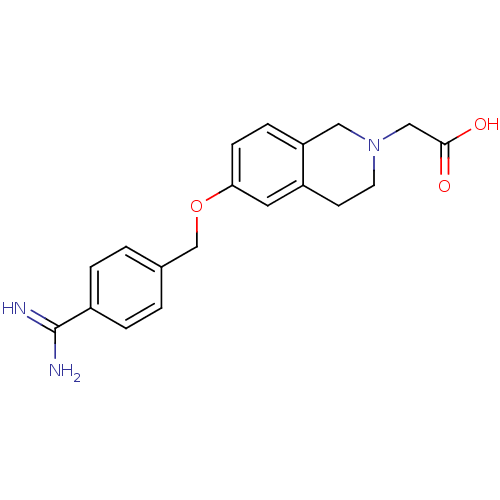
(CHEMBL439620 | [6-(4-Carbamimidoyl-benzyloxy)-3,4-...)Show InChI InChI=1S/C19H21N3O3/c20-19(21)14-3-1-13(2-4-14)12-25-17-6-5-16-10-22(11-18(23)24)8-7-15(16)9-17/h1-6,9H,7-8,10-12H2,(H3,20,21)(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to purified human alpha IIb beta3 integrin (GP IIb-IIIa) |
J Med Chem 42: 4875-89 (1999)
BindingDB Entry DOI: 10.7270/Q2NC60DZ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128284
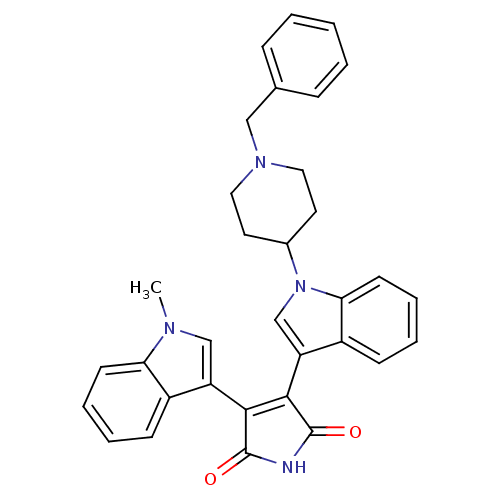
(3-(1-(1-benzylpiperidin-4-yl)-1H-indol-3-yl)-4-(1-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(C3CCN(Cc4ccccc4)CC3)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C33H30N4O2/c1-35-20-26(24-11-5-7-13-28(24)35)30-31(33(39)34-32(30)38)27-21-37(29-14-8-6-12-25(27)29)23-15-17-36(18-16-23)19-22-9-3-2-4-10-22/h2-14,20-21,23H,15-19H2,1H3,(H,34,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128285

(3-(1-Methyl-1H-indol-3-yl)-4-[1-(1-pyridin-2-ylmet...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(C3CCN(Cc4ccccn4)CC3)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C32H29N5O2/c1-35-19-25(23-9-2-4-11-27(23)35)29-30(32(39)34-31(29)38)26-20-37(28-12-5-3-10-24(26)28)22-13-16-36(17-14-22)18-21-8-6-7-15-33-21/h2-12,15,19-20,22H,13-14,16-18H2,1H3,(H,34,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15465
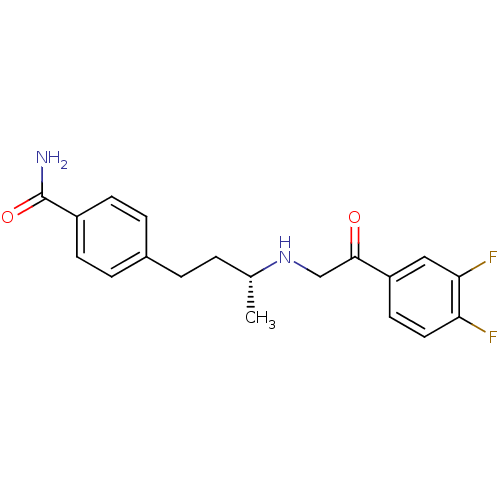
(4-[(3R)-3-{[2-(3,4-difluorophenyl)-2-oxoethyl]amin...)Show SMILES C[C@H](CCc1ccc(cc1)C(N)=O)NCC(=O)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C19H20F2N2O2/c1-12(2-3-13-4-6-14(7-5-13)19(22)25)23-11-18(24)15-8-9-16(20)17(21)10-15/h4-10,12,23H,2-3,11H2,1H3,(H2,22,25)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
Bioorg Med Chem Lett 17: 1765-8 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.074
BindingDB Entry DOI: 10.7270/Q247483F |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50082926
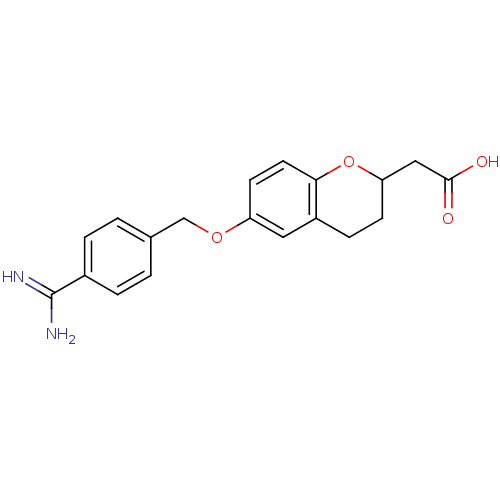
(CHEMBL144408 | [6-(4-Carbamimidoyl-benzyloxy)-chro...)Show InChI InChI=1S/C19H20N2O4/c20-19(21)13-3-1-12(2-4-13)11-24-15-7-8-17-14(9-15)5-6-16(25-17)10-18(22)23/h1-4,7-9,16H,5-6,10-11H2,(H3,20,21)(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to purified human alpha IIb beta3 integrin (GP IIb-IIIa) |
J Med Chem 42: 4875-89 (1999)
BindingDB Entry DOI: 10.7270/Q2NC60DZ |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50128280

(3-[1-(1-Ethyl-piperidin-4-yl)-1H-indol-3-yl]-4-(1-...)Show SMILES CCN1CCC(CC1)n1cc(C2=C(C(=O)NC2=O)c2cn(C)c3ccccc23)c2ccccc12 |t:12| Show InChI InChI=1S/C28H28N4O2/c1-3-31-14-12-18(13-15-31)32-17-22(20-9-5-7-11-24(20)32)26-25(27(33)29-28(26)34)21-16-30(2)23-10-6-4-8-19(21)23/h4-11,16-18H,3,12-15H2,1-2H3,(H,29,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C eta |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50128282

(3-(1H-Indol-3-yl)-4-[1-(1-methyl-piperidin-4-yl)-1...)Show SMILES CN1CCC(CC1)n1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc12 |t:11| Show InChI InChI=1S/C26H24N4O2/c1-29-12-10-16(11-13-29)30-15-20(18-7-3-5-9-22(18)30)24-23(25(31)28-26(24)32)19-14-27-21-8-4-2-6-17(19)21/h2-9,14-16,27H,10-13H2,1H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C eta |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50128283

(3-[1-(1-Benzyl-pyrrolidin-3-yl)-1H-indol-3-yl]-4-(...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(C3CCN(Cc4ccccc4)C3)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C32H28N4O2/c1-34-19-25(23-11-5-7-13-27(23)34)29-30(32(38)33-31(29)37)26-20-36(28-14-8-6-12-24(26)28)22-15-16-35(18-22)17-21-9-3-2-4-10-21/h2-14,19-20,22H,15-18H2,1H3,(H,33,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C eta |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128284

(3-(1-(1-benzylpiperidin-4-yl)-1H-indol-3-yl)-4-(1-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(C3CCN(Cc4ccccc4)CC3)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C33H30N4O2/c1-35-20-26(24-11-5-7-13-28(24)35)30-31(33(39)34-32(30)38)27-21-37(29-14-8-6-12-25(27)29)23-15-17-36(18-16-23)19-22-9-3-2-4-10-22/h2-14,20-21,23H,15-19H2,1H3,(H,34,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 1 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50128282

(3-(1H-Indol-3-yl)-4-[1-(1-methyl-piperidin-4-yl)-1...)Show SMILES CN1CCC(CC1)n1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc12 |t:11| Show InChI InChI=1S/C26H24N4O2/c1-29-12-10-16(11-13-29)30-15-20(18-7-3-5-9-22(18)30)24-23(25(31)28-26(24)32)19-14-27-21-8-4-2-6-17(19)21/h2-9,14-16,27H,10-13H2,1H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C epsilon |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50128287

(3-(1-Methyl-1H-indol-3-yl)-4-[1-(1-methyl-piperidi...)Show SMILES CN1CCC(CC1)n1cc(C2=C(C(=O)NC2=O)c2cn(C)c3ccccc23)c2ccccc12 |t:11| Show InChI InChI=1S/C27H26N4O2/c1-29-13-11-17(12-14-29)31-16-21(19-8-4-6-10-23(19)31)25-24(26(32)28-27(25)33)20-15-30(2)22-9-5-3-7-18(20)22/h3-10,15-17H,11-14H2,1-2H3,(H,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C eta |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50128290

(3-[1-(1-Cyclopropylmethyl-piperidin-4-yl)-1H-indol...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(C3CCN(CC4CC4)CC3)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C30H30N4O2/c1-32-17-23(21-6-2-4-8-25(21)32)27-28(30(36)31-29(27)35)24-18-34(26-9-5-3-7-22(24)26)20-12-14-33(15-13-20)16-19-10-11-19/h2-9,17-20H,10-16H2,1H3,(H,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C eta |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50128281

(18-dimethylaminomethyl-(18S)-17-oxa-4,14,21-triaza...)Show SMILES CN(C)C[C@@H]1CCn2cc(C3=C(C(=O)NC3=O)c3cn(CCO1)c1ccccc31)c1ccccc21 |t:10| Show InChI InChI=1S/C28H28N4O3/c1-30(2)15-18-11-12-31-16-21(19-7-3-5-9-23(19)31)25-26(28(34)29-27(25)33)22-17-32(13-14-35-18)24-10-6-4-8-20(22)24/h3-10,16-18H,11-15H2,1-2H3,(H,29,33,34)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C eta |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50128289

(4-{3-[4-(1-Methyl-1H-indol-3-yl)-2,5-dioxo-2,5-dih...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(C3CCN(CC3)C(=O)OC(C)(C)C)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C31H32N4O4/c1-31(2,3)39-30(38)34-15-13-19(14-16-34)35-18-23(21-10-6-8-12-25(21)35)27-26(28(36)32-29(27)37)22-17-33(4)24-11-7-5-9-20(22)24/h5-12,17-19H,13-16H2,1-4H3,(H,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15464
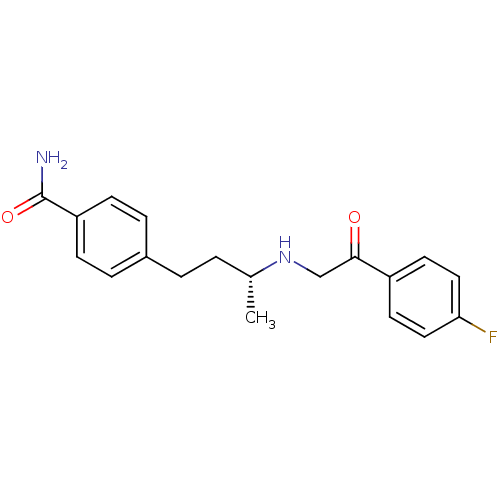
(4-[(3R)-3-{[2-(4-fluorophenyl)-2-oxoethyl]amino}bu...)Show SMILES C[C@H](CCc1ccc(cc1)C(N)=O)NCC(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H21FN2O2/c1-13(2-3-14-4-6-16(7-5-14)19(21)24)22-12-18(23)15-8-10-17(20)11-9-15/h4-11,13,22H,2-3,12H2,1H3,(H2,21,24)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
Bioorg Med Chem Lett 17: 1765-8 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.074
BindingDB Entry DOI: 10.7270/Q247483F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15469
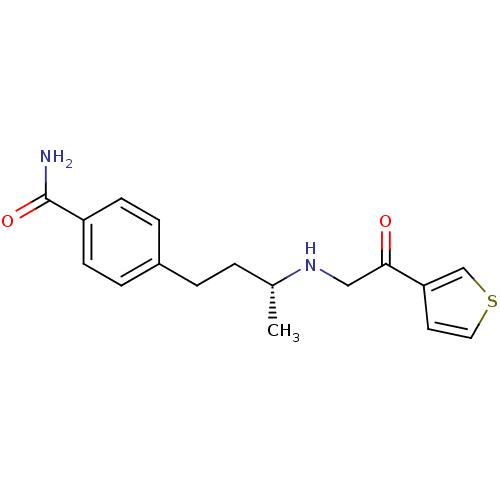
(4-[(3R)-3-{[2-oxo-2-(thiophen-3-yl)ethyl]amino}but...)Show SMILES C[C@H](CCc1ccc(cc1)C(N)=O)NCC(=O)c1ccsc1 |r| Show InChI InChI=1S/C17H20N2O2S/c1-12(19-10-16(20)15-8-9-22-11-15)2-3-13-4-6-14(7-5-13)17(18)21/h4-9,11-12,19H,2-3,10H2,1H3,(H2,18,21)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
Bioorg Med Chem Lett 17: 1765-8 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.074
BindingDB Entry DOI: 10.7270/Q247483F |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C gamma |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50128286

(3-[1-(1-Isopropyl-piperidin-4-yl)-1H-indol-3-yl]-4...)Show SMILES CC(C)N1CCC(CC1)n1cc(C2=C(C(=O)NC2=O)c2cn(C)c3ccccc23)c2ccccc12 |t:13| Show InChI InChI=1S/C29H30N4O2/c1-18(2)32-14-12-19(13-15-32)33-17-23(21-9-5-7-11-25(21)33)27-26(28(34)30-29(27)35)22-16-31(3)24-10-6-4-8-20(22)24/h4-11,16-19H,12-15H2,1-3H3,(H,30,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C eta |
Bioorg Med Chem Lett 13: 1857-9 (2003)
BindingDB Entry DOI: 10.7270/Q2319V87 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data