 Found 429 hits with Last Name = 'murakami' and Initial = 'm'
Found 429 hits with Last Name = 'murakami' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50095778
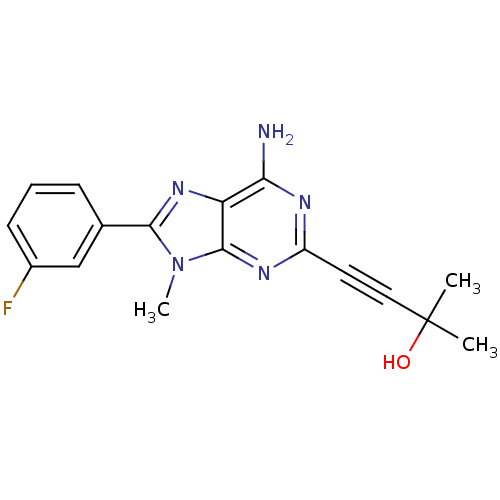
(4-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC(C)(C)O)-c1cccc(F)c1 Show InChI InChI=1S/C17H16FN5O/c1-17(2,24)8-7-12-20-14(19)13-16(21-12)23(3)15(22-13)10-5-4-6-11(18)9-10/h4-6,9,24H,1-3H3,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50095790
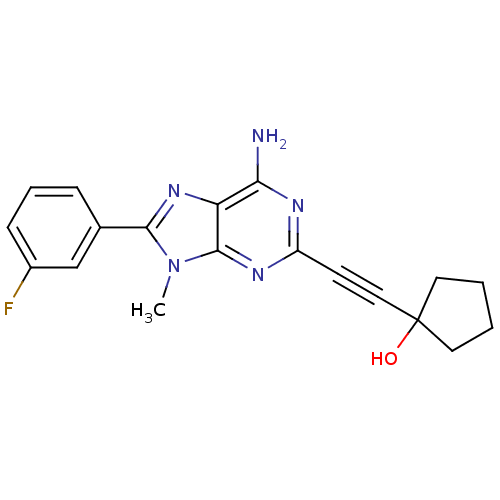
(1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC1(O)CCCC1)-c1cccc(F)c1 Show InChI InChI=1S/C19H18FN5O/c1-25-17(12-5-4-6-13(20)11-12)24-15-16(21)22-14(23-18(15)25)7-10-19(26)8-2-3-9-19/h4-6,11,26H,2-3,8-9H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50095786
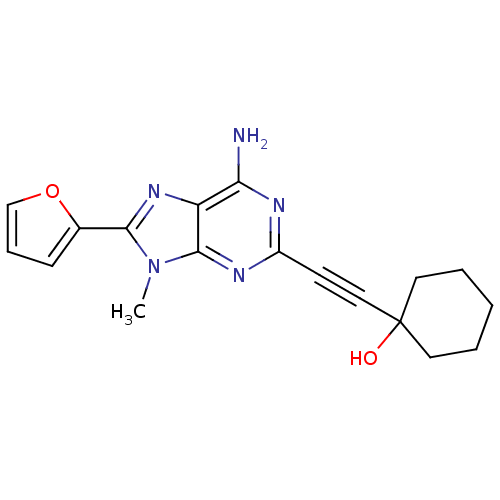
(1-(6-Amino-8-furan-2-yl-9-methyl-9H-purin-2-ylethy...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC1(O)CCCCC1)-c1ccco1 Show InChI InChI=1S/C18H19N5O2/c1-23-16(12-6-5-11-25-12)22-14-15(19)20-13(21-17(14)23)7-10-18(24)8-3-2-4-9-18/h5-6,11,24H,2-4,8-9H2,1H3,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50095787
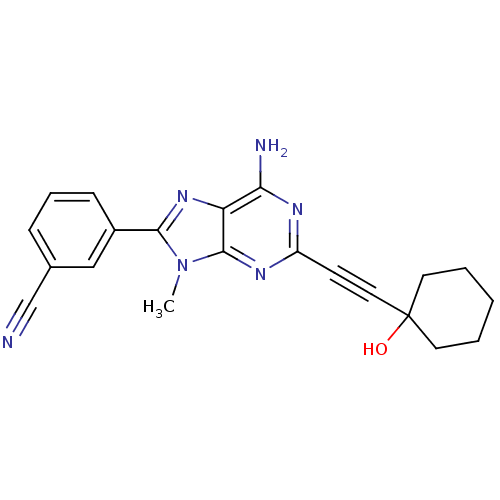
(3-[6-Amino-2-(1-hydroxy-cyclohexylethynyl)-9-methy...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC1(O)CCCCC1)-c1cccc(c1)C#N Show InChI InChI=1S/C21H20N6O/c1-27-19(15-7-5-6-14(12-15)13-22)26-17-18(23)24-16(25-20(17)27)8-11-21(28)9-3-2-4-10-21/h5-7,12,28H,2-4,9-10H2,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50059376
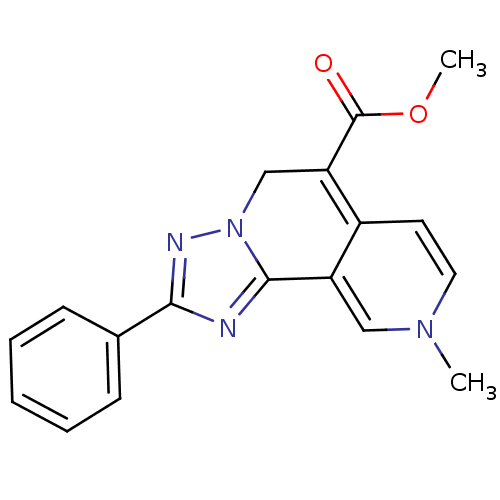
(9-Methyl-2-phenyl-5,9-dihydro-[1,2,4]triazolo[5,1-...)Show SMILES COC(=O)C1=C2C=CN(C)C=C2c2nc(nn2C1)-c1ccccc1 |c:4,6,10| Show InChI InChI=1S/C18H16N4O2/c1-21-9-8-13-14(10-21)17-19-16(12-6-4-3-5-7-12)20-22(17)11-15(13)18(23)24-2/h3-10H,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50095784
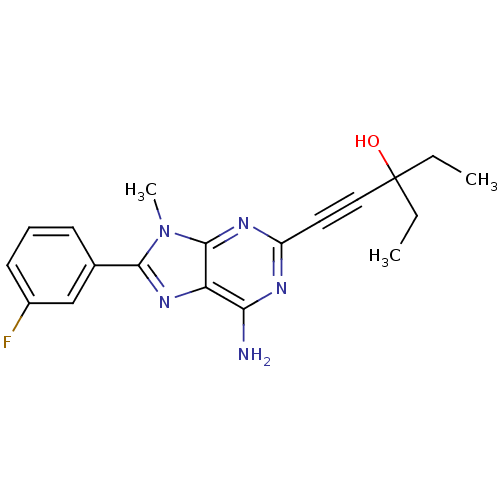
(1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...)Show SMILES CCC(O)(CC)C#Cc1nc(N)c2nc(-c3cccc(F)c3)n(C)c2n1 Show InChI InChI=1S/C19H20FN5O/c1-4-19(26,5-2)10-9-14-22-16(21)15-18(23-14)25(3)17(24-15)12-7-6-8-13(20)11-12/h6-8,11,26H,4-5H2,1-3H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50095793
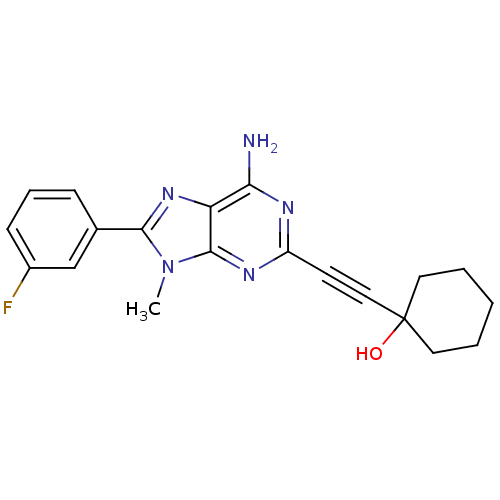
(1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC1(O)CCCCC1)-c1cccc(F)c1 Show InChI InChI=1S/C20H20FN5O/c1-26-18(13-6-5-7-14(21)12-13)25-16-17(22)23-15(24-19(16)26)8-11-20(27)9-3-2-4-10-20/h5-7,12,27H,2-4,9-10H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50095788
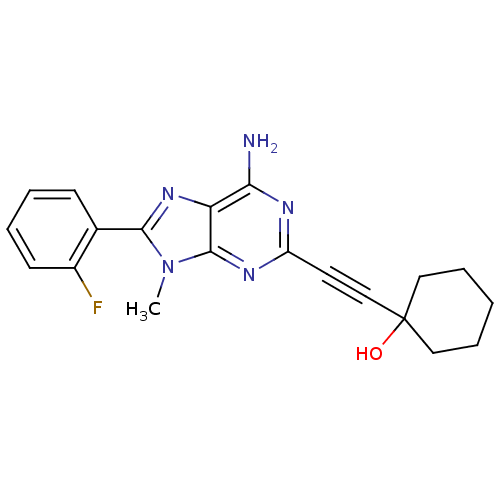
(1-[6-Amino-8-(2-fluoro-phenyl)-9-methyl-9H-purin-2...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC1(O)CCCCC1)-c1ccccc1F Show InChI InChI=1S/C20H20FN5O/c1-26-18(13-7-3-4-8-14(13)21)25-16-17(22)23-15(24-19(16)26)9-12-20(27)10-5-2-6-11-20/h3-4,7-8,27H,2,5-6,10-11H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50095793

(1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC1(O)CCCCC1)-c1cccc(F)c1 Show InChI InChI=1S/C20H20FN5O/c1-26-18(13-6-5-7-14(21)12-13)25-16-17(22)23-15(24-19(16)26)8-11-20(27)9-3-2-4-10-20/h5-7,12,27H,2-4,9-10H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50079652
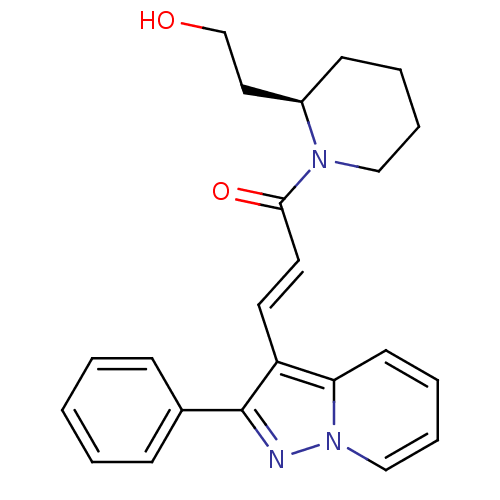
((E)-1-[(R)-2-(2-Hydroxy-ethyl)-piperidin-1-yl]-3-(...)Show SMILES OCC[C@H]1CCCCN1C(=O)\C=C\c1c(nn2ccccc12)-c1ccccc1 Show InChI InChI=1S/C23H25N3O2/c27-17-14-19-10-4-6-15-25(19)22(28)13-12-20-21-11-5-7-16-26(21)24-23(20)18-8-2-1-3-9-18/h1-3,5,7-9,11-13,16,19,27H,4,6,10,14-15,17H2/b13-12+/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50095788

(1-[6-Amino-8-(2-fluoro-phenyl)-9-methyl-9H-purin-2...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC1(O)CCCCC1)-c1ccccc1F Show InChI InChI=1S/C20H20FN5O/c1-26-18(13-7-3-4-8-14(13)21)25-16-17(22)23-15(24-19(16)26)9-12-20(27)10-5-2-6-11-20/h3-4,7-8,27H,2,5-6,10-11H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50095781
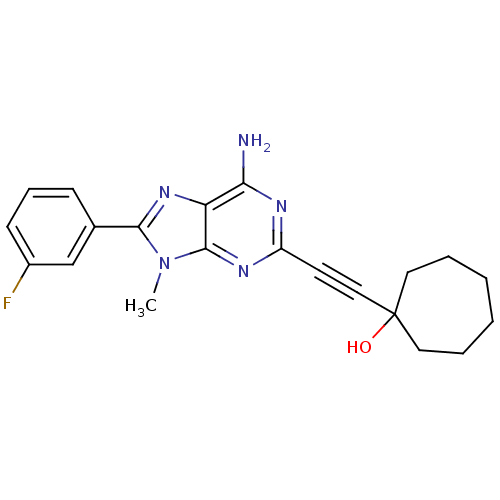
(1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC1(O)CCCCCC1)-c1cccc(F)c1 Show InChI InChI=1S/C21H22FN5O/c1-27-19(14-7-6-8-15(22)13-14)26-17-18(23)24-16(25-20(17)27)9-12-21(28)10-4-2-3-5-11-21/h6-8,13,28H,2-5,10-11H2,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50095790

(1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC1(O)CCCC1)-c1cccc(F)c1 Show InChI InChI=1S/C19H18FN5O/c1-25-17(12-5-4-6-13(20)11-12)24-15-16(21)22-14(23-18(15)25)7-10-19(26)8-2-3-9-19/h4-6,11,26H,2-3,8-9H2,1H3,(H2,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50095786

(1-(6-Amino-8-furan-2-yl-9-methyl-9H-purin-2-ylethy...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC1(O)CCCCC1)-c1ccco1 Show InChI InChI=1S/C18H19N5O2/c1-23-16(12-6-5-11-25-12)22-14-15(19)20-13(21-17(14)23)7-10-18(24)8-3-2-4-9-18/h5-6,11,24H,2-4,8-9H2,1H3,(H2,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50095781

(1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC1(O)CCCCCC1)-c1cccc(F)c1 Show InChI InChI=1S/C21H22FN5O/c1-27-19(14-7-6-8-15(22)13-14)26-17-18(23)24-16(25-20(17)27)9-12-21(28)10-4-2-3-5-11-21/h6-8,13,28H,2-5,10-11H2,1H3,(H2,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50095784

(1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...)Show SMILES CCC(O)(CC)C#Cc1nc(N)c2nc(-c3cccc(F)c3)n(C)c2n1 Show InChI InChI=1S/C19H20FN5O/c1-4-19(26,5-2)10-9-14-22-16(21)15-18(23-14)25(3)17(24-15)12-7-6-8-13(20)11-12/h6-8,11,26H,4-5H2,1-3H3,(H2,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50095778

(4-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC(C)(C)O)-c1cccc(F)c1 Show InChI InChI=1S/C17H16FN5O/c1-17(2,24)8-7-12-20-14(19)13-16(21-12)23(3)15(22-13)10-5-4-6-11(18)9-10/h4-6,9,24H,1-3H3,(H2,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50006710
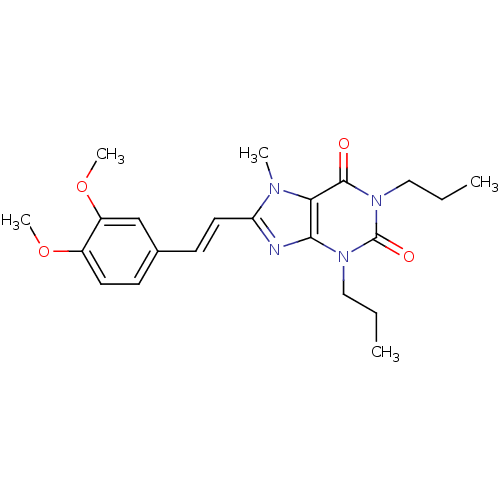
(8-[(E)-2-(3,4-Dimethoxy-phenyl)-vinyl]-7-methyl-1,...)Show SMILES CCCn1c2nc(\C=C\c3ccc(OC)c(OC)c3)n(C)c2c(=O)n(CCC)c1=O Show InChI InChI=1S/C22H28N4O4/c1-6-12-25-20-19(21(27)26(13-7-2)22(25)28)24(3)18(23-20)11-9-15-8-10-16(29-4)17(14-15)30-5/h8-11,14H,6-7,12-13H2,1-5H3/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity for adenosine A2A receptor expressed in HEK-293 cells compared to [3H]-CGS-21,680 |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50095787

(3-[6-Amino-2-(1-hydroxy-cyclohexylethynyl)-9-methy...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC1(O)CCCCC1)-c1cccc(c1)C#N Show InChI InChI=1S/C21H20N6O/c1-27-19(15-7-5-6-14(12-15)13-22)26-17-18(23)24-16(25-20(17)27)8-11-21(28)9-3-2-4-10-21/h5-7,12,28H,2-4,9-10H2,1H3,(H2,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50603613
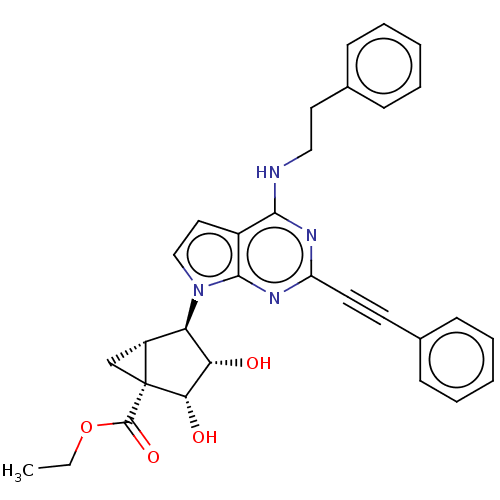
(CHEMBL5169644)Show SMILES [H][C@]12C[C@@]1([C@@H](O)[C@@H](O)[C@@H]2n1ccc2c(NCCc3ccccc3)nc(nc12)C#Cc1ccccc1)C(=O)OCC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114103
BindingDB Entry DOI: 10.7270/Q2PV6QGP |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50095784

(1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...)Show SMILES CCC(O)(CC)C#Cc1nc(N)c2nc(-c3cccc(F)c3)n(C)c2n1 Show InChI InChI=1S/C19H20FN5O/c1-4-19(26,5-2)10-9-14-22-16(21)15-18(23-14)25(3)17(24-15)12-7-6-8-13(20)11-12/h6-8,11,26H,4-5H2,1-3H3,(H2,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50603613

(CHEMBL5169644)Show SMILES [H][C@]12C[C@@]1([C@@H](O)[C@@H](O)[C@@H]2n1ccc2c(NCCc3ccccc3)nc(nc12)C#Cc1ccccc1)C(=O)OCC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 483 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114103
BindingDB Entry DOI: 10.7270/Q2PV6QGP |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50095793

(1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC1(O)CCCCC1)-c1cccc(F)c1 Show InChI InChI=1S/C20H20FN5O/c1-26-18(13-6-5-7-14(21)12-13)25-16-17(22)23-15(24-19(16)26)8-11-20(27)9-3-2-4-10-20/h5-7,12,27H,2-4,9-10H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50095790

(1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC1(O)CCCC1)-c1cccc(F)c1 Show InChI InChI=1S/C19H18FN5O/c1-25-17(12-5-4-6-13(20)11-12)24-15-16(21)22-14(23-18(15)25)7-10-19(26)8-2-3-9-19/h4-6,11,26H,2-3,8-9H2,1H3,(H2,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50079652

((E)-1-[(R)-2-(2-Hydroxy-ethyl)-piperidin-1-yl]-3-(...)Show SMILES OCC[C@H]1CCCCN1C(=O)\C=C\c1c(nn2ccccc12)-c1ccccc1 Show InChI InChI=1S/C23H25N3O2/c27-17-14-19-10-4-6-15-25(19)22(28)13-12-20-21-11-5-7-16-26(21)24-23(20)18-8-2-1-3-9-18/h1-3,5,7-9,11-13,16,19,27H,4,6,10,14-15,17H2/b13-12+/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity for adenosine A2A receptor expressed in HEK-293 cells compared to [3H]-CGS-21,680 |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50095778

(4-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...)Show SMILES Cn1c(nc2c(N)nc(nc12)C#CC(C)(C)O)-c1cccc(F)c1 Show InChI InChI=1S/C17H16FN5O/c1-17(2,24)8-7-12-20-14(19)13-16(21-12)23(3)15(22-13)10-5-4-6-11(18)9-10/h4-6,9,24H,1-3H3,(H2,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50006710

(8-[(E)-2-(3,4-Dimethoxy-phenyl)-vinyl]-7-methyl-1,...)Show SMILES CCCn1c2nc(\C=C\c3ccc(OC)c(OC)c3)n(C)c2c(=O)n(CCC)c1=O Show InChI InChI=1S/C22H28N4O4/c1-6-12-25-20-19(21(27)26(13-7-2)22(25)28)24(3)18(23-20)11-9-15-8-10-16(29-4)17(14-15)30-5/h8-11,14H,6-7,12-13H2,1-5H3/b11-9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50079652

((E)-1-[(R)-2-(2-Hydroxy-ethyl)-piperidin-1-yl]-3-(...)Show SMILES OCC[C@H]1CCCCN1C(=O)\C=C\c1c(nn2ccccc12)-c1ccccc1 Show InChI InChI=1S/C23H25N3O2/c27-17-14-19-10-4-6-15-25(19)22(28)13-12-20-21-11-5-7-16-26(21)24-23(20)18-8-2-1-3-9-18/h1-3,5,7-9,11-13,16,19,27H,4,6,10,14-15,17H2/b13-12+/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50603612

(CHEMBL5171777)Show SMILES COc1cc(CCNC2=NC(=NC3=NC=NC23)C#Cc2ccc(Cl)s2)ccc1O |c:10,14,t:8,12| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114103
BindingDB Entry DOI: 10.7270/Q2PV6QGP |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50059376

(9-Methyl-2-phenyl-5,9-dihydro-[1,2,4]triazolo[5,1-...)Show SMILES COC(=O)C1=C2C=CN(C)C=C2c2nc(nn2C1)-c1ccccc1 |c:4,6,10| Show InChI InChI=1S/C18H16N4O2/c1-21-9-8-13-14(10-21)17-19-16(12-6-4-3-5-7-12)20-22(17)11-15(13)18(23)24-2/h3-10H,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50006710

(8-[(E)-2-(3,4-Dimethoxy-phenyl)-vinyl]-7-methyl-1,...)Show SMILES CCCn1c2nc(\C=C\c3ccc(OC)c(OC)c3)n(C)c2c(=O)n(CCC)c1=O Show InChI InChI=1S/C22H28N4O4/c1-6-12-25-20-19(21(27)26(13-7-2)22(25)28)24(3)18(23-20)11-9-15-8-10-16(29-4)17(14-15)30-5/h8-11,14H,6-7,12-13H2,1-5H3/b11-9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50059376

(9-Methyl-2-phenyl-5,9-dihydro-[1,2,4]triazolo[5,1-...)Show SMILES COC(=O)C1=C2C=CN(C)C=C2c2nc(nn2C1)-c1ccccc1 |c:4,6,10| Show InChI InChI=1S/C18H16N4O2/c1-21-9-8-13-14(10-21)17-19-16(12-6-4-3-5-7-12)20-22(17)11-15(13)18(23)24-2/h3-10H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding Affinity for adenosine A2A receptor expressed in HEK-293 cells compared to [3H]-CGS-21,680 |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Mus musculus) | BDBM50603613

(CHEMBL5169644)Show SMILES [H][C@]12C[C@@]1([C@@H](O)[C@@H](O)[C@@H]2n1ccc2c(NCCc3ccccc3)nc(nc12)C#Cc1ccccc1)C(=O)OCC |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114103
BindingDB Entry DOI: 10.7270/Q2PV6QGP |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50035609
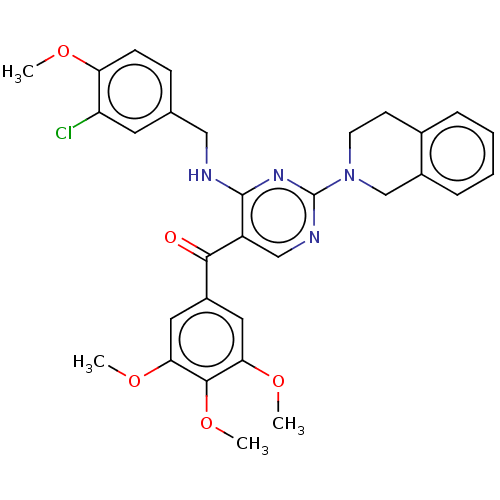
(CHEMBL3338455)Show SMILES COc1ccc(CNc2nc(ncc2C(=O)c2cc(OC)c(OC)c(OC)c2)N2CCc3ccccc3C2)cc1Cl Show InChI InChI=1S/C31H31ClN4O5/c1-38-25-10-9-19(13-24(25)32)16-33-30-23(28(37)22-14-26(39-2)29(41-4)27(15-22)40-3)17-34-31(35-30)36-12-11-20-7-5-6-8-21(20)18-36/h5-10,13-15,17H,11-12,16,18H2,1-4H3,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of canine lung PDE5 using [3H]cGMP substrate by radiolabeled nucleotide method |
Bioorg Med Chem Lett 24: 5175-80 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.082
BindingDB Entry DOI: 10.7270/Q2F76F5Z |
More data for this
Ligand-Target Pair | |
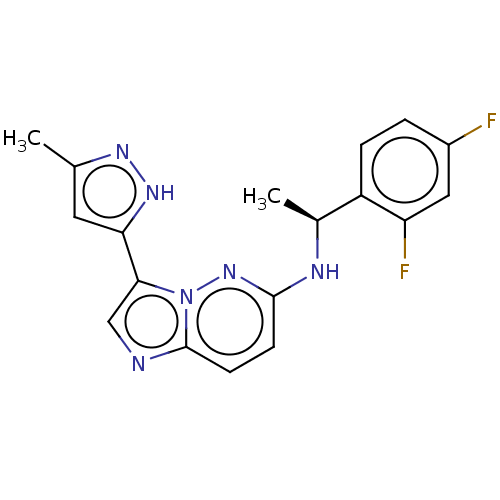
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50524981
(CHEMBL4562879)Show SMILES C[C@H](Nc1ccc2ncc(-c3cc(C)n[nH]3)n2n1)c1ccc(F)cc1F |r| Show InChI InChI=1S/C18H16F2N6/c1-10-7-15(24-23-10)16-9-21-18-6-5-17(25-26(16)18)22-11(2)13-4-3-12(19)8-14(13)20/h3-9,11H,1-2H3,(H,22,25)(H,23,24)/t11-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50035620

(CHEMBL3338445)Show SMILES COc1ccc(CNc2nc(OCc3ccccn3)ncc2C(=O)c2cc(OC)c(OC)c(OC)c2)cc1Cl Show InChI InChI=1S/C28H27ClN4O6/c1-35-22-9-8-17(11-21(22)29)14-31-27-20(15-32-28(33-27)39-16-19-7-5-6-10-30-19)25(34)18-12-23(36-2)26(38-4)24(13-18)37-3/h5-13,15H,14,16H2,1-4H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of canine lung PDE5 using [3H]cGMP substrate by radiolabeled nucleotide method |
Bioorg Med Chem Lett 24: 5175-80 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.082
BindingDB Entry DOI: 10.7270/Q2F76F5Z |
More data for this
Ligand-Target Pair | |
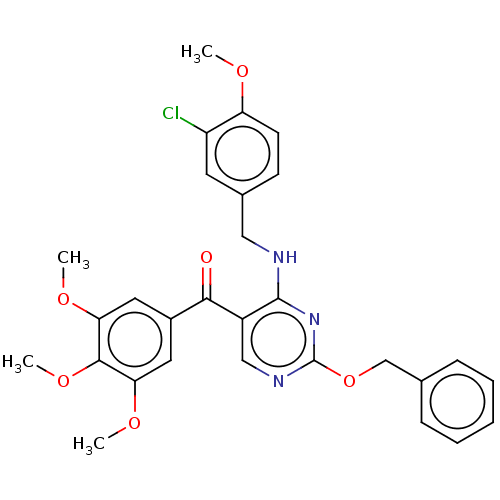
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50035617
(CHEMBL3338448)Show SMILES COc1ccc(CNc2nc(OCc3ccccc3)ncc2C(=O)c2cc(OC)c(OC)c(OC)c2)cc1Cl Show InChI InChI=1S/C29H28ClN3O6/c1-35-23-11-10-19(12-22(23)30)15-31-28-21(16-32-29(33-28)39-17-18-8-6-5-7-9-18)26(34)20-13-24(36-2)27(38-4)25(14-20)37-3/h5-14,16H,15,17H2,1-4H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of canine lung PDE5 using [3H]cGMP substrate by radiolabeled nucleotide method |
Bioorg Med Chem Lett 24: 5175-80 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.082
BindingDB Entry DOI: 10.7270/Q2F76F5Z |
More data for this
Ligand-Target Pair | |
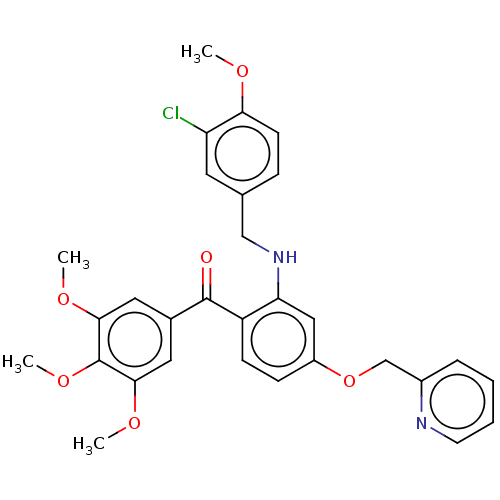
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50035621
(CHEMBL3338444)Show SMILES COc1ccc(CNc2cc(OCc3ccccn3)ccc2C(=O)c2cc(OC)c(OC)c(OC)c2)cc1Cl Show InChI InChI=1S/C30H29ClN2O6/c1-35-26-11-8-19(13-24(26)31)17-33-25-16-22(39-18-21-7-5-6-12-32-21)9-10-23(25)29(34)20-14-27(36-2)30(38-4)28(15-20)37-3/h5-16,33H,17-18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of canine lung PDE5 using [3H]cGMP substrate by radiolabeled nucleotide method |
Bioorg Med Chem Lett 24: 5175-80 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.082
BindingDB Entry DOI: 10.7270/Q2F76F5Z |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50035613
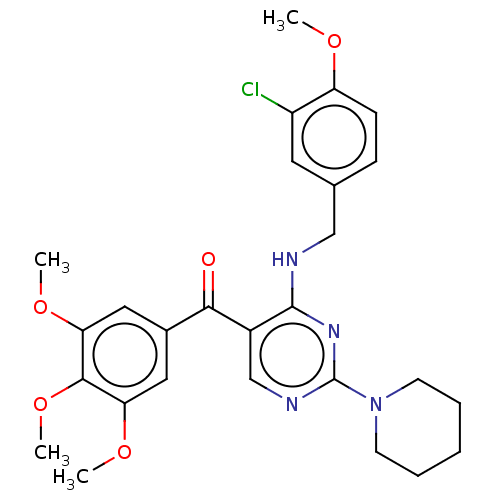
(CHEMBL3338452)Show SMILES COc1ccc(CNc2nc(ncc2C(=O)c2cc(OC)c(OC)c(OC)c2)N2CCCCC2)cc1Cl Show InChI InChI=1S/C27H31ClN4O5/c1-34-21-9-8-17(12-20(21)28)15-29-26-19(16-30-27(31-26)32-10-6-5-7-11-32)24(33)18-13-22(35-2)25(37-4)23(14-18)36-3/h8-9,12-14,16H,5-7,10-11,15H2,1-4H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of canine lung PDE5 using [3H]cGMP substrate by radiolabeled nucleotide method |
Bioorg Med Chem Lett 24: 5175-80 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.082
BindingDB Entry DOI: 10.7270/Q2F76F5Z |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50036633
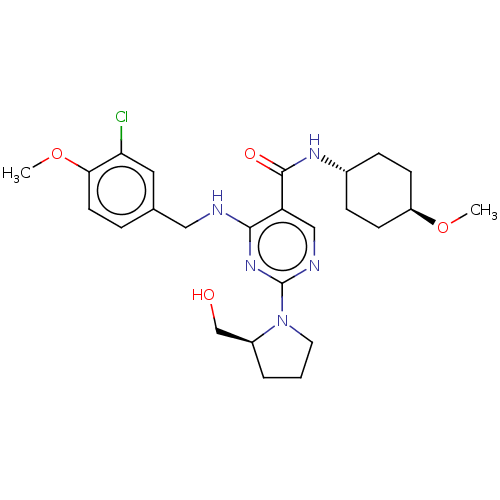
(CHEMBL3354285)Show SMILES CO[C@H]1CC[C@@H](CC1)NC(=O)c1cnc(nc1NCc1ccc(OC)c(Cl)c1)N1CCC[C@H]1CO |r,wU:5.8,32.36,wD:2.1,(10.17,-30.98,;10.17,-29.44,;8.84,-28.67,;8.84,-27.13,;7.5,-26.35,;6.18,-27.13,;6.18,-28.67,;7.5,-29.44,;4.85,-26.36,;4.84,-24.82,;6.17,-24.05,;3.5,-24.05,;2.17,-24.83,;.84,-24.06,;.84,-22.52,;2.16,-21.74,;3.5,-22.51,;4.83,-21.73,;4.82,-20.19,;6.15,-19.41,;6.13,-17.88,;7.45,-17.1,;8.8,-17.86,;10.13,-17.09,;11.47,-17.85,;8.81,-19.41,;10.14,-20.17,;7.48,-20.18,;-.5,-21.75,;-1.92,-22.38,;-2.94,-21.24,;-2.18,-19.9,;-.67,-20.22,;.48,-19.19,;1.94,-19.66,)| Show InChI InChI=1S/C25H34ClN5O4/c1-34-19-8-6-17(7-9-19)29-24(33)20-14-28-25(31-11-3-4-18(31)15-32)30-23(20)27-13-16-5-10-22(35-2)21(26)12-16/h5,10,12,14,17-19,32H,3-4,6-9,11,13,15H2,1-2H3,(H,29,33)(H,27,28,30)/t17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of dog lungs PDE5 using [3H]cGMP as substrate after 30 mins by scintillation counting analysis |
Bioorg Med Chem Lett 24: 5460-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.10.008
BindingDB Entry DOI: 10.7270/Q2JS9S1W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50006710

(8-[(E)-2-(3,4-Dimethoxy-phenyl)-vinyl]-7-methyl-1,...)Show SMILES CCCn1c2nc(\C=C\c3ccc(OC)c(OC)c3)n(C)c2c(=O)n(CCC)c1=O Show InChI InChI=1S/C22H28N4O4/c1-6-12-25-20-19(21(27)26(13-7-2)22(25)28)24(3)18(23-20)11-9-15-8-10-16(29-4)17(14-15)30-5/h8-11,14H,6-7,12-13H2,1-5H3/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclic AMP production in rat Adenosine A2A receptor assay |
J Med Chem 44: 170-9 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q0X |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50100662

(CHEMBL553371 | CHEMBL77971 | methyl 2-(4-aminophen...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3ccccn3)cc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C32H29N3O7/c1-38-26-15-19(16-27(39-2)30(26)40-3)28-24-13-12-23(42-18-21-7-5-6-14-34-21)17-25(24)31(36)35(29(28)32(37)41-4)22-10-8-20(33)9-11-22/h5-17H,18,33H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) |
Bioorg Med Chem Lett 24: 5175-80 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.082
BindingDB Entry DOI: 10.7270/Q2F76F5Z |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50036625

(CHEMBL3354279)Show SMILES COc1ccc(CNc2nc(ncc2C(=O)NCc2ccccn2)N2CCC[C@H]2CO)cc1Cl |r| Show InChI InChI=1S/C24H27ClN6O3/c1-34-21-8-7-16(11-20(21)25)12-27-22-19(23(33)28-13-17-5-2-3-9-26-17)14-29-24(30-22)31-10-4-6-18(31)15-32/h2-3,5,7-9,11,14,18,32H,4,6,10,12-13,15H2,1H3,(H,28,33)(H,27,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of dog lungs PDE5 using [3H]cGMP as substrate after 30 mins by scintillation counting analysis |
Bioorg Med Chem Lett 24: 5460-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.10.008
BindingDB Entry DOI: 10.7270/Q2JS9S1W |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50524985
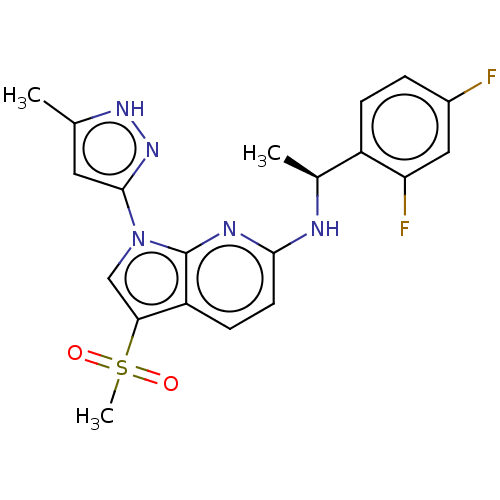
(CHEMBL4460367)Show SMILES C[C@H](Nc1ccc2c(cn(-c3cc(C)[nH]n3)c2n1)S(C)(=O)=O)c1ccc(F)cc1F |r| Show InChI InChI=1S/C20H19F2N5O2S/c1-11-8-19(26-25-11)27-10-17(30(3,28)29)15-6-7-18(24-20(15)27)23-12(2)14-5-4-13(21)9-16(14)22/h4-10,12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50035611
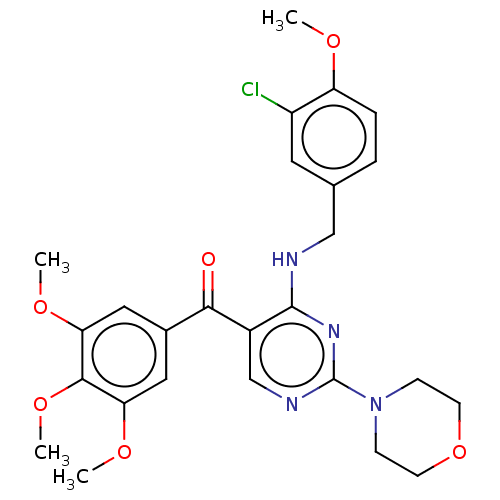
(CHEMBL3338453)Show SMILES COc1ccc(CNc2nc(ncc2C(=O)c2cc(OC)c(OC)c(OC)c2)N2CCOCC2)cc1Cl Show InChI InChI=1S/C26H29ClN4O6/c1-33-20-6-5-16(11-19(20)27)14-28-25-18(15-29-26(30-25)31-7-9-37-10-8-31)23(32)17-12-21(34-2)24(36-4)22(13-17)35-3/h5-6,11-13,15H,7-10,14H2,1-4H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of canine lung PDE5 using [3H]cGMP substrate by radiolabeled nucleotide method |
Bioorg Med Chem Lett 24: 5175-80 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.082
BindingDB Entry DOI: 10.7270/Q2F76F5Z |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50035618
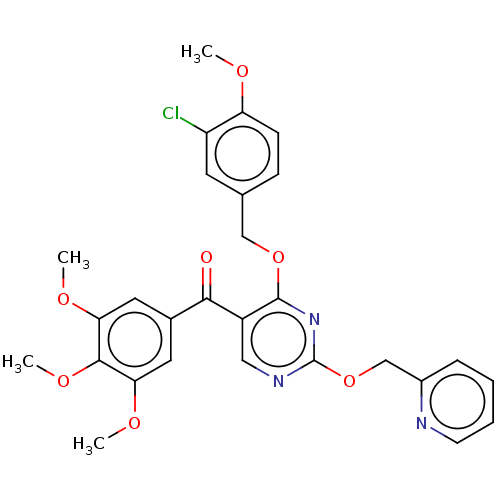
(CHEMBL3338447)Show SMILES COc1ccc(COc2nc(OCc3ccccn3)ncc2C(=O)c2cc(OC)c(OC)c(OC)c2)cc1Cl Show InChI InChI=1S/C28H26ClN3O7/c1-34-22-9-8-17(11-21(22)29)15-38-27-20(14-31-28(32-27)39-16-19-7-5-6-10-30-19)25(33)18-12-23(35-2)26(37-4)24(13-18)36-3/h5-14H,15-16H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of canine lung PDE5 using [3H]cGMP substrate by radiolabeled nucleotide method |
Bioorg Med Chem Lett 24: 5175-80 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.082
BindingDB Entry DOI: 10.7270/Q2F76F5Z |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) |
Bioorg Med Chem Lett 24: 5175-80 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.082
BindingDB Entry DOI: 10.7270/Q2F76F5Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50035615
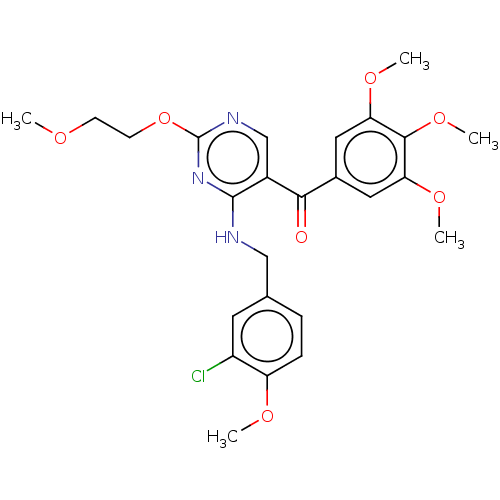
(CHEMBL3338450)Show SMILES COCCOc1ncc(C(=O)c2cc(OC)c(OC)c(OC)c2)c(NCc2ccc(OC)c(Cl)c2)n1 Show InChI InChI=1S/C25H28ClN3O7/c1-31-8-9-36-25-28-14-17(22(30)16-11-20(33-3)23(35-5)21(12-16)34-4)24(29-25)27-13-15-6-7-19(32-2)18(26)10-15/h6-7,10-12,14H,8-9,13H2,1-5H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of canine lung PDE5 using [3H]cGMP substrate by radiolabeled nucleotide method |
Bioorg Med Chem Lett 24: 5175-80 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.082
BindingDB Entry DOI: 10.7270/Q2F76F5Z |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50036632

(CHEMBL3354284)Show SMILES COc1ccc(CNc2nc(ncc2C(=O)N[C@H]2CC[C@H](O)CC2)N2CCC[C@H]2CO)cc1Cl |r,wU:17.17,28.31,wD:20.21,(27.61,-1.23,;26.27,-.47,;24.94,-1.24,;23.59,-.48,;22.27,-1.26,;22.29,-2.79,;20.96,-3.57,;20.97,-5.11,;19.64,-5.89,;18.3,-5.12,;16.98,-5.9,;16.98,-7.44,;18.31,-8.21,;19.64,-7.43,;20.98,-8.2,;22.31,-7.43,;20.98,-9.74,;22.32,-10.51,;23.64,-9.73,;24.98,-10.51,;24.98,-12.05,;26.31,-12.82,;23.64,-12.82,;22.31,-12.05,;15.65,-5.13,;14.23,-5.76,;13.2,-4.62,;13.97,-3.28,;15.48,-3.6,;16.61,-2.57,;18.08,-3.03,;23.62,-3.56,;24.95,-2.79,;26.28,-3.55,)| Show InChI InChI=1S/C24H32ClN5O4/c1-34-21-9-4-15(11-20(21)25)12-26-22-19(23(33)28-16-5-7-18(32)8-6-16)13-27-24(29-22)30-10-2-3-17(30)14-31/h4,9,11,13,16-18,31-32H,2-3,5-8,10,12,14H2,1H3,(H,28,33)(H,26,27,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of dog lungs PDE5 using [3H]cGMP as substrate after 30 mins by scintillation counting analysis |
Bioorg Med Chem Lett 24: 5460-5 (2015)
Article DOI: 10.1016/j.bmcl.2014.10.008
BindingDB Entry DOI: 10.7270/Q2JS9S1W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data