

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000485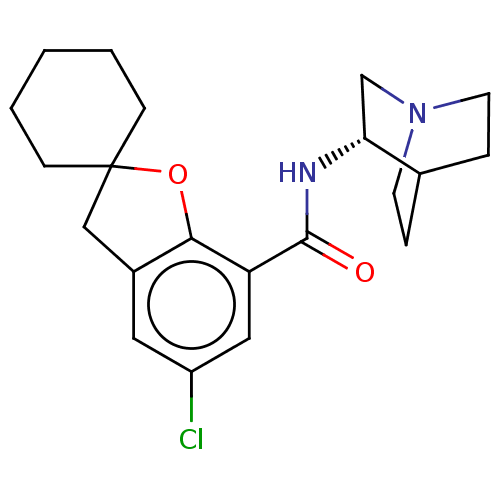 ((S)-2-(5-chlorospiro[2,3-dihydrobenzo[b]furan-2,1'...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000482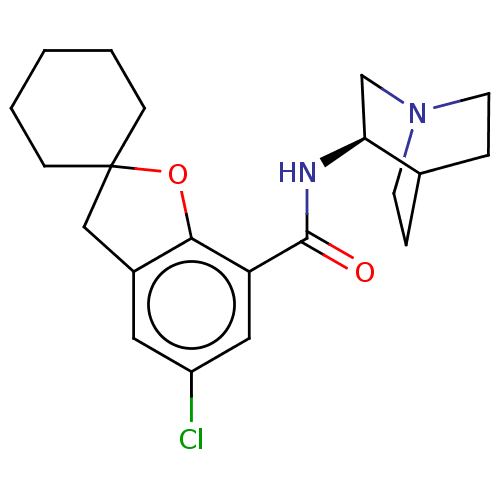 ((R)-2-(5-chlorospiro[2,3-dihydrobenzo[b]furan-2,1'...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000480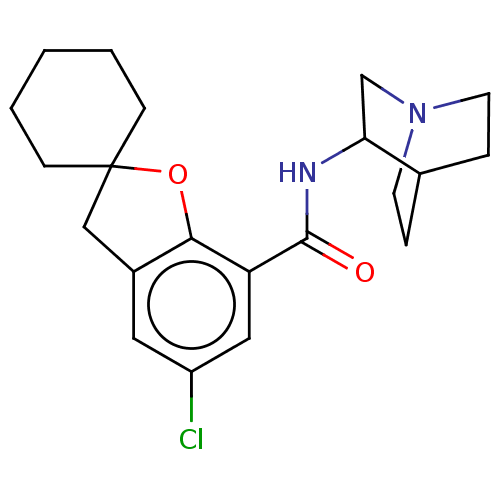 (2-(5-chlorospiro[2,3-dihydrobenzo[b]furan-2,1'-cyc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000479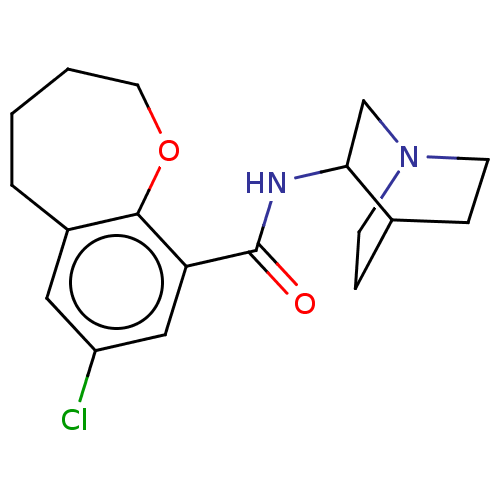 (7-Chloro-2,3,4,5-tetrahydro-benzo[b]oxepine-9-carb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000492 ((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000495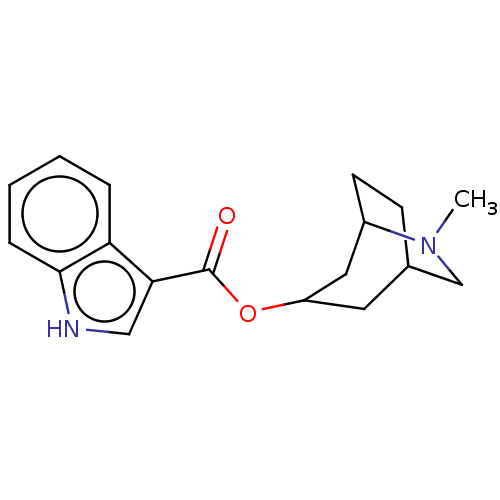 ((ICS 205-930)1H-Indole-3-carboxylic acid 6-methyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000483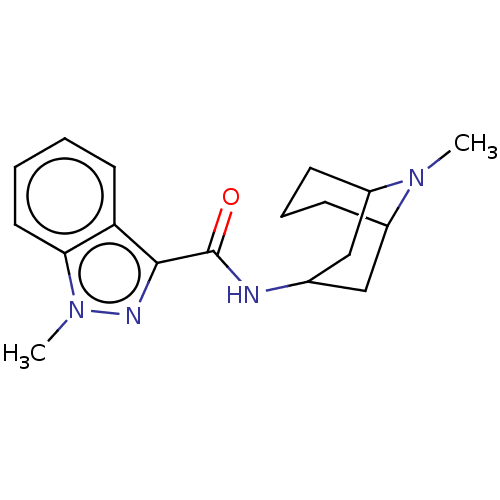 ((BRL 43694)1-Methyl-1H-indazole-3-carboxylic acid ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000494 (7-Bromo-2,3,4,5-tetrahydro-benzo[b]oxepine-9-carbo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000487 (8-Chloro-3,4,5,6-tetrahydro-2H-benzo[b]oxocine-10-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000489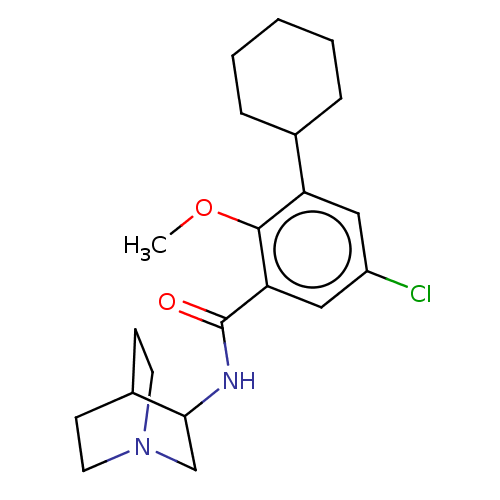 (CHEMBL353993 | N-(1-Aza-bicyclo[2.2.2]oct-3-yl)-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM85330 (CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000478 (2,3,4,5-Tetrahydro-benzo[b]oxepine-9-carboxylic ac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000484 (7-Chloro-2,2-dimethyl-2,3,4,5-tetrahydro-benzo[b]o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000486 (CHEMBL368992 | N-(1-Aza-bicyclo[2.2.2]oct-3-yl)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000488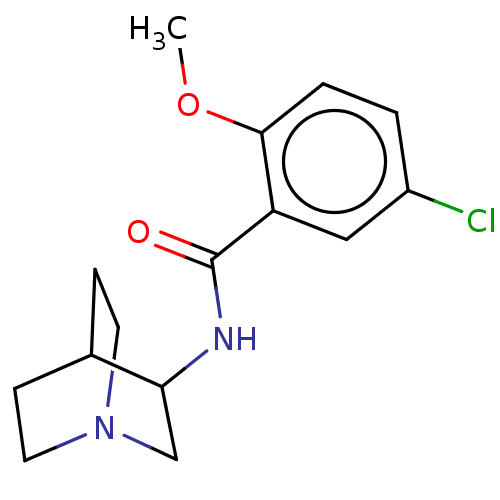 (CHEMBL171070 | N-(1-Aza-bicyclo[2.2.2]oct-3-yl)-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000490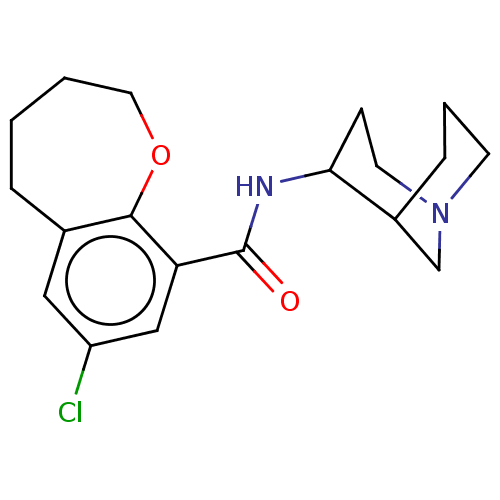 (7-Chloro-2,3,4,5-tetrahydro-benzo[b]oxepine-9-carb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000481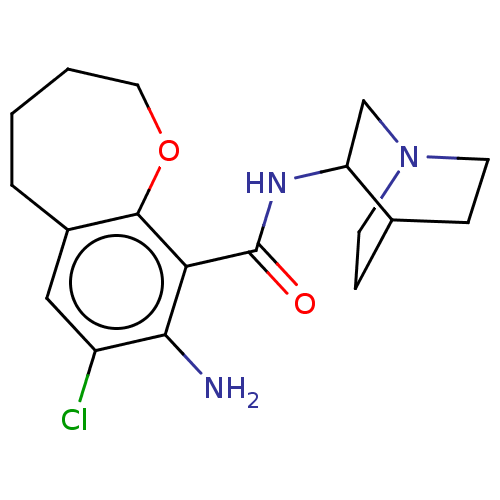 (8-Amino-7-chloro-2,3,4,5-tetrahydro-benzo[b]oxepin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50006819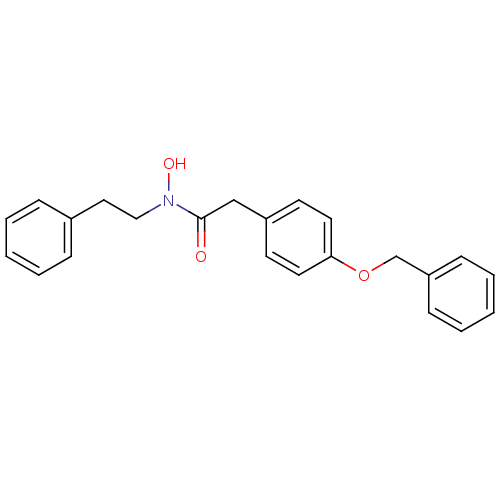 (2-(4-Benzyloxy-phenyl)-N-hydroxy-N-phenethyl-aceta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Invitro inhibitory activity against polymorphonuclear leukocyte 5-lipoxygenase in human cell | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50016673 (CHEMBL52896 | N-Hydroxy-N-methyl-2-[4-(quinolin-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Invitro inhibitory activity against polymorphonuclear leukocyte 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM48320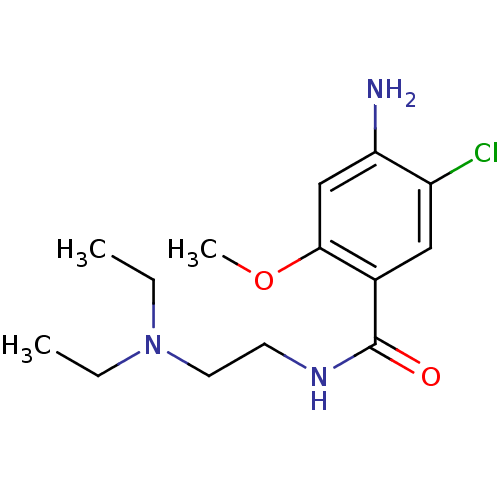 (4-amino-5-chloro-N-[2-(diethylamino)ethyl]-2-metho...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | 995 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. | J Med Chem 35: 895-903 (1992) BindingDB Entry DOI: 10.7270/Q2SJ1M77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50016673 (CHEMBL52896 | N-Hydroxy-N-methyl-2-[4-(quinolin-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Activity of the compound to bind against [3H]-LTD4 radioligand in guinea pig | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid lipoxygenase ALOX15 (Rattus norvegicus) | BDBM50006819 (2-(4-Benzyloxy-phenyl)-N-hydroxy-N-phenethyl-aceta...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat platelet 12-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50016687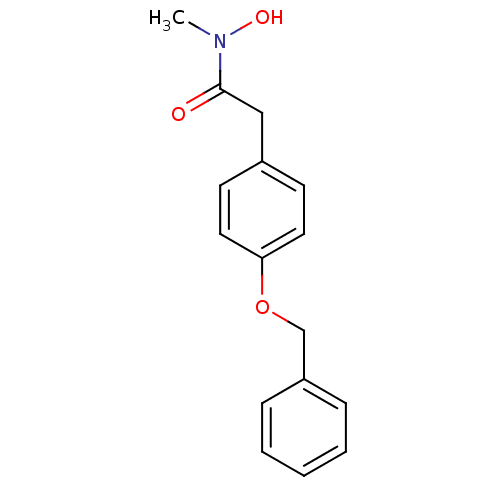 (2-(4-Benzyloxy-phenyl)-N-hydroxy-N-methyl-acetamid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Activity of the compound to bind against [3H]-LTD4 radioligand in guinea pig | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50016671 (2-(4-Benzyloxy-phenyl)-N-hydroxy-N-(1-phenyl-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory activity against polymorphonuclear leukocyte 5-lipoxygenase using guinea pig supernatant | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50016680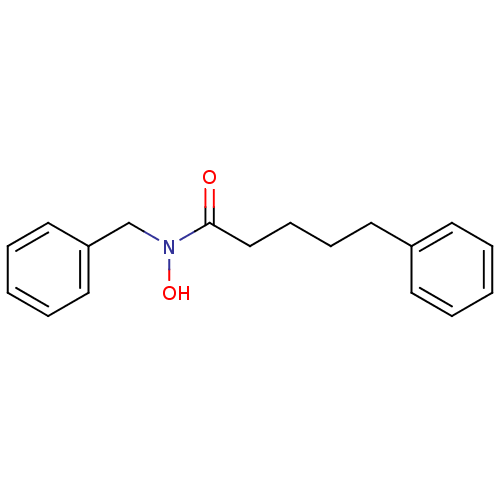 (5-Phenyl-pentanoic acid benzyl-hydroxy-amide | CHE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat polymorphonuclear leukocyte 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50016694 (4-Benzyloxy-N-hydroxy-N-phenethyl-benzamide | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory activity against polymorphonuclear leukocyte 5-lipoxygenase using guinea pig supernatant | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50016679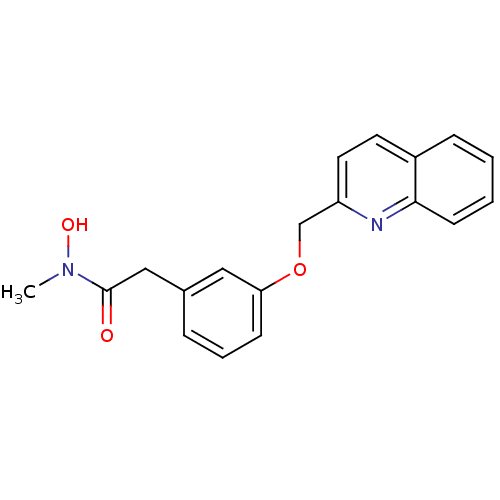 (CHEMBL52085 | N-Hydroxy-N-methyl-2-[3-(quinolin-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat polymorphonuclear leukocyte 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50016683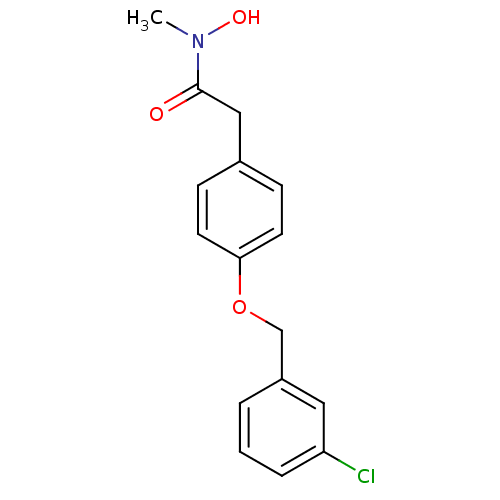 (2-[4-(3-Chloro-benzyloxy)-phenyl]-N-hydroxy-N-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat polymorphonuclear leukocyte 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50016686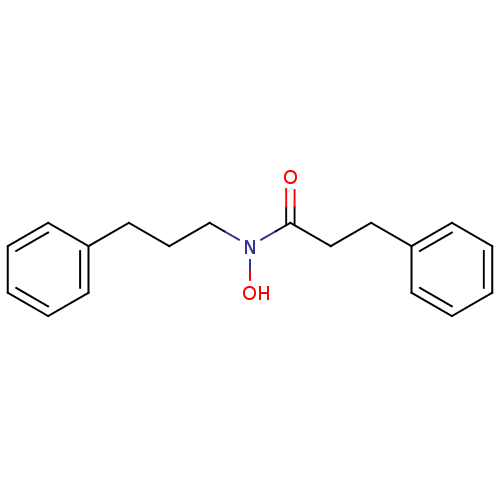 (CHEMBL54013 | N-Hydroxy-3-phenyl-N-(3-phenyl-propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat polymorphonuclear leukocyte 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50016687 (2-(4-Benzyloxy-phenyl)-N-hydroxy-N-methyl-acetamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat polymorphonuclear leukocyte 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50016672 (2-(4-Benzyloxy-phenyl)-N-hydroxy-N-propyl-acetamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat polymorphonuclear leukocyte 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50016688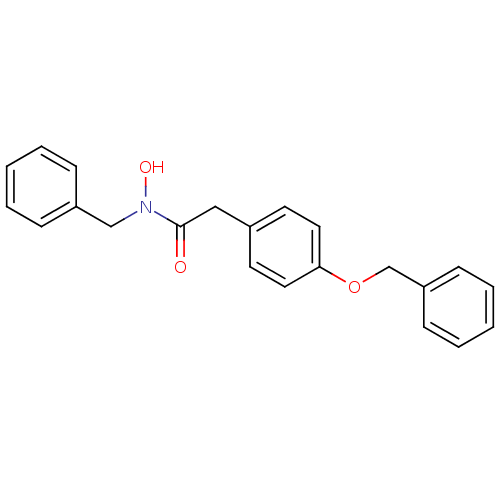 (CHEMBL52749 | N-Benzyl-2-(4-benzyloxy-phenyl)-N-hy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat polymorphonuclear leukocyte 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50016686 (CHEMBL54013 | N-Hydroxy-3-phenyl-N-(3-phenyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Invitro inhibition of polymorphonuclear leukocyte derived human 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50016681 (2-(3-Benzyloxy-phenyl)-N-hydroxy-N-phenethyl-propi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Invitro inhibition of polymorphonuclear leukocyte derived human 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Invitro inhibition of polymorphonuclear leukocyte derived human 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50006819 (2-(4-Benzyloxy-phenyl)-N-hydroxy-N-phenethyl-aceta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory activity against polymorphonuclear leukocyte 5-lipoxygenase using guinea pig supernatant | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50016690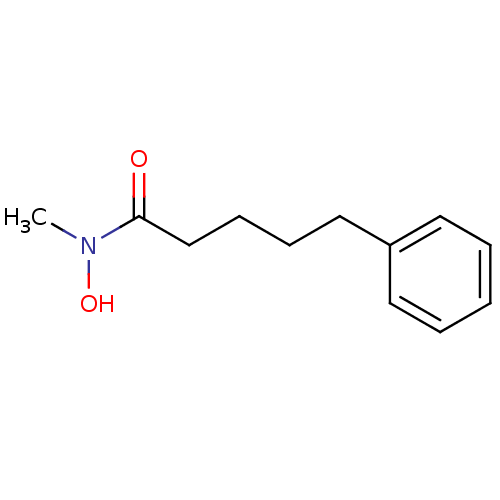 (5-Phenyl-pentanoic acid hydroxy-methyl-amide | CHE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat polymorphonuclear leukocyte 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50016676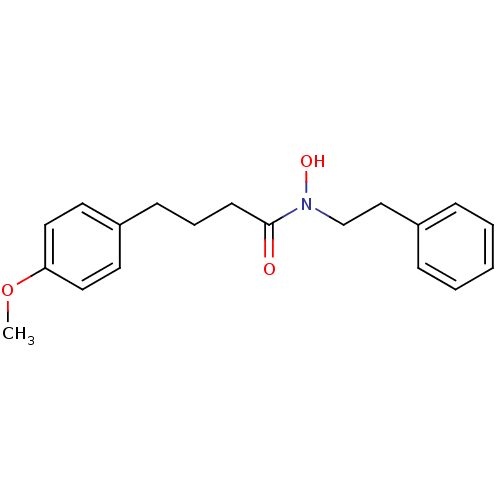 (CHEMBL293264 | N-Hydroxy-4-(4-methoxy-phenyl)-N-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat polymorphonuclear leukocyte 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50016688 (CHEMBL52749 | N-Benzyl-2-(4-benzyloxy-phenyl)-N-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Invitro inhibition of polymorphonuclear leukocyte derived human 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50016675 (CHEMBL55053 | N-Hydroxy-N-phenethyl-4-phenyl-butyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory activity against polymorphonuclear leukocyte 5-lipoxygenase using guinea pig supernatant | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50016682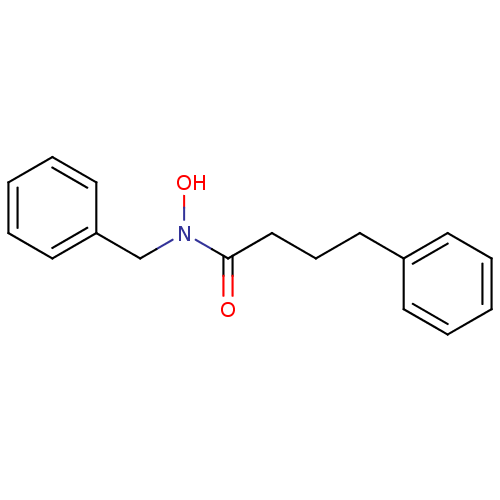 (CHEMBL53028 | N-Benzyl-N-hydroxy-4-phenyl-butyrami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Invitro inhibition of polymorphonuclear leukocyte derived human 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50016682 (CHEMBL53028 | N-Benzyl-N-hydroxy-4-phenyl-butyrami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat polymorphonuclear leukocyte 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50016680 (5-Phenyl-pentanoic acid benzyl-hydroxy-amide | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Invitro inhibition of polymorphonuclear leukocyte derived human 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50016672 (2-(4-Benzyloxy-phenyl)-N-hydroxy-N-propyl-acetamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Invitro inhibition of polymorphonuclear leukocyte derived human 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50016675 (CHEMBL55053 | N-Hydroxy-N-phenethyl-4-phenyl-butyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Invitro inhibition of polymorphonuclear leukocyte derived human 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50016687 (2-(4-Benzyloxy-phenyl)-N-hydroxy-N-methyl-acetamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat platelet 12-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50016683 (2-[4-(3-Chloro-benzyloxy)-phenyl]-N-hydroxy-N-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Invitro inhibition of polymorphonuclear leukocyte derived human 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50016690 (5-Phenyl-pentanoic acid hydroxy-methyl-amide | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Invitro inhibition of polymorphonuclear leukocyte derived human 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50016676 (CHEMBL293264 | N-Hydroxy-4-(4-methoxy-phenyl)-N-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Invitro inhibition of polymorphonuclear leukocyte derived human 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50016685 (CHEMBL54838 | N-Hydroxy-N-phenethyl-3-phenoxy-prop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat polymorphonuclear leukocyte 5-lipoxygenase | J Med Chem 32: 1836-42 (1989) BindingDB Entry DOI: 10.7270/Q2VT1R21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 108 total ) | Next | Last >> |