

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic receptor M1 (Bos taurus) | BDBM50055976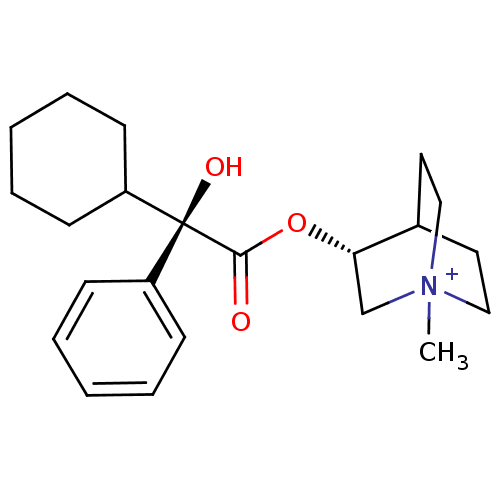 ((R)-3-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards Muscarinic acetylcholine receptor M1 was determined in calf brain membrane | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50055978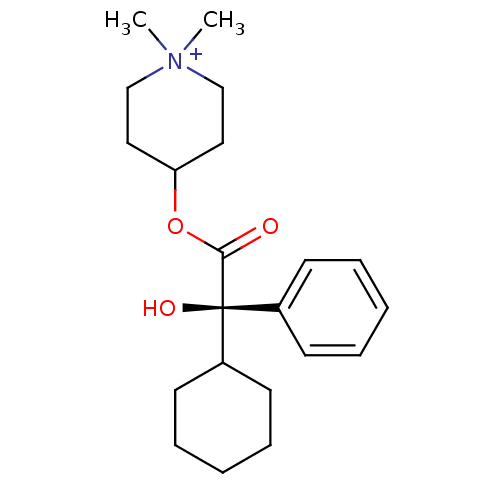 (4-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy)-1,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards rat Muscarinic acetylcholine receptor M1 was determined | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50055976 ((R)-3-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards rat Muscarinic acetylcholine receptor M1 was determined | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50055978 (4-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy)-1,...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards Muscarinic acetylcholine receptor M1 was determined in calf brain membrane | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (Cavia porcellus) | BDBM50055978 (4-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards Muscarinic acetylcholine receptor M3 was determined in guinea pig ileum | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50055976 ((R)-3-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards rat Muscarinic acetylcholine receptor M2 was determined | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (Cavia porcellus) | BDBM50055976 ((R)-3-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards Muscarinic acetylcholine receptor M3 was determined in guinea pig ileum | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50369230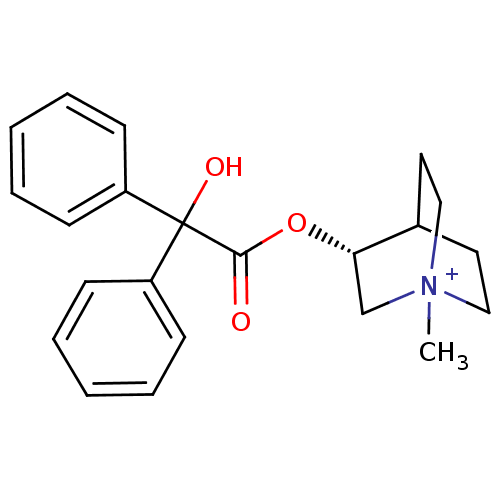 (CHEMBL1398637 | CLIDINIUM) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards rat Muscarinic acetylcholine receptor M1 was determined | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50055978 (4-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards rat Muscarinic acetylcholine receptor M2 was determined | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50452855 (Isoptpo Hyoscine | Scopolamine) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards Muscarinic acetylcholine receptor M1 was determined in calf brain membrane | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50369230 (CHEMBL1398637 | CLIDINIUM) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards rat Muscarinic acetylcholine receptor M2 was determined | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50452855 (Isoptpo Hyoscine | Scopolamine) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards rat Muscarinic acetylcholine receptor M1 was determined | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (Cavia porcellus) | BDBM50369230 (CHEMBL1398637 | CLIDINIUM) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards Muscarinic acetylcholine receptor M3 was determined in guinea pig ileum | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50452855 (Isoptpo Hyoscine | Scopolamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards rat Muscarinic acetylcholine receptor M2 was determined | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (Cavia porcellus) | BDBM50176065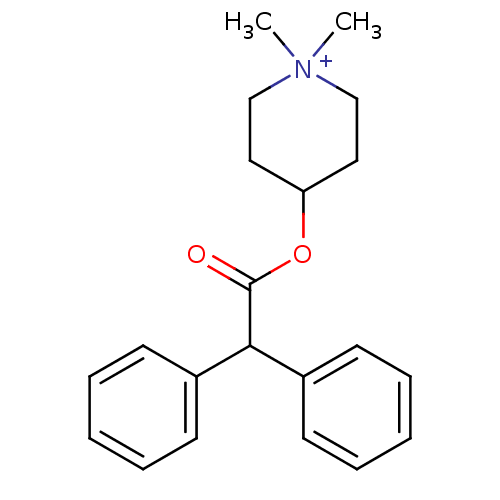 (4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards Muscarinic acetylcholine receptor M3 was determined in guinea pig ileum | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM251572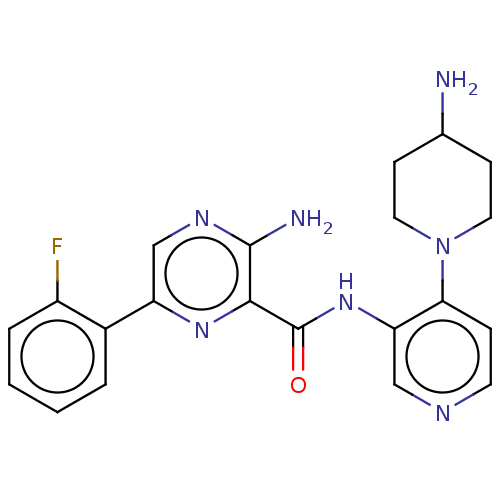 (US9452998, Table 4 Compound 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.0 | 25 |
NOVARTIS AG US Patent | Assay Description Types of GSK-3 assay used to test the selectivity/off target potential compounds of the invention with respect to PKC α/θ inhibition activity inclu... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B (Homo sapiens (Human)) | BDBM50344013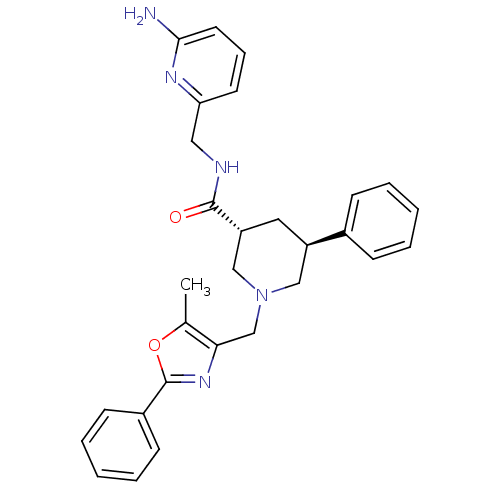 (CHEMBL1780094 | trans-(3R,5S)-N-((6-aminopyridin-2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PDE8B | Bioorg Med Chem Lett 21: 3095-8 (2011) Article DOI: 10.1016/j.bmcl.2011.03.022 BindingDB Entry DOI: 10.7270/Q20002F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251508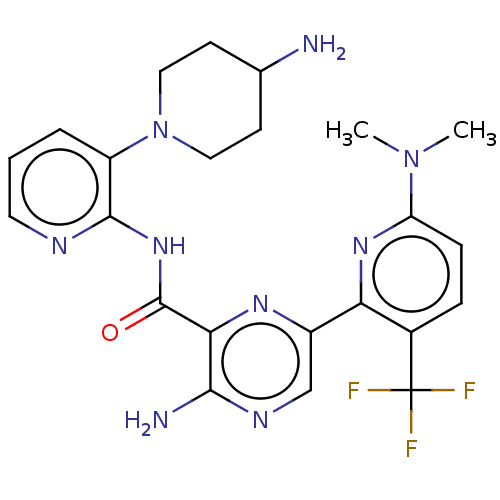 (US9452998, 57) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251509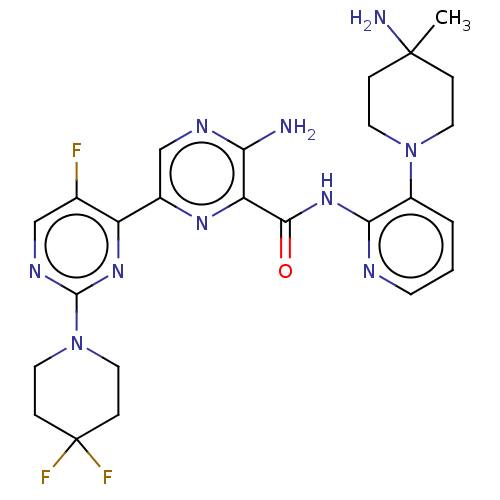 (US9452998, 58) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251512 (US9452998, 61) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251513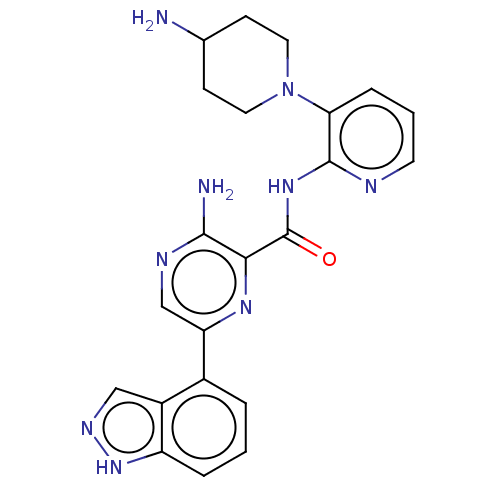 (US9452998, 62) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251515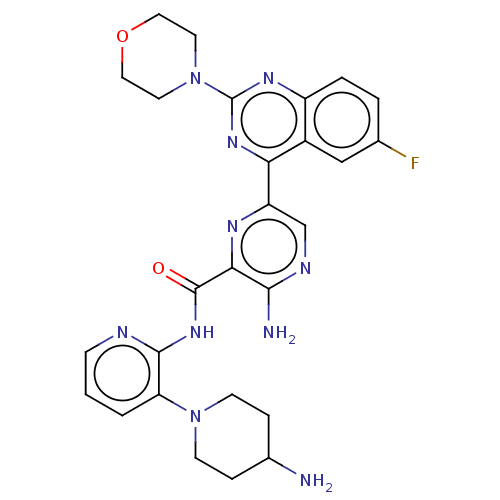 (US9452998, 64) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251516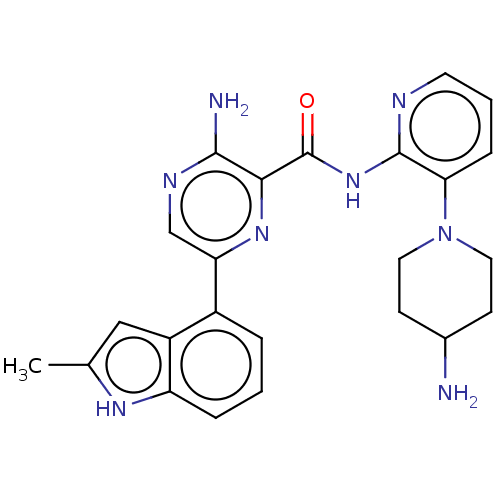 (US9452998, 65) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251517 (US9452998, 66 | US9452998, 77) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251522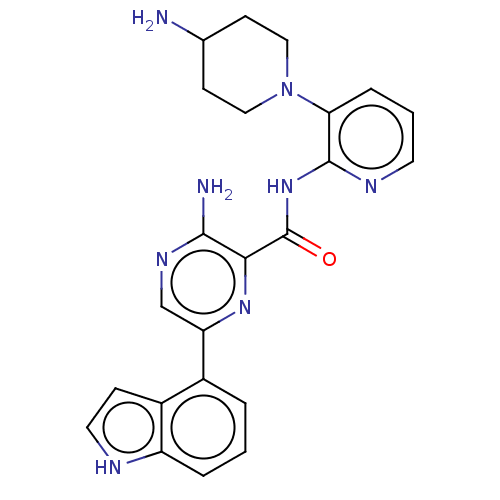 (US9452998, 71) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251523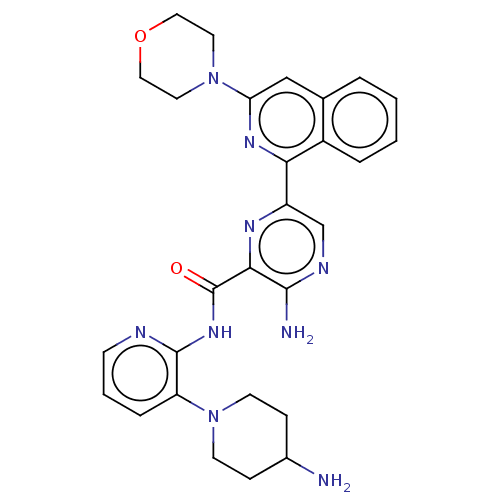 (US9452998, 72 | US9452998, 78) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251524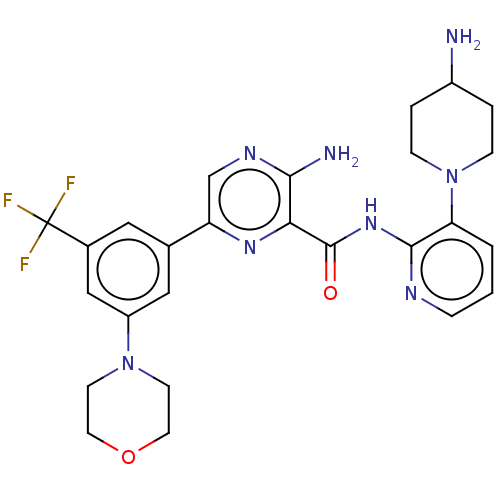 (US9452998, 73) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251507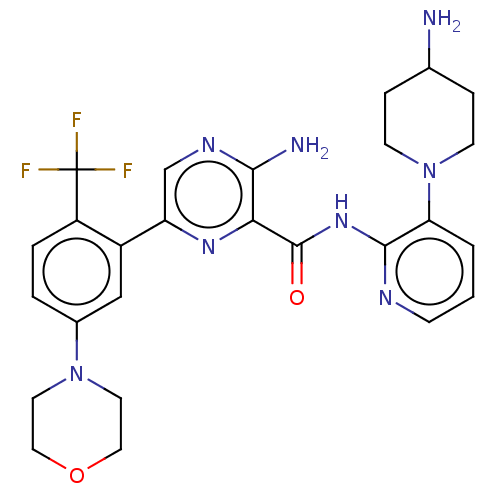 (US9452998, 56 | US9452998, 76) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251517 (US9452998, 66 | US9452998, 77) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251523 (US9452998, 72 | US9452998, 78) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251544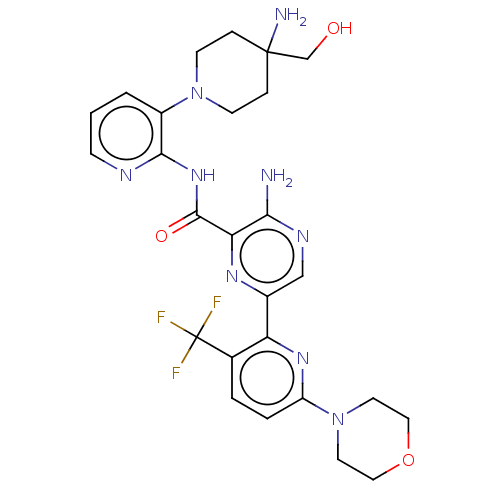 (US9452998, 93) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251455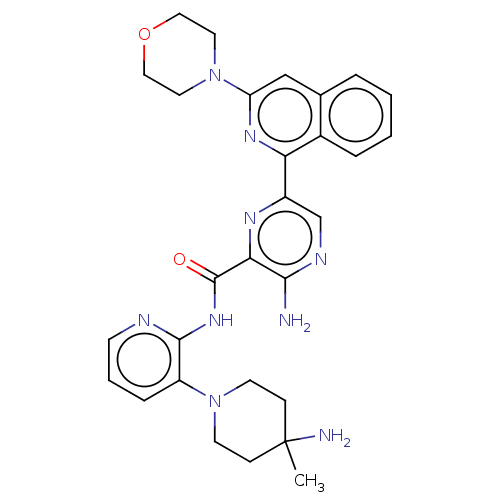 (US9452998, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251458 (US9452998, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251491 (US9452998, 40 | US9452998, 51) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251572 (US9452998, Table 4 Compound 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251574 (US9452998, Table 4 Compound 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251497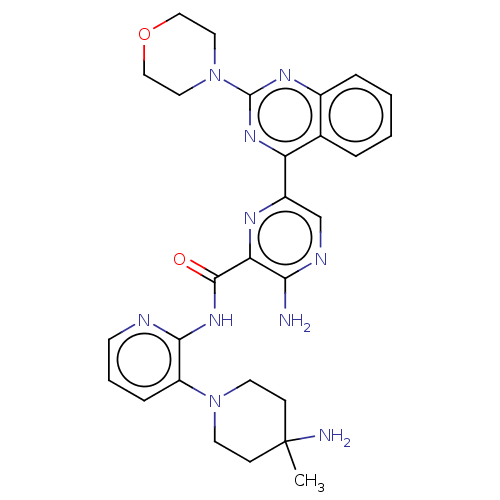 (US9452998, 46) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251506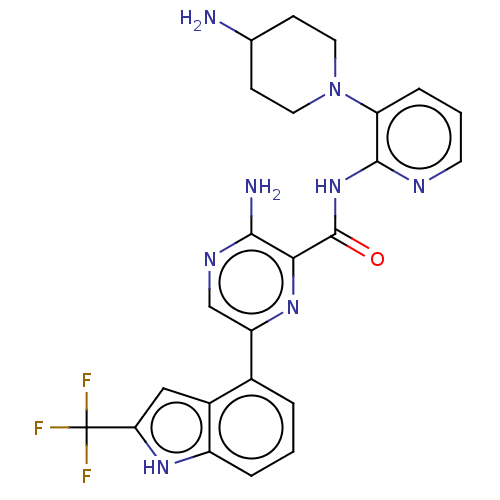 (US9452998, 55) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251507 (US9452998, 56 | US9452998, 76) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM251525 (US9452998, 74) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM251544 (US9452998, 93) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251496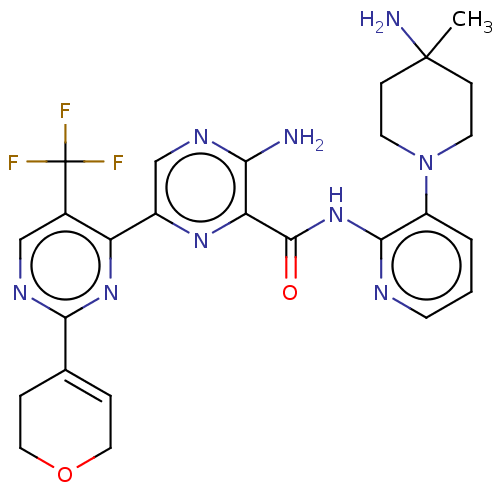 (US9452998, 45) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251511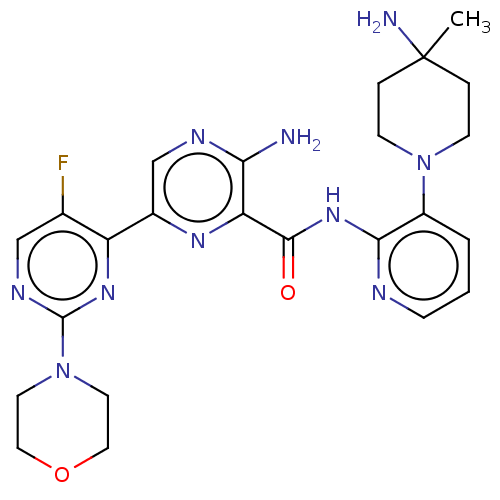 (US9452998, 60) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251573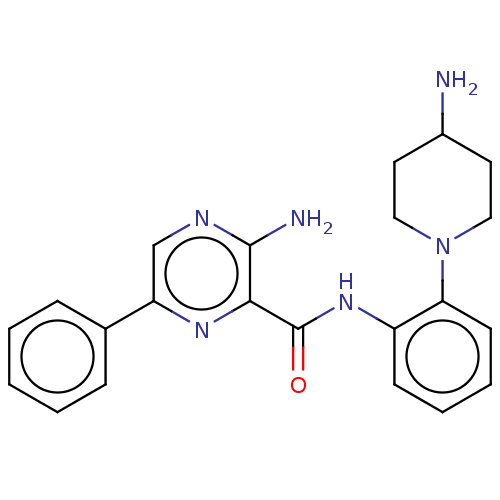 (US9452998, Table 4 Compound 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM251524 (US9452998, 73) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251540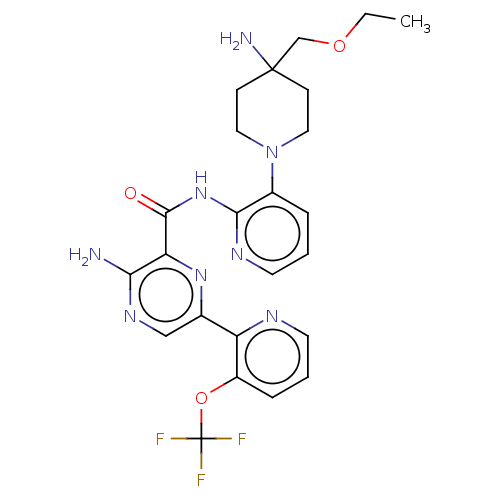 (US9452998, 89) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251493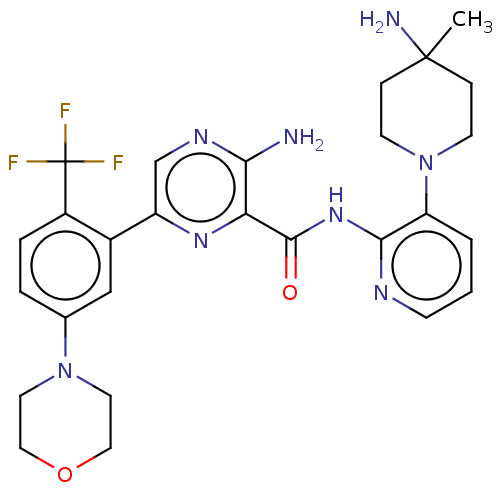 (US9452998, 42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251501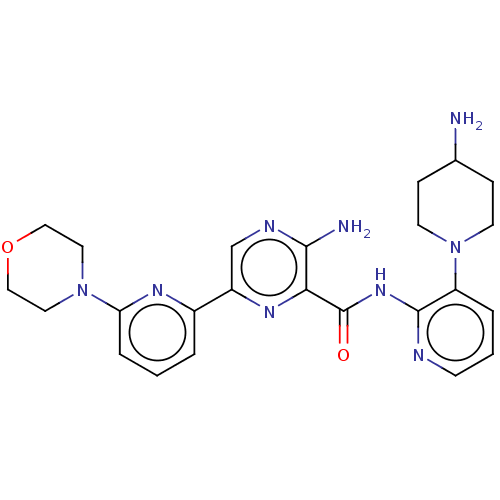 (US9452998, 50) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251575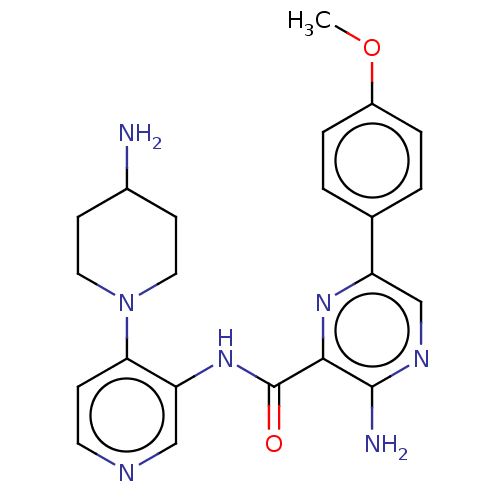 (US9452998, Table 4 Compound 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NOVARTIS AG US Patent | Assay Description The compounds of formula I were tested for their activity on different PKC isoforms according to a published method (D. Geiges et al. Biochem. Pharma... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM251574 (US9452998, Table 4 Compound 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.0 | 25 |
NOVARTIS AG US Patent | Assay Description Types of GSK-3 assay used to test the selectivity/off target potential compounds of the invention with respect to PKC α/θ inhibition activity inclu... | US Patent US9452998 (2016) BindingDB Entry DOI: 10.7270/Q2C82870 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1000 total ) | Next | Last >> |