

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143951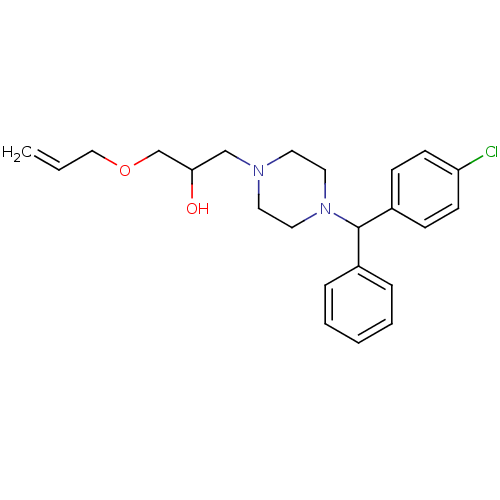 (1-Allyloxy-3-{4-[(4-chloro-phenyl)-phenyl-methyl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50119202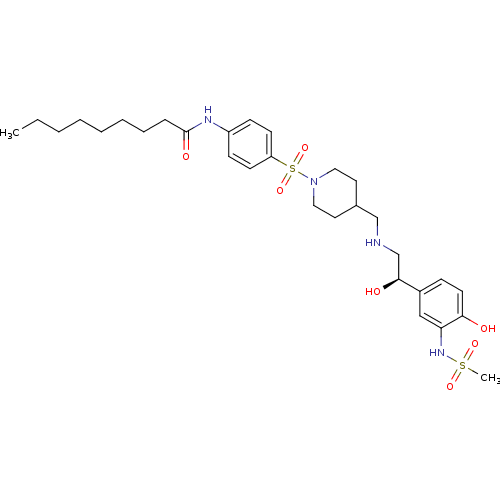 (CHEMBL99599 | Nonanoic acid [4-(4-{[2-hydroxy-2-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound against Beta-2 adrenergic receptor was determined | Bioorg Med Chem Lett 12: 2963-7 (2002) BindingDB Entry DOI: 10.7270/Q2D21WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50119195 (CHEMBL317003 | {1-[4-(4-{[(R)-2-Hydroxy-2-(4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound against Beta-2 adrenergic receptor was determined | Bioorg Med Chem Lett 12: 2963-7 (2002) BindingDB Entry DOI: 10.7270/Q2D21WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50119195 (CHEMBL317003 | {1-[4-(4-{[(R)-2-Hydroxy-2-(4-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound against Beta-1 adrenergic receptor was determined | Bioorg Med Chem Lett 12: 2963-7 (2002) BindingDB Entry DOI: 10.7270/Q2D21WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143948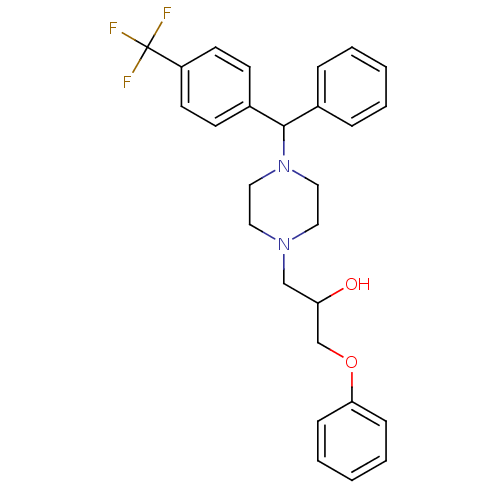 (1-Phenoxy-3-{4-[phenyl-(4-trifluoromethyl-phenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143952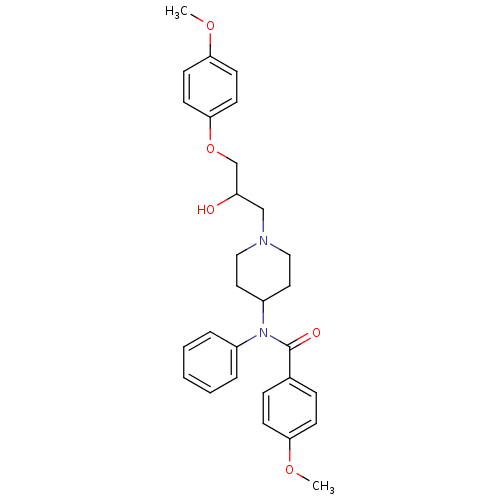 (CHEMBL59712 | N-{1-[2-Hydroxy-3-(4-methoxy-phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143962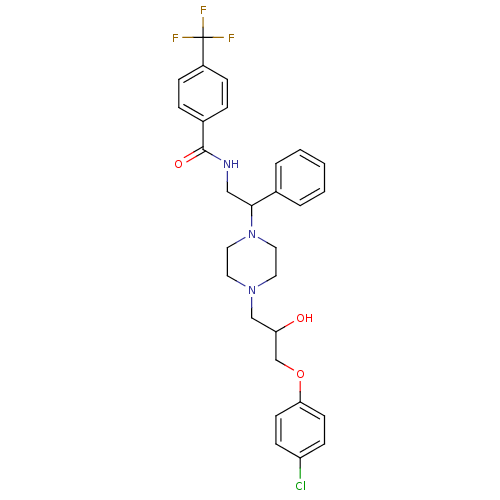 (CHEMBL62878 | N-(2-{4-[3-(4-Chloro-phenoxy)-2-hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143945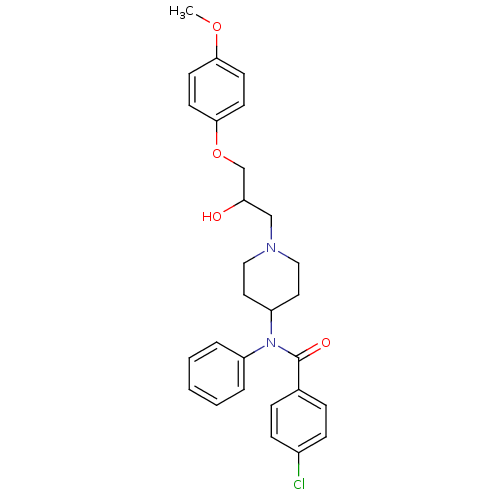 (4-Chloro-N-{1-[2-hydroxy-3-(4-methoxy-phenoxy)-pro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143949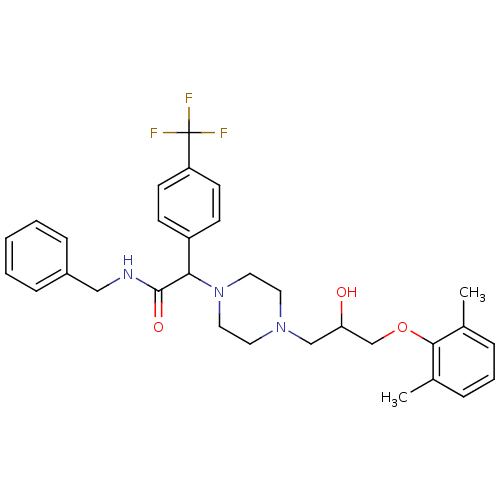 (CHEMBL60843 | N-Benzyl-2-{4-[3-(2,6-dimethyl-pheno...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143958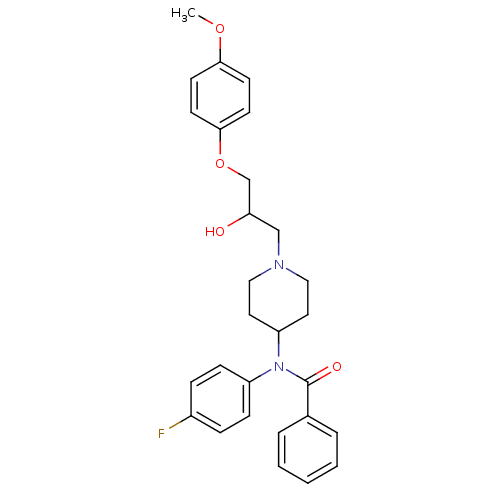 (CHEMBL63060 | N-(4-Fluoro-phenyl)-N-{1-[2-hydroxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143950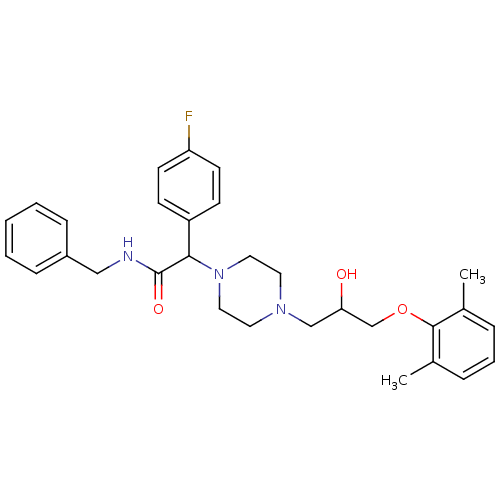 (CHEMBL305591 | N-Benzyl-2-{4-[3-(2,6-dimethyl-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143971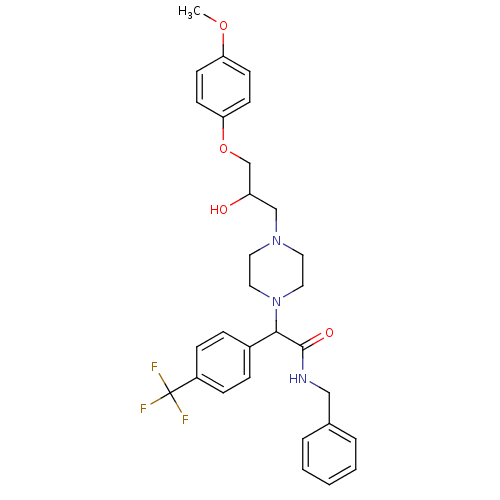 (CHEMBL59644 | N-Benzyl-2-{4-[2-hydroxy-3-(4-methox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143967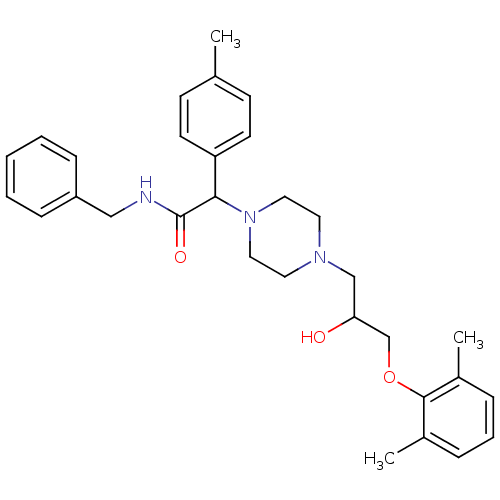 (CHEMBL60568 | N-Benzyl-2-{4-[3-(2,6-dimethyl-pheno...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143974 (2-{4-[3-(2,6-Dimethyl-phenoxy)-2-hydroxy-propyl]-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143961 (CHEMBL62240 | N-{1-[2-Hydroxy-3-(4-methoxy-phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143964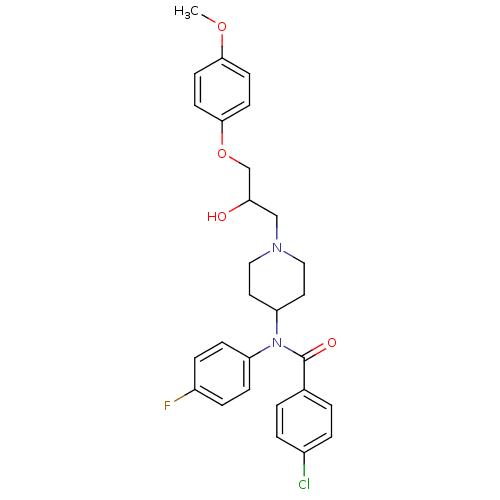 (4-Chloro-N-(4-fluoro-phenyl)-N-{1-[2-hydroxy-3-(4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143968 (4-Fluoro-N-(2-{4-[2-hydroxy-3-(4-methoxy-phenoxy)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143970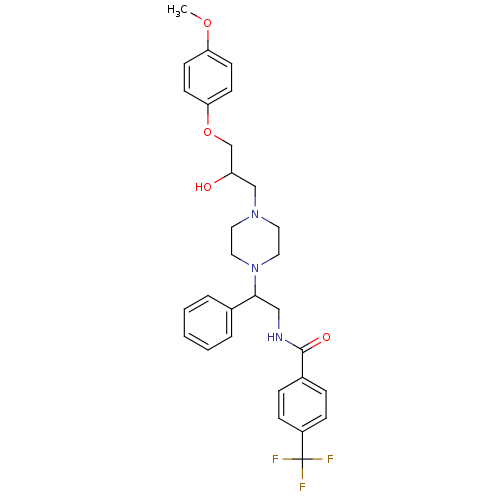 (CHEMBL61336 | N-(2-{4-[2-Hydroxy-3-(4-methoxy-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143956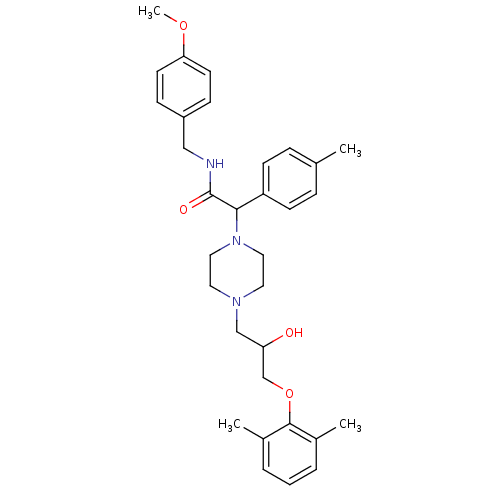 (2-{4-[3-(2,6-Dimethyl-phenoxy)-2-hydroxy-propyl]-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143954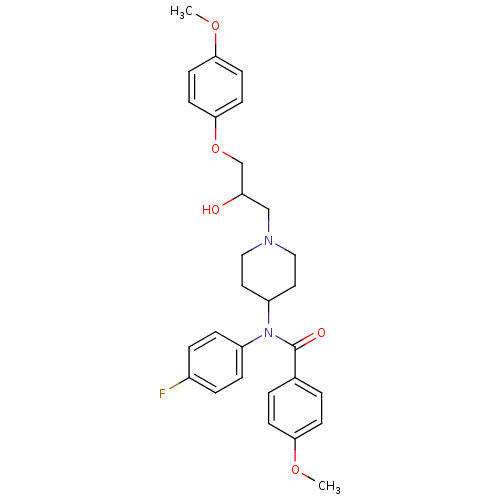 (CHEMBL433100 | N-(4-Fluoro-phenyl)-N-{1-[2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143976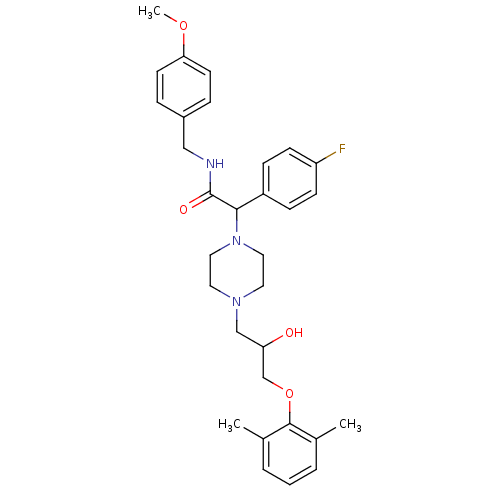 (2-{4-[3-(2,6-Dimethyl-phenoxy)-2-hydroxy-propyl]-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143973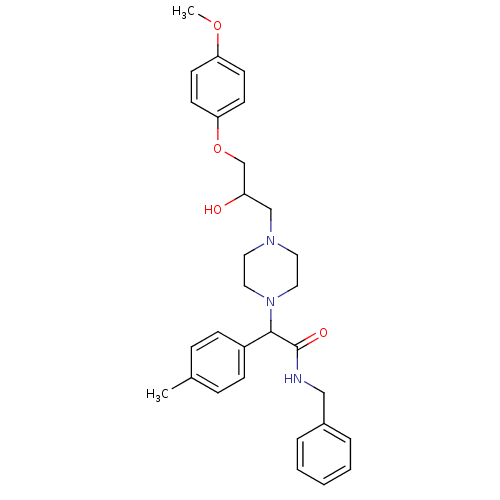 (CHEMBL291629 | N-Benzyl-2-{4-[2-hydroxy-3-(4-metho...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143955 (2-{4-[2-Hydroxy-3-(4-methoxy-phenoxy)-propyl]-pipe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143946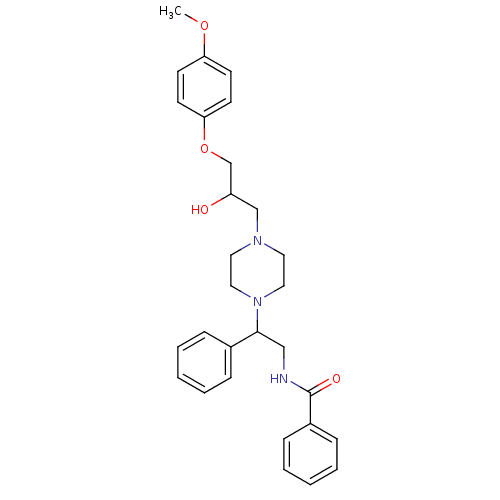 (CHEMBL64177 | N-(2-{4-[2-Hydroxy-3-(4-methoxy-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143959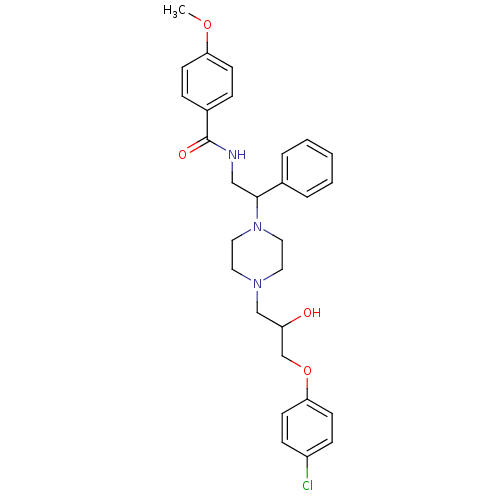 (CHEMBL61283 | N-(2-{4-[3-(4-Chloro-phenoxy)-2-hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143957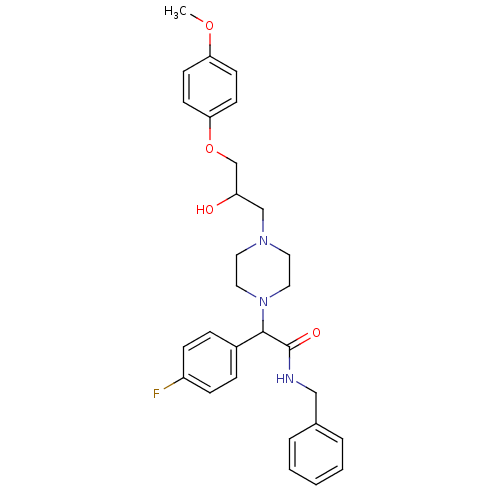 (CHEMBL60847 | N-Benzyl-2-(4-fluoro-phenyl)-2-{4-[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143972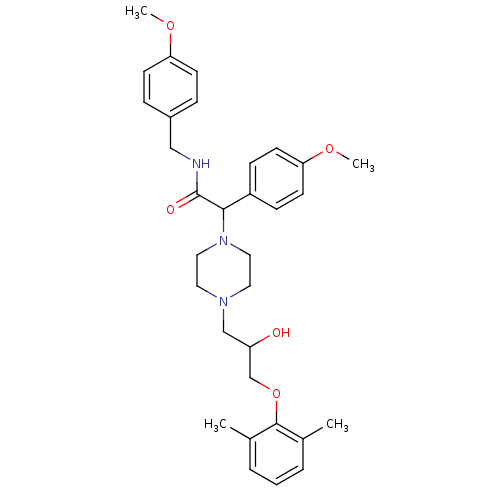 (2-{4-[3-(2,6-Dimethyl-phenoxy)-2-hydroxy-propyl]-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50119202 (CHEMBL99599 | Nonanoic acid [4-(4-{[2-hydroxy-2-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound against Beta-1 adrenergic receptor was determined | Bioorg Med Chem Lett 12: 2963-7 (2002) BindingDB Entry DOI: 10.7270/Q2D21WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143965 (CHEMBL60666 | N-(2-{4-[3-(4-Chloro-phenoxy)-2-hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143947 (4-Fluoro-N-{2-[4-(2-hydroxy-3-phenoxy-propyl)-pipe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143975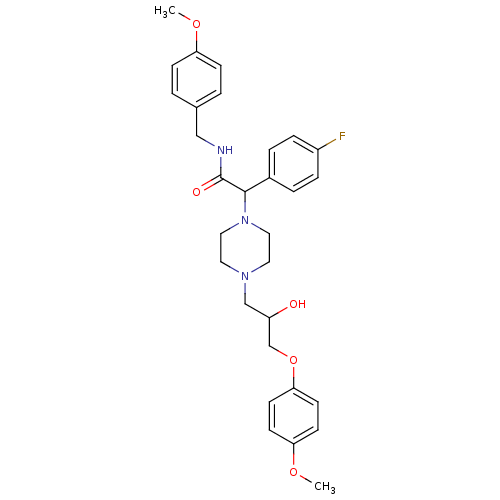 (2-(4-Fluoro-phenyl)-2-{4-[2-hydroxy-3-(4-methoxy-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143953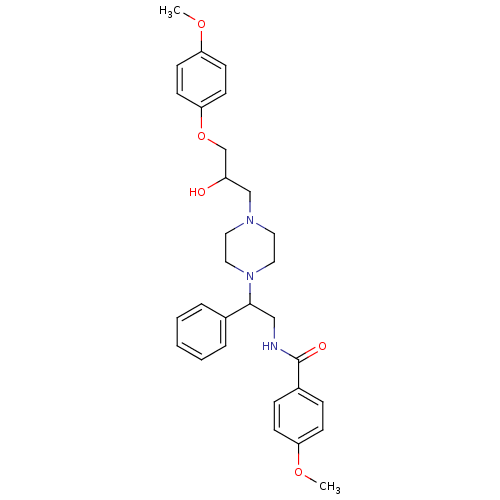 (CHEMBL64831 | N-(2-{4-[2-Hydroxy-3-(4-methoxy-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143960 (CHEMBL64688 | N-(2-{4-[2-Hydroxy-3-(4-methoxy-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143963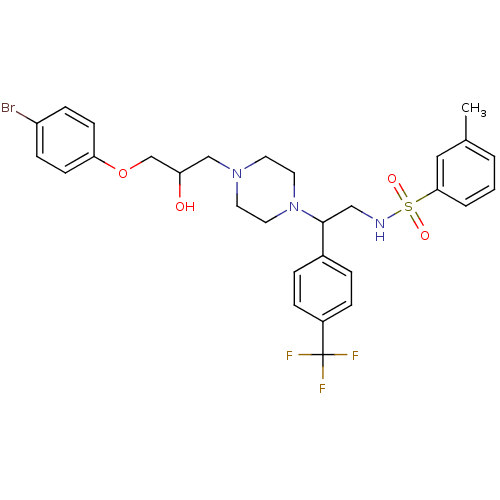 (CHEMBL64869 | N-[2-{4-[3-(4-Bromo-phenoxy)-2-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143966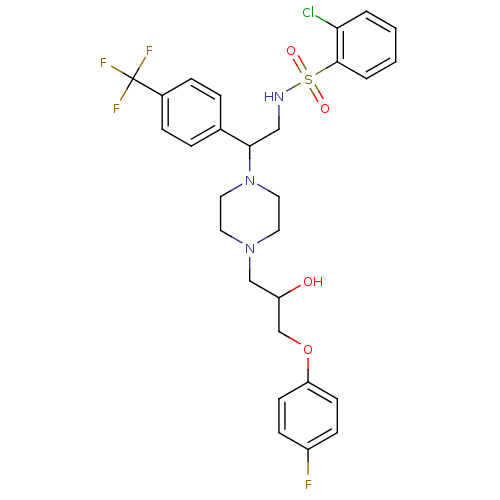 (2-Chloro-N-[2-{4-[3-(4-fluoro-phenoxy)-2-hydroxy-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50143969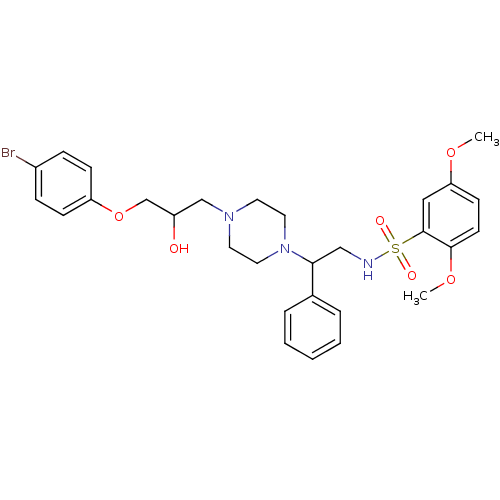 (CHEMBL64583 | N-(2-{4-[3-(4-Bromo-phenoxy)-2-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc Curated by ChEMBL | Assay Description Predictive competitive inhibition of cytochrome P450 2D6 was determined in silico | Bioorg Med Chem Lett 14: 2025-30 (2004) Article DOI: 10.1016/j.bmcl.2004.02.078 BindingDB Entry DOI: 10.7270/Q22J6B8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087670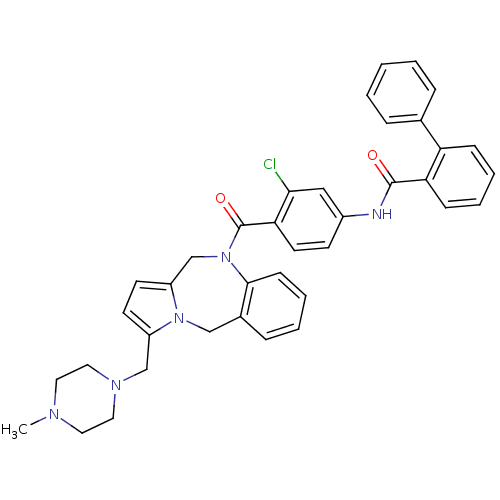 (Biphenyl-2-carboxylic acid {3-chloro-4-[3-(4-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108434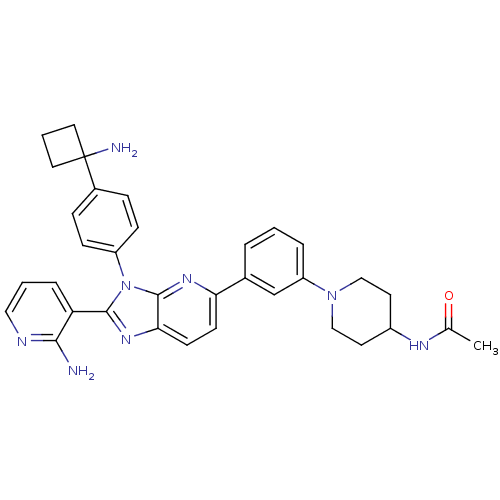 (US8609688, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.44 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108434 (US8609688, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.44 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108430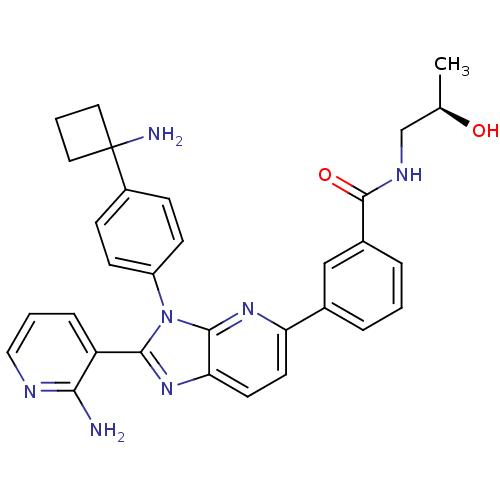 (US8609688, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108430 (US8609688, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108428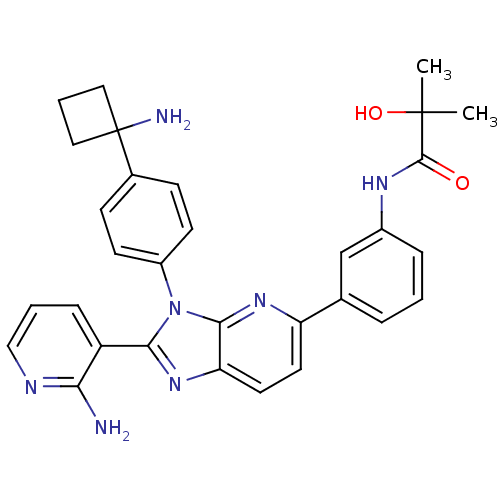 (US8609688, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108428 (US8609688, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM130225 (US8815854, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.69 | n/a | n/a | n/a | n/a | 8.0 | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8815854 (2014) BindingDB Entry DOI: 10.7270/Q2J67FMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108426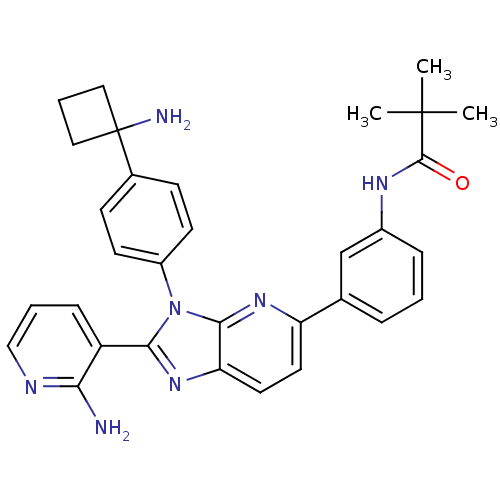 (US8609688, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108426 (US8609688, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108440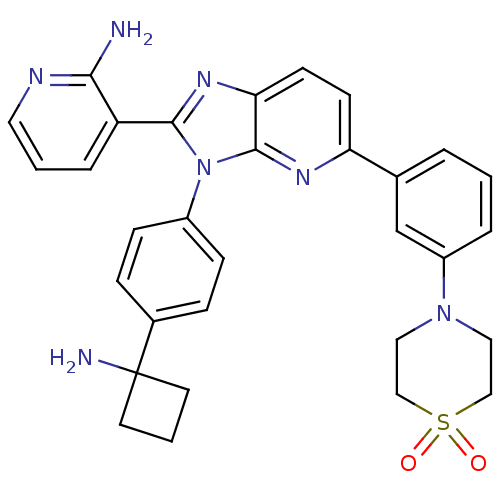 (US8609688, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.79 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108440 (US8609688, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108436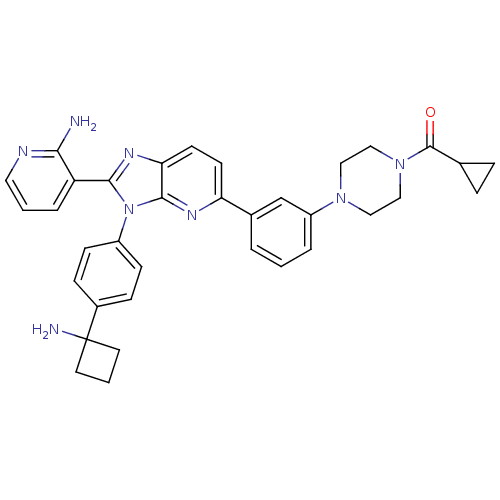 (US8609688, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.84 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 872 total ) | Next | Last >> |