 Found 426 hits with Last Name = 'rahim' and Initial = 'ma'
Found 426 hits with Last Name = 'rahim' and Initial = 'ma' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Corticoliberin
(Homo sapiens) | BDBM50610720

(CHEMBL5286255)Show SMILES CCCC(CCC)c1c(CC)nn2c(nc(C)nc12)-c1cc(OC)c(OC)cc1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Corticoliberin
(RAT) | BDBM50610716
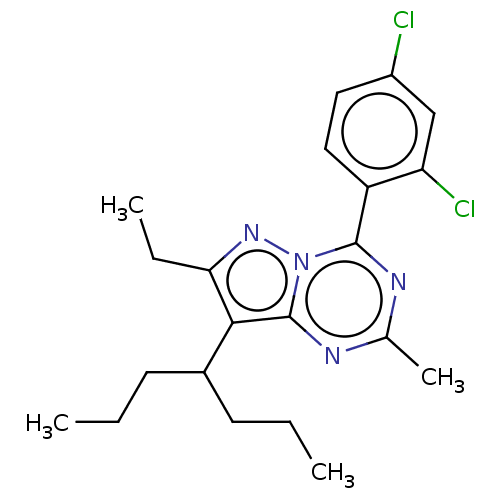
(CHEMBL5279390)Show SMILES CCCC(CCC)c1c(CC)nn2c(nc(C)nc12)-c1ccc(Cl)cc1Cl | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Corticoliberin
(RAT) | BDBM50610717

(CHEMBL5279909)Show SMILES CCCC(COC)c1c(CC)nn2c(nc(C)nc12)-c1ccc(Cl)cc1Cl | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50084875
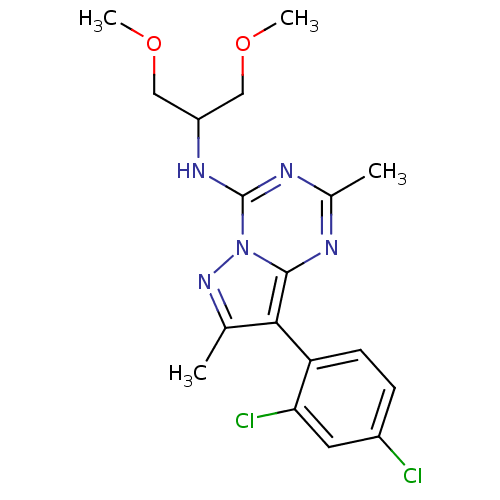
(8-(2,4-dichlorophenyl)-N-(1,3-dimethoxypropan-2-yl...)Show SMILES COCC(COC)Nc1nc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1Cl |(-3.04,-6.69,;-3.03,-8.23,;-4.37,-9,;-4.37,-10.54,;-3.03,-11.31,;-1.7,-10.54,;-1.7,-9.01,;-5.7,-11.31,;-5.7,-12.85,;-7.03,-13.63,;-7.03,-15.17,;-8.36,-15.94,;-5.7,-15.93,;-4.37,-15.17,;-2.91,-15.64,;-2,-14.4,;-.46,-14.4,;-2.91,-13.15,;-4.37,-13.63,;-2.18,-17.01,;-3.01,-18.3,;-2.29,-19.67,;-.75,-19.73,;-.03,-21.08,;.06,-18.41,;-.65,-17.05,;.16,-15.75,)| Show InChI InChI=1S/C18H21Cl2N5O2/c1-10-16(14-6-5-12(19)7-15(14)20)17-21-11(2)22-18(25(17)24-10)23-13(8-26-3)9-27-4/h5-7,13H,8-9H2,1-4H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Corticoliberin
(RAT) | BDBM50610718

(CHEMBL5290774)Show SMILES CCCC(COC)c1c(CC)nn2c(nc(C)nc12)-c1cc(OC)c(OC)cc1Cl | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Corticoliberin
(RAT) | BDBM50610719
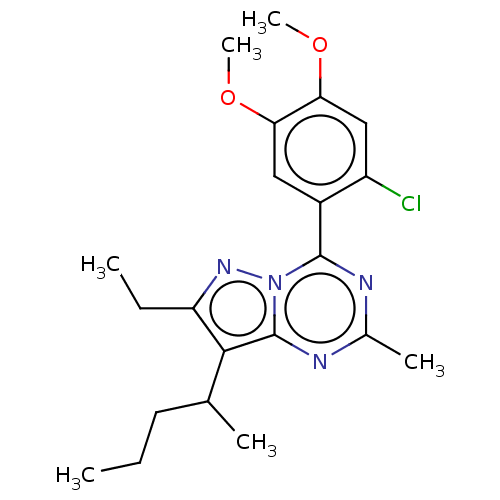
(CHEMBL5291384)Show SMILES CCCC(C)c1c(CC)nn2c(nc(C)nc12)-c1cc(OC)c(OC)cc1Cl | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM621185
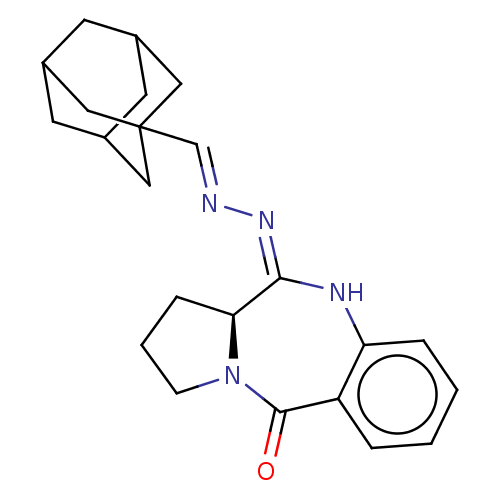
(US20230303581, Compound 4q)Show SMILES O=C1N2CCC[C@H]2\C(Nc2ccccc12)=N/N=C/C12CC3CC(CC(C3)C1)C2 |r,TLB:27:18:25:21.22.23,17:18:21:25.24.23,THB:27:22:25:18.26.19,26:18:21:25.24.23,26:24:21:18.27.19| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM621184
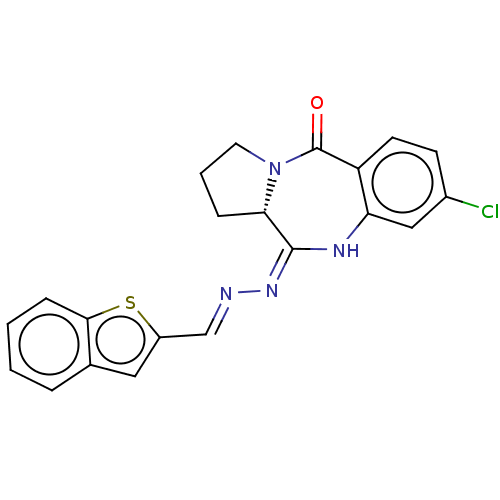
(US20230303581, Compound 4k)Show SMILES Clc1ccc2c(N\C(=N\N=C\c3cc4ccccc4s3)[C@@H]3CCCN3C2=O)c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114068
BindingDB Entry DOI: 10.7270/Q29W0KH4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Bos taurus) | BDBM50094943
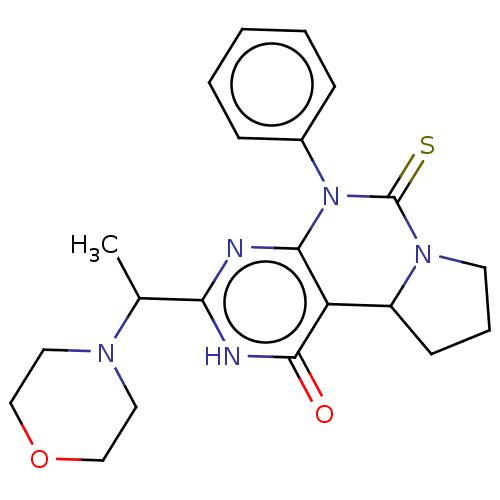
(CHEMBL3589372)Show SMILES CC(N1CCOCC1)c1nc2N(C(=S)N3CCCC3c2c(=O)[nH]1)c1ccccc1 Show InChI InChI=1S/C19H19N3OS/c1-19(2,3)22-18(23)15-12-21(11-13-7-6-10-24-13)16-9-5-4-8-14(16)17(15)20-22/h4-10,12H,11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged bovine p110alpha expressed in Sf9/Baculovirus system using [gamma-33P]ATP by scintillation proximity assay |
Eur J Med Chem 99: 1-13 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.036
BindingDB Entry DOI: 10.7270/Q2SF2XWT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Bos taurus) | BDBM50094956
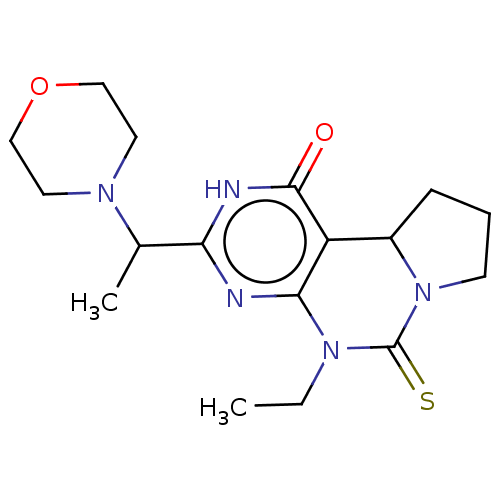
(CHEMBL3589370)Show SMILES CCN1C(=S)N2CCCC2c2c1nc([nH]c2=O)C(C)N1CCOCC1 Show InChI InChI=1S/C24H25N3O/c28-24-21-17-26(15-18-9-3-1-4-10-18)22-14-8-7-13-20(22)23(21)25-27(24)16-19-11-5-2-6-12-19/h1,3-4,7-10,13-14,17,19H,2,5-6,11-12,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged bovine p110alpha expressed in Sf9/Baculovirus system using [gamma-33P]ATP by scintillation proximity assay |
Eur J Med Chem 99: 1-13 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.036
BindingDB Entry DOI: 10.7270/Q2SF2XWT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Bos taurus) | BDBM50094942
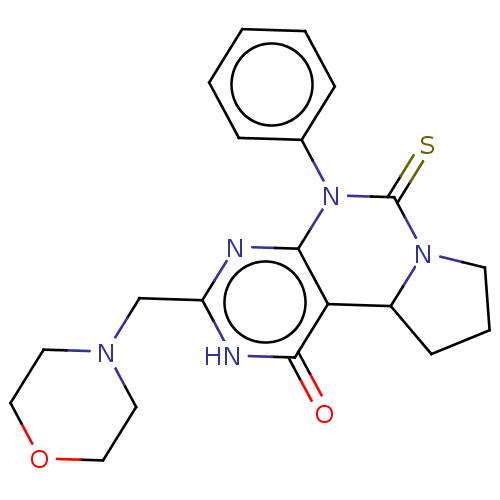
(CHEMBL3589371)Show SMILES O=c1[nH]c(CN2CCOCC2)nc2N(C(=S)N3CCCC3c12)c1ccccc1 Show InChI InChI=1S/C20H19N3OS/c24-20-17-13-22(12-15-8-5-11-25-15)18-10-4-3-9-16(18)19(17)21-23(20)14-6-1-2-7-14/h3-5,8-11,13-14H,1-2,6-7,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged bovine p110alpha expressed in Sf9/Baculovirus system using [gamma-33P]ATP by scintillation proximity assay |
Eur J Med Chem 99: 1-13 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.036
BindingDB Entry DOI: 10.7270/Q2SF2XWT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Bos taurus) | BDBM50094955
(CHEMBL3589324)Show InChI InChI=1S/C21H20ClN3O/c1-2-3-11-25-21(26)18-14-24(13-15-7-6-8-16(22)12-15)19-10-5-4-9-17(19)20(18)23-25/h4-10,12,14H,2-3,11,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged bovine p110alpha expressed in Sf9/Baculovirus system using [gamma-33P]ATP by scintillation proximity assay |
Eur J Med Chem 99: 1-13 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.036
BindingDB Entry DOI: 10.7270/Q2SF2XWT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402421
(CHEMBL2208035)Show SMILES O=C(Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1)[C@H]1CCC(=O)N1 |r| Show InChI InChI=1S/C25H26N6O3/c32-23-10-9-22(29-23)24(33)27-18-3-1-17(2-4-18)21-11-12-26-25(30-21)28-19-5-7-20(8-6-19)31-13-15-34-16-14-31/h1-8,11-12,22H,9-10,13-16H2,(H,27,33)(H,29,32)(H,26,28,30)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Bos taurus) | BDBM50094948

(CHEMBL3589377)Show SMILES CCN1C(=S)N2CCCC2c2c1nc(-c1ccc(OC)cc1)c(C#N)c2N1CCOCC1 Show InChI InChI=1S/C21H27N3O/c1-2-3-13-24-21(25)18-15-23(14-16-9-5-4-6-10-16)19-12-8-7-11-17(19)20(18)22-24/h7-8,11-12,15-16H,2-6,9-10,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged bovine p110alpha expressed in Sf9/Baculovirus system using [gamma-33P]ATP by scintillation proximity assay |
Eur J Med Chem 99: 1-13 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.036
BindingDB Entry DOI: 10.7270/Q2SF2XWT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50118683

(Acetic acid 4-[4-(4-methanesulfonyl-phenyl)-2-oxo-...)Show SMILES CC(=O)Oc1ccc(cc1)C1=C(COC1=O)c1ccc(cc1)S(C)(=O)=O |t:11| Show InChI InChI=1S/C19H16O6S/c1-12(20)25-15-7-3-14(4-8-15)18-17(11-24-19(18)21)13-5-9-16(10-6-13)26(2,22)23/h3-10H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of compound required to inhibit Prostaglandin G/H synthase 2 enzyme was determined |
Bioorg Med Chem Lett 12: 2753-6 (2002)
BindingDB Entry DOI: 10.7270/Q27H1HXN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50118684

(Acetic acid 3-[4-(4-methanesulfonyl-phenyl)-2-oxo-...)Show SMILES CC(=O)Oc1cccc(c1)C1=C(COC1=O)c1ccc(cc1)S(C)(=O)=O |t:11| Show InChI InChI=1S/C19H16O6S/c1-12(20)25-15-5-3-4-14(10-15)18-17(11-24-19(18)21)13-6-8-16(9-7-13)26(2,22)23/h3-10H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of compound required to inhibit Prostaglandin G/H synthase 2 enzyme was determined |
Bioorg Med Chem Lett 12: 2753-6 (2002)
BindingDB Entry DOI: 10.7270/Q27H1HXN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402413

(CHEMBL2208032)Show SMILES C[C@@H](N)C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C23H26N6O2/c1-16(24)22(30)26-18-4-2-17(3-5-18)21-10-11-25-23(28-21)27-19-6-8-20(9-7-19)29-12-14-31-15-13-29/h2-11,16H,12-15,24H2,1H3,(H,26,30)(H,25,27,28)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Bos taurus) | BDBM50094950
(CHEMBL3589379)Show SMILES CCN1C(=S)N2CCCC2c2c1nc(-c1ccc(OC)c(OC)c1)c(C#N)c2N1CCOCC1 Show InChI InChI=1S/C20H19N3O/c1-2-3-13-23-20(24)17-14-22(15-9-5-4-6-10-15)18-12-8-7-11-16(18)19(17)21-23/h4-12,14H,2-3,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged bovine p110alpha expressed in Sf9/Baculovirus system using [gamma-33P]ATP by scintillation proximity assay |
Eur J Med Chem 99: 1-13 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.036
BindingDB Entry DOI: 10.7270/Q2SF2XWT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402409
(CHEMBL2208034)Show SMILES O=C(Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1)[C@H]1CCCN1 |r| Show InChI InChI=1S/C25H28N6O2/c32-24(23-2-1-12-26-23)28-19-5-3-18(4-6-19)22-11-13-27-25(30-22)29-20-7-9-21(10-8-20)31-14-16-33-17-15-31/h3-11,13,23,26H,1-2,12,14-17H2,(H,28,32)(H,27,29,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402412
(CHEMBL2208033)Show SMILES N[C@H](CO)C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C23H26N6O3/c24-20(15-30)22(31)26-17-3-1-16(2-4-17)21-9-10-25-23(28-21)27-18-5-7-19(8-6-18)29-11-13-32-14-12-29/h1-10,20,30H,11-15,24H2,(H,26,31)(H,25,27,28)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM23928
(1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...)Show InChI InChI=1S/C17H18O3/c1-18-15-8-6-13(7-9-15)4-5-14-10-16(19-2)12-17(11-14)20-3/h4-12H,1-3H3/b5-4+ | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50118681

(Acetic acid 2-[4-(4-methanesulfonyl-phenyl)-2-oxo-...)Show SMILES CC(=O)Oc1ccccc1C1=C(COC1=O)c1ccc(cc1)S(C)(=O)=O |t:11| Show InChI InChI=1S/C19H16O6S/c1-12(20)25-17-6-4-3-5-15(17)18-16(11-24-19(18)21)13-7-9-14(10-8-13)26(2,22)23/h3-10H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of compound required to inhibit Prostaglandin G/H synthase 2 enzyme was determined |
Bioorg Med Chem Lett 12: 2753-6 (2002)
BindingDB Entry DOI: 10.7270/Q27H1HXN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50310998

(CHEMBL1077458 | N-(4-(2-(4-morpholinophenylamino)p...)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 Show InChI InChI=1S/C22H23N5O2/c1-16(28)24-18-4-2-17(3-5-18)21-10-11-23-22(26-21)25-19-6-8-20(9-7-19)27-12-14-29-15-13-27/h2-11H,12-15H2,1H3,(H,24,28)(H,23,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Bos taurus) | BDBM50094947

(CHEMBL3589376)Show SMILES CCN1C(=S)N2CCCC2c2c1nc(-c1cccc(OC)c1)c(C#N)c2N1CCOCC1 Show InChI InChI=1S/C22H21N3O/c26-22-19-15-24(13-16-7-2-1-3-8-16)20-12-5-4-11-18(20)21(19)23-25(22)14-17-9-6-10-17/h1-5,7-8,11-12,15,17H,6,9-10,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged bovine p110alpha expressed in Sf9/Baculovirus system using [gamma-33P]ATP by scintillation proximity assay |
Eur J Med Chem 99: 1-13 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.036
BindingDB Entry DOI: 10.7270/Q2SF2XWT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Bos taurus) | BDBM50094949
(CHEMBL3589378)Show SMILES CCN1C(=S)N2CCCC2c2c1nc(-c1ccc(OC)c(O)c1)c(C#N)c2N1CCOCC1 Show InChI InChI=1S/C24H27N5O3S/c1-3-28-23-20(17-5-4-8-29(17)24(28)33)22(27-9-11-32-12-10-27)16(14-25)21(26-23)15-6-7-19(31-2)18(30)13-15/h6-7,13,17,30H,3-5,8-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged bovine p110alpha expressed in Sf9/Baculovirus system using [gamma-33P]ATP by scintillation proximity assay |
Eur J Med Chem 99: 1-13 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.036
BindingDB Entry DOI: 10.7270/Q2SF2XWT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402416

(CHEMBL2208025)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2cccc(c2)N2CCOCC2)n1 Show InChI InChI=1S/C22H23N5O2/c1-16(28)24-18-7-5-17(6-8-18)21-9-10-23-22(26-21)25-19-3-2-4-20(15-19)27-11-13-29-14-12-27/h2-10,15H,11-14H2,1H3,(H,24,28)(H,23,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402424

(CHEMBL2208027)Show SMILES CC(=O)Nc1ccc(cc1)-c1nc(Nc2ccc(cc2)N2CCOCC2)ncc1F Show InChI InChI=1S/C22H22FN5O2/c1-15(29)25-17-4-2-16(3-5-17)21-20(23)14-24-22(27-21)26-18-6-8-19(9-7-18)28-10-12-30-13-11-28/h2-9,14H,10-13H2,1H3,(H,25,29)(H,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380413

(CHEMBL2018573)Show SMILES Fc1ccc(C[C@@H]2C[C@H](N(C2)C(=O)Cn2nccn2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C28H25F2N5O3/c29-21-3-1-19(2-4-21)15-20-16-26(34(17-20)27(36)18-35-31-13-14-32-35)28(37)33-23-7-11-25(12-8-23)38-24-9-5-22(30)6-10-24/h1-14,20,26H,15-18H2,(H,33,37)/t20-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402415
(CHEMBL2208028)Show SMILES CC(=O)Nc1ccc(cc1)-c1nc(Nc2ccc(cc2)N2CCOCC2)ncc1C Show InChI InChI=1S/C23H25N5O2/c1-16-15-24-23(27-22(16)18-3-5-19(6-4-18)25-17(2)29)26-20-7-9-21(10-8-20)28-11-13-30-14-12-28/h3-10,15H,11-14H2,1-2H3,(H,25,29)(H,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402411

(CHEMBL2207759)Show SMILES CN1CCC[C@@H]1C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C26H30N6O2/c1-31-14-2-3-24(31)25(33)28-20-6-4-19(5-7-20)23-12-13-27-26(30-23)29-21-8-10-22(11-9-21)32-15-17-34-18-16-32/h4-13,24H,2-3,14-18H2,1H3,(H,28,33)(H,27,29,30)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402420

(CHEMBL2207758)Show SMILES O=C(Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1)[C@H]1CCNC1 |r| Show InChI InChI=1S/C25H28N6O2/c32-24(19-9-11-26-17-19)28-20-3-1-18(2-4-20)23-10-12-27-25(30-23)29-21-5-7-22(8-6-21)31-13-15-33-16-14-31/h1-8,10,12,19,26H,9,11,13-17H2,(H,28,32)(H,27,29,30)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380418

(CHEMBL2018571)Show SMILES Fc1ccc(C[C@@H]2C[C@H](N(C2)C(=O)Cn2ccnn2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C28H25F2N5O3/c29-21-3-1-19(2-4-21)15-20-16-26(35(17-20)27(36)18-34-14-13-31-33-34)28(37)32-23-7-11-25(12-8-23)38-24-9-5-22(30)6-10-24/h1-14,20,26H,15-18H2,(H,32,37)/t20-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380417
(CHEMBL2018485)Show SMILES Fc1ccc(C[C@@H]2C[C@H](N(C2)C(=O)Cn2cncn2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C28H25F2N5O3/c29-21-3-1-19(2-4-21)13-20-14-26(35(15-20)27(36)16-34-18-31-17-32-34)28(37)33-23-7-11-25(12-8-23)38-24-9-5-22(30)6-10-24/h1-12,17-18,20,26H,13-16H2,(H,33,37)/t20-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402417
(CHEMBL2208024)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2cccc(c2)N2CCCCC2)n1 Show InChI InChI=1S/C23H25N5O/c1-17(29)25-19-10-8-18(9-11-19)22-12-13-24-23(27-22)26-20-6-5-7-21(16-20)28-14-3-2-4-15-28/h5-13,16H,2-4,14-15H2,1H3,(H,25,29)(H,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380410

(CHEMBL2018484)Show SMILES Fc1ccc(Oc2ccc(NC(=O)[C@@H]3C[C@@H](Cc4ccccc4)CN3C(=O)Cn3cncn3)cc2)cc1 |r| Show InChI InChI=1S/C28H26FN5O3/c29-22-6-10-24(11-7-22)37-25-12-8-23(9-13-25)32-28(36)26-15-21(14-20-4-2-1-3-5-20)16-34(26)27(35)17-33-19-30-18-31-33/h1-13,18-19,21,26H,14-17H2,(H,32,36)/t21-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402427
(CHEMBL2207766)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2cccc(NC(=O)c3ccccc3Cl)c2)n1 Show InChI InChI=1S/C25H20ClN5O2/c1-16(32)28-18-11-9-17(10-12-18)23-13-14-27-25(31-23)30-20-6-4-5-19(15-20)29-24(33)21-7-2-3-8-22(21)26/h2-15H,1H3,(H,28,32)(H,29,33)(H,27,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Bos taurus) | BDBM50094946

(CHEMBL3589375)Show SMILES CCN1C(=S)N2CCCC2c2c1nc(-c1cccc(O)c1)c(C#N)c2N1CCOCC1 Show InChI InChI=1S/C21H27N3O/c1-21(2,3)24-20(25)17-14-23(13-15-9-5-4-6-10-15)18-12-8-7-11-16(18)19(17)22-24/h7-8,11-12,14-15H,4-6,9-10,13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged bovine p110alpha expressed in Sf9/Baculovirus system using [gamma-33P]ATP by scintillation proximity assay |
Eur J Med Chem 99: 1-13 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.036
BindingDB Entry DOI: 10.7270/Q2SF2XWT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402414
(CHEMBL2208031)Show SMILES C[C@H](N)C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C23H26N6O2/c1-16(24)22(30)26-18-4-2-17(3-5-18)21-10-11-25-23(28-21)27-19-6-8-20(9-7-19)29-12-14-31-15-13-29/h2-11,16H,12-15,24H2,1H3,(H,26,30)(H,25,27,28)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402410
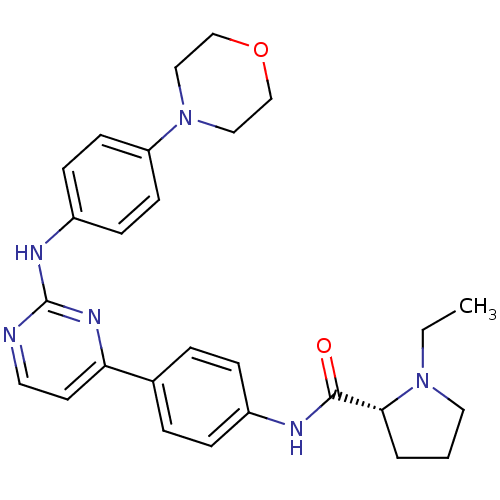
(CHEMBL2207760)Show SMILES CCN1CCC[C@@H]1C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C27H32N6O2/c1-2-32-15-3-4-25(32)26(34)29-21-7-5-20(6-8-21)24-13-14-28-27(31-24)30-22-9-11-23(12-10-22)33-16-18-35-19-17-33/h5-14,25H,2-4,15-19H2,1H3,(H,29,34)(H,28,30,31)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50152068
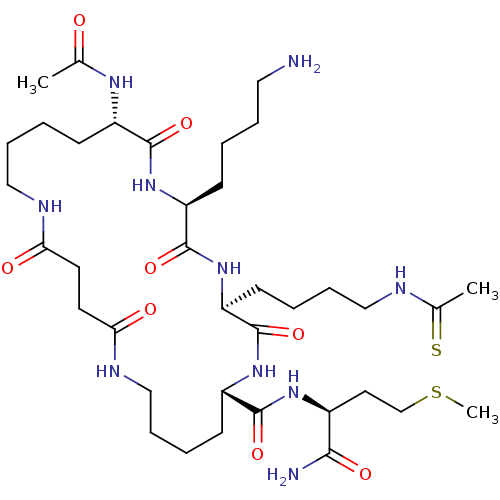
(CHEMBL3781485)Show SMILES CSCC[C@H](NC(=O)[C@@H]1CCCCNC(=O)CCC(=O)NCCCC[C@H](NC(C)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCNC(C)=S)C(=O)N1)C(N)=O |r| Show InChI InChI=1S/C37H66N10O8S2/c1-24(48)43-27-13-6-10-21-41-31(49)16-17-32(50)42-22-11-7-15-29(35(53)44-26(33(39)51)18-23-57-3)46-37(55)30(14-5-9-20-40-25(2)56)47-36(54)28(45-34(27)52)12-4-8-19-38/h26-30H,4-23,38H2,1-3H3,(H2,39,51)(H,40,56)(H,41,49)(H,42,50)(H,43,48)(H,44,53)(H,45,52)(H,46,55)(H,47,54)/t26-,27-,28-,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402426

(CHEMBL2207767)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2cccc(NC(=O)c3ccccc3)c2)n1 Show InChI InChI=1S/C25H21N5O2/c1-17(31)27-20-12-10-18(11-13-20)23-14-15-26-25(30-23)29-22-9-5-8-21(16-22)28-24(32)19-6-3-2-4-7-19/h2-16H,1H3,(H,27,31)(H,28,32)(H,26,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402419
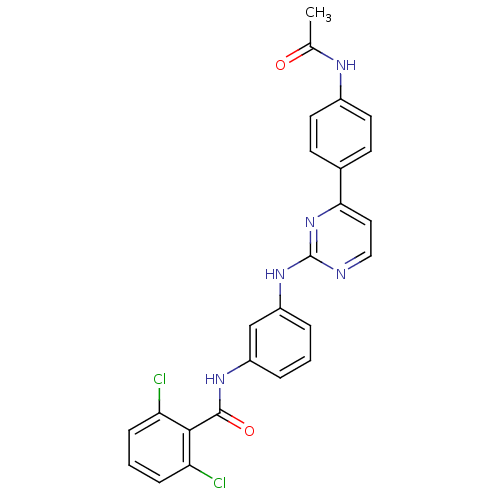
(CHEMBL2207764)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2cccc(NC(=O)c3c(Cl)cccc3Cl)c2)n1 Show InChI InChI=1S/C25H19Cl2N5O2/c1-15(33)29-17-10-8-16(9-11-17)22-12-13-28-25(32-22)31-19-5-2-4-18(14-19)30-24(34)23-20(26)6-3-7-21(23)27/h2-14H,1H3,(H,29,33)(H,30,34)(H,28,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402418
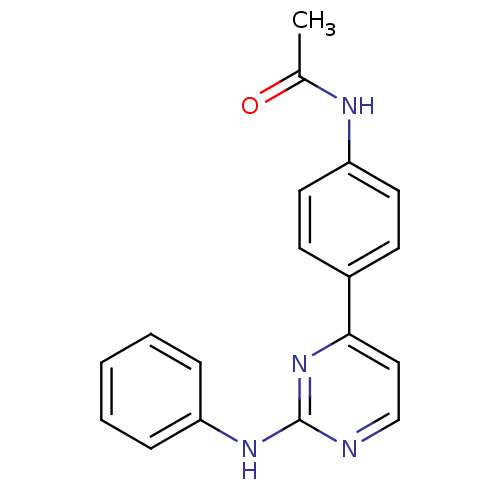
(CHEMBL2208023)Show InChI InChI=1S/C18H16N4O/c1-13(23)20-16-9-7-14(8-10-16)17-11-12-19-18(22-17)21-15-5-3-2-4-6-15/h2-12H,1H3,(H,20,23)(H,19,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380414

(CHEMBL2018467)Show SMILES Fc1ccc(Oc2ccc(NC(=O)[C@H](COCc3ccccc3)NC(=O)Cc3cnc[nH]3)cc2)cc1 |r| Show InChI InChI=1S/C27H25FN4O4/c28-20-6-10-23(11-7-20)36-24-12-8-21(9-13-24)31-27(34)25(17-35-16-19-4-2-1-3-5-19)32-26(33)14-22-15-29-18-30-22/h1-13,15,18,25H,14,16-17H2,(H,29,30)(H,31,34)(H,32,33)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380417

(CHEMBL2018485)Show SMILES Fc1ccc(C[C@@H]2C[C@H](N(C2)C(=O)Cn2cncn2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C28H25F2N5O3/c29-21-3-1-19(2-4-21)13-20-14-26(35(15-20)27(36)16-34-18-31-17-32-34)28(37)33-23-7-11-25(12-8-23)38-24-9-5-22(30)6-10-24/h1-12,17-18,20,26H,13-16H2,(H,33,37)/t20-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced tango beta-arrestin recruitment preincubated for... |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380437
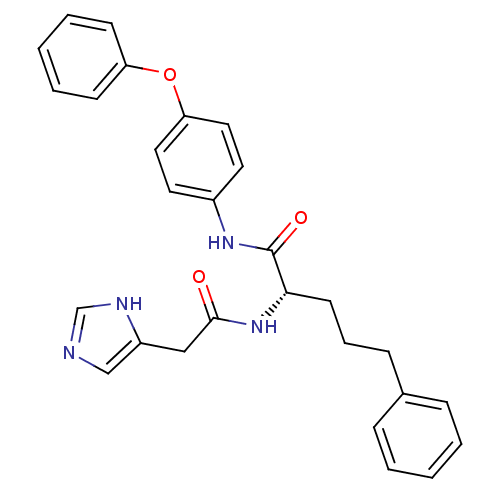
(CHEMBL2018474)Show SMILES O=C(Cc1cnc[nH]1)N[C@@H](CCCc1ccccc1)C(=O)Nc1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C28H28N4O3/c33-27(18-23-19-29-20-30-23)32-26(13-7-10-21-8-3-1-4-9-21)28(34)31-22-14-16-25(17-15-22)35-24-11-5-2-6-12-24/h1-6,8-9,11-12,14-17,19-20,26H,7,10,13,18H2,(H,29,30)(H,31,34)(H,32,33)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380413

(CHEMBL2018573)Show SMILES Fc1ccc(C[C@@H]2C[C@H](N(C2)C(=O)Cn2nccn2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C28H25F2N5O3/c29-21-3-1-19(2-4-21)15-20-16-26(34(17-20)27(36)18-35-31-13-14-32-35)28(37)33-23-7-11-25(12-8-23)38-24-9-5-22(30)6-10-24/h1-14,20,26H,15-18H2,(H,33,37)/t20-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced tango beta-arrestin recruitment preincubated for... |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380418

(CHEMBL2018571)Show SMILES Fc1ccc(C[C@@H]2C[C@H](N(C2)C(=O)Cn2ccnn2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C28H25F2N5O3/c29-21-3-1-19(2-4-21)15-20-16-26(35(17-20)27(36)18-34-14-13-31-33-34)28(37)32-23-7-11-25(12-8-23)38-24-9-5-22(30)6-10-24/h1-14,20,26H,15-18H2,(H,32,37)/t20-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced tango beta-arrestin recruitment preincubated for... |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50540056
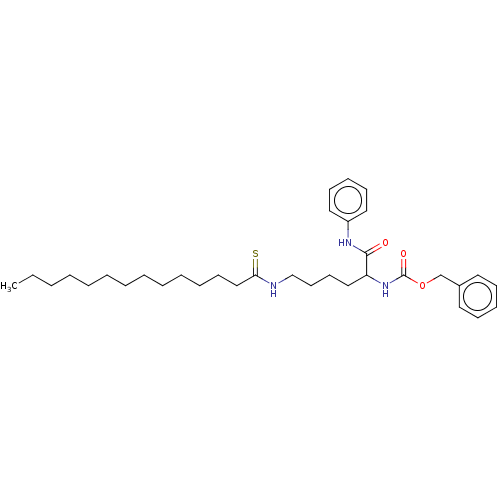
(CHEMBL4638983)Show SMILES CCCCCCCCCCCCCC(=S)NCCCCC(NC(=O)OCc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H51N3O3S/c1-2-3-4-5-6-7-8-9-10-11-18-26-32(41)35-27-20-19-25-31(33(38)36-30-23-16-13-17-24-30)37-34(39)40-28-29-21-14-12-15-22-29/h12-17,21-24,31H,2-11,18-20,25-28H2,1H3,(H,35,41)(H,36,38)(H,37,39) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data