 Found 466 hits with Last Name = 'robers' and Initial = 'mb'
Found 466 hits with Last Name = 'robers' and Initial = 'mb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human HCK using KVEKIGEGTYGVVYK as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM202553
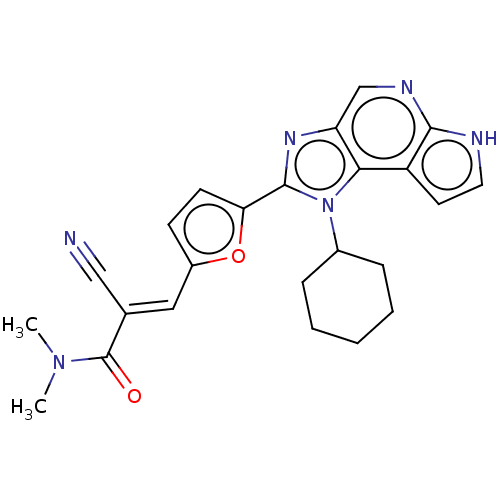
((E/Z)-2-cyano-3-(5-(1-cyclohexyl-1,6-dihydroimidaz...)Show SMILES CN(C)C(=O)C(=C\c1ccc(o1)-c1nc2cnc3[nH]ccc3c2n1C1CCCCC1)\C#N Show InChI InChI=1S/C24H24N6O2/c1-29(2)24(31)15(13-25)12-17-8-9-20(32-17)23-28-19-14-27-22-18(10-11-26-22)21(19)30(23)16-6-4-3-5-7-16/h8-12,14,16H,3-7H2,1-2H3,(H,26,27)/b15-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.127 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
| Assay Description
A commercial assay by Reaction Biology Corp. |
Cell Chem Biol 23: 1335-1340 (2016)
Article DOI: 10.1016/j.chembiol.2016.10.008
BindingDB Entry DOI: 10.7270/Q2WS8S22 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM202554
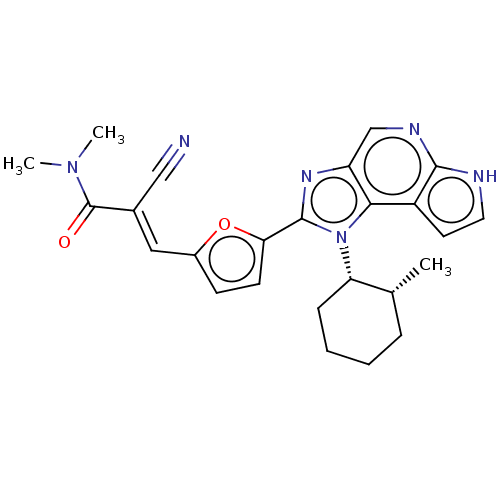
((E/Z)-2-cyano-N,N-dimethyl-3-(5-(1-((1S,2R)-2-meth...)Show SMILES C[C@@H]1CCCC[C@@H]1n1c(nc2cnc3[nH]ccc3c12)-c1ccc(\C=C(/C#N)C(=O)N(C)C)o1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.154 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
| Assay Description
A commercial assay by Reaction Biology Corp. |
Cell Chem Biol 23: 1335-1340 (2016)
Article DOI: 10.1016/j.chembiol.2016.10.008
BindingDB Entry DOI: 10.7270/Q2WS8S22 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to LYN (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50238177

(CHEMBL4098072)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C25H22FN5O2S/c1-4-22(32)28-18-9-10-20(33-2)19(14-18)29-21-13-16(11-12-27-21)24-23(30-25(31-24)34-3)15-5-7-17(26)8-6-15/h4-14H,1H2,2-3H3,(H,27,29)(H,28,32)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00848
BindingDB Entry DOI: 10.7270/Q2Q52TQZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human LCK using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50247556
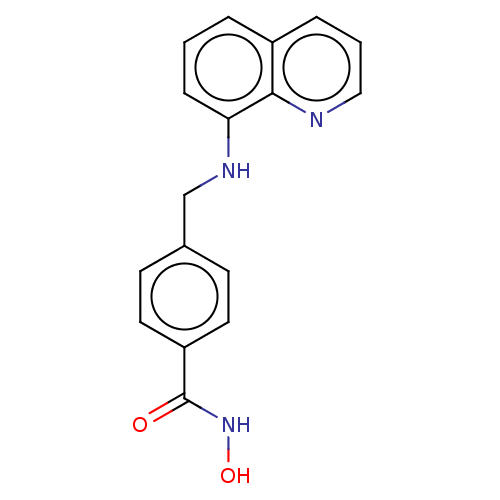
(CHEMBL4104117)Show InChI InChI=1S/C17H15N3O2/c21-17(20-22)14-8-6-12(7-9-14)11-19-15-5-1-3-13-4-2-10-18-16(13)15/h1-10,19,22H,11H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) |
J Med Chem 62: 8557-8577 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00946
BindingDB Entry DOI: 10.7270/Q2542RXH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.292 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
| Assay Description
A commercial assay by Reaction Biology Corp. |
Cell Chem Biol 23: 1335-1340 (2016)
Article DOI: 10.1016/j.chembiol.2016.10.008
BindingDB Entry DOI: 10.7270/Q2WS8S22 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50571526
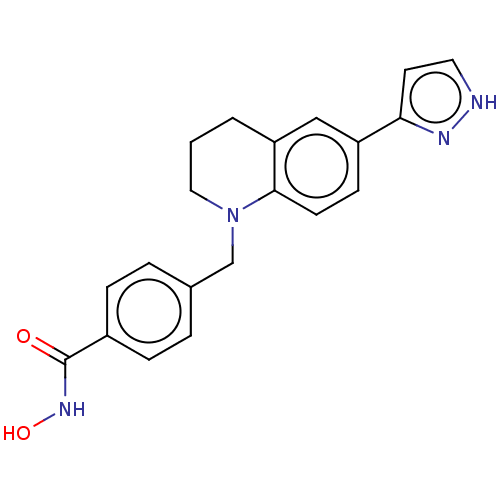
(CHEMBL4863515)Show SMILES ONC(=O)c1ccc(CN2CCCc3cc(ccc23)-c2cc[nH]n2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50274998
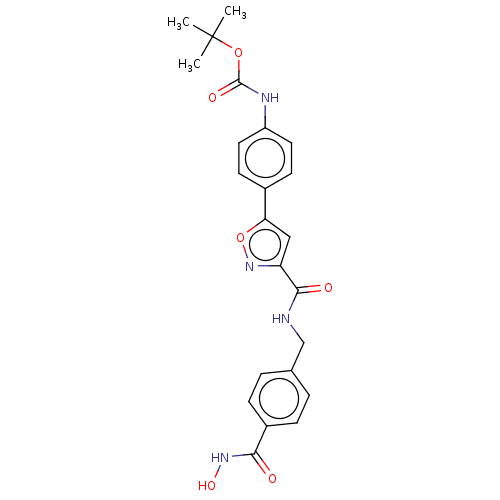
(CHEMBL4126811)Show SMILES CC(C)(C)OC(=O)Nc1ccc(cc1)-c1cc(no1)C(=O)NCc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C23H24N4O6/c1-23(2,3)32-22(30)25-17-10-8-15(9-11-17)19-12-18(27-33-19)21(29)24-13-14-4-6-16(7-5-14)20(28)26-31/h4-12,31H,13H2,1-3H3,(H,24,29)(H,25,30)(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human HDAC6 (1 to 1215 residues) expressed in baculovirus infected sf9 insect cells using p53 (379 to... |
J Med Chem 62: 8557-8577 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00946
BindingDB Entry DOI: 10.7270/Q2542RXH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human FYN using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ABL1 using EAIYAAPFAKKK as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.496 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
| Assay Description
A commercial assay by Reaction Biology Corp. |
Cell Chem Biol 23: 1335-1340 (2016)
Article DOI: 10.1016/j.chembiol.2016.10.008
BindingDB Entry DOI: 10.7270/Q2WS8S22 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM417049
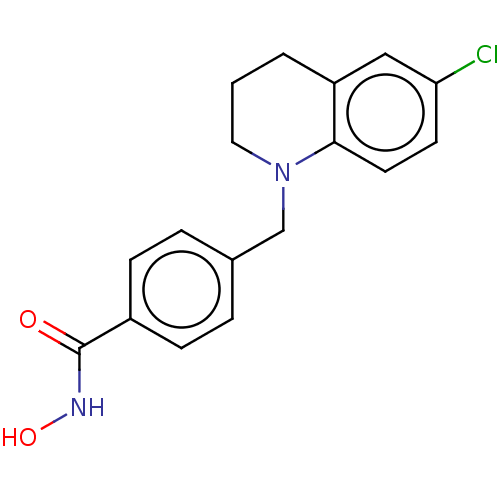
(4-((6-chloro-3,4-dihydroquinolin-1(2H)-yl)methyl)-...)Show InChI InChI=1S/C17H17ClN2O2/c18-15-7-8-16-14(10-15)2-1-9-20(16)11-12-3-5-13(6-4-12)17(21)19-22/h3-8,10,22H,1-2,9,11H2,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50571528
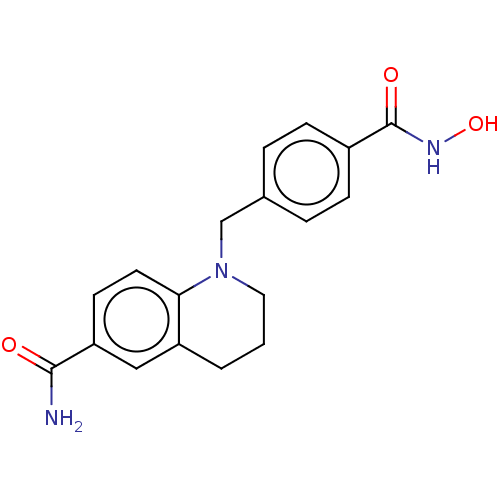
(CHEMBL4846435) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50571517
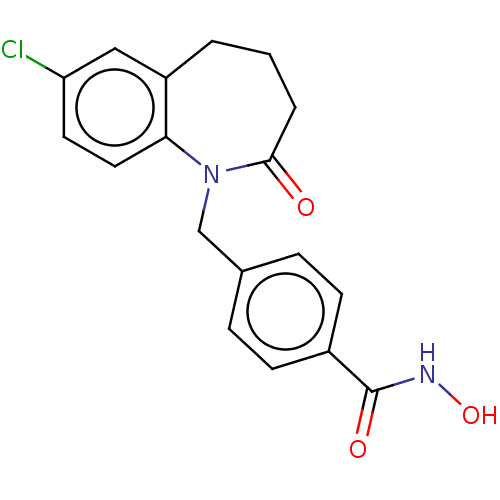
(CHEMBL4846714) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50252391
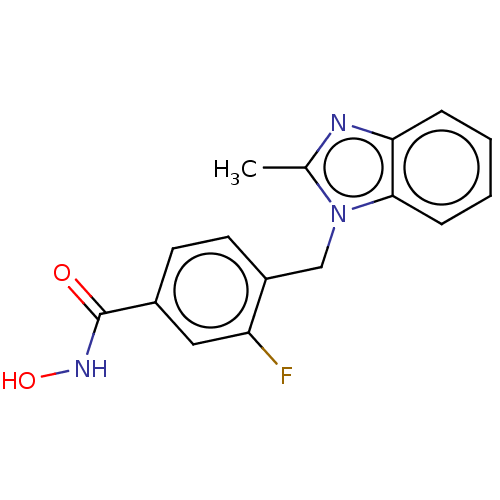
(CHEMBL4080014)Show InChI InChI=1S/C16H14FN3O2/c1-10-18-14-4-2-3-5-15(14)20(10)9-12-7-6-11(8-13(12)17)16(21)19-22/h2-8,22H,9H2,1H3,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human HDAC6 (1 to 1215 residues) expressed in baculovirus infected sf9 insect cells using RHK-K(Ac)-A... |
J Med Chem 62: 8557-8577 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00946
BindingDB Entry DOI: 10.7270/Q2542RXH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human YES using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50571523
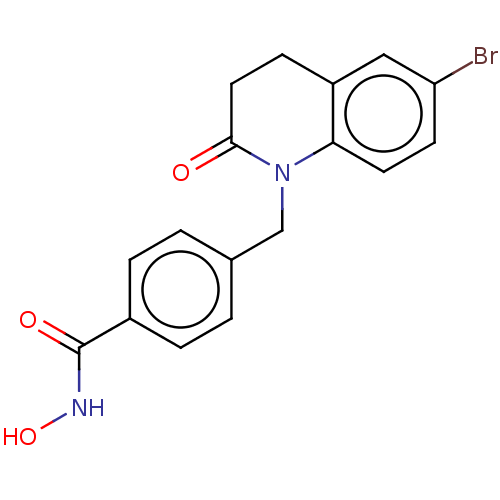
(CHEMBL4857825) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50567701
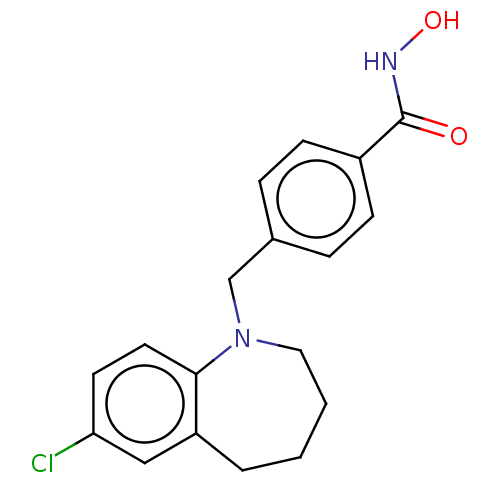
(CHEMBL4856735) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human PDGFRalpha using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50602487
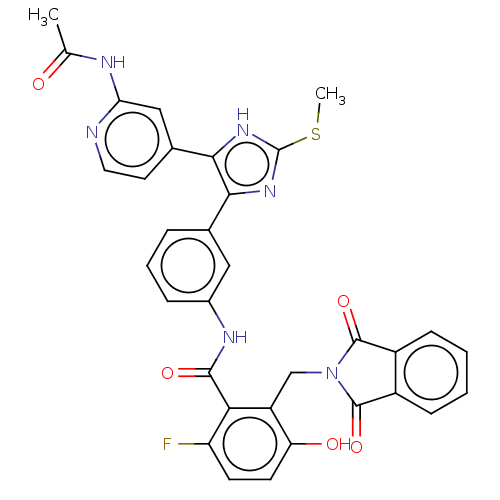
(CHEMBL5183286)Show SMILES CSc1nc(c([nH]1)-c1ccnc(NC(C)=O)c1)-c1cccc(NC(=O)c2c(F)ccc(O)c2CN2C(=O)c3ccccc3C2=O)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00848
BindingDB Entry DOI: 10.7270/Q2Q52TQZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase FRK
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human FRK using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50571527
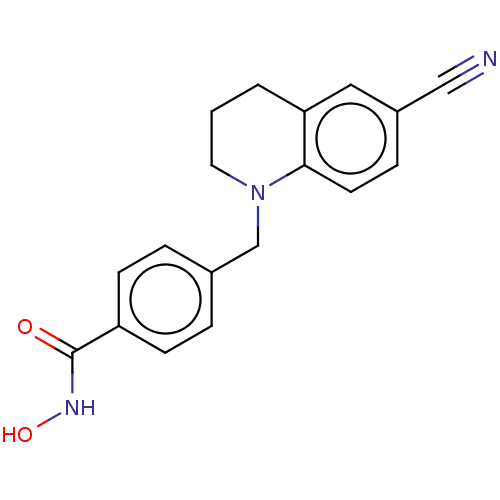
(CHEMBL4874875) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length recombinant human HDAC1 expressed in baculovirus infected Sf9 insect cells using RHKKAc fluorogenic peptide as substrate pr... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00848
BindingDB Entry DOI: 10.7270/Q2Q52TQZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50398716
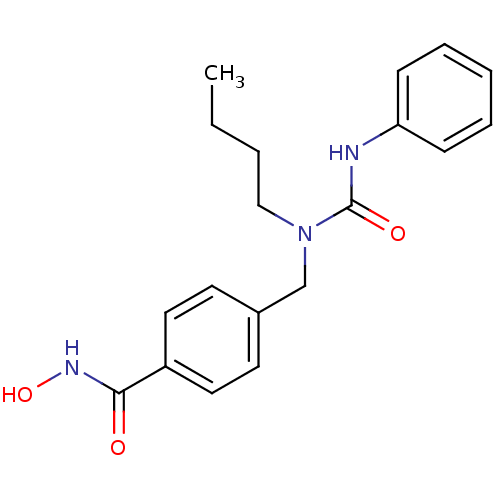
(CHEMBL2179618 | US10227295, Compound 5g | US940985...)Show InChI InChI=1S/C19H23N3O3/c1-2-3-13-22(19(24)20-17-7-5-4-6-8-17)14-15-9-11-16(12-10-15)18(23)21-25/h4-12,25H,2-3,13-14H2,1H3,(H,20,24)(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Oplophorus-luciferin 2-monooxygenase catalytic subunit
(Oplophorus gracilirostris (Luminous shrimp)) | BDBM223183
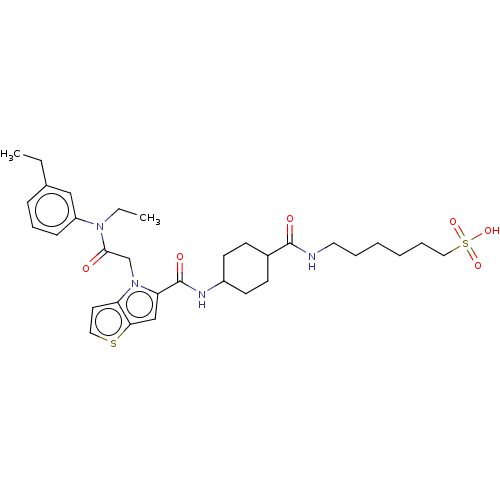
(Sodium 6-(trans-4-(1-(2-(ethyl(3-ethylphenyl)amino...)Show SMILES CCN(C(=O)Cn1c(cc2sccc12)C(=O)NC1CCC(CC1)C(=O)NCCCCCCS(O)(=O)=O)c1cccc(CC)c1 |(54.27,-31.84,;54.27,-30.3,;52.94,-29.53,;52.94,-27.99,;51.6,-27.22,;54.27,-27.22,;54.27,-25.68,;55.52,-24.77,;55.04,-23.31,;53.5,-23.31,;52.25,-22.4,;51.01,-23.31,;51.48,-24.77,;53.02,-24.77,;56.98,-25.25,;57.3,-26.75,;58.13,-24.22,;59.59,-24.69,;59.91,-26.2,;61.37,-26.68,;62.52,-25.65,;62.2,-24.14,;60.73,-23.66,;63.98,-26.12,;65.13,-25.09,;64.3,-27.63,;65.77,-28.1,;66.09,-29.61,;67.55,-30.09,;67.87,-31.59,;69.34,-32.07,;69.66,-33.57,;71.12,-34.05,;71.52,-32.56,;72.61,-34.45,;70.72,-35.54,;51.6,-30.3,;50.27,-29.53,;48.94,-30.3,;48.94,-31.84,;50.27,-32.61,;50.27,-34.15,;51.6,-34.92,;51.6,-31.84,)| Show InChI InChI=1S/C32H44N4O6S2/c1-3-23-10-9-11-26(20-23)35(4-2)30(37)22-36-27-16-18-43-29(27)21-28(36)32(39)34-25-14-12-24(13-15-25)31(38)33-17-7-5-6-8-19-44(40,41)42/h9-11,16,18,20-21,24-25H,3-8,12-15,17,19,22H2,1-2H3,(H,33,38)(H,34,39)(H,40,41,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Promega Biosciences LLC
| Assay Description
NanoLuc enzyme was diluted to 0.4 ng/mL in CO2 independent media + 10% FBS to make the detection reagent. A 3× dilution series of each inhibitor was ... |
ACS Chem Biol 12: 1028-1037 (2017)
Article DOI: 10.1021/acschembio.6b01129
BindingDB Entry DOI: 10.7270/Q2SF2V1S |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50513915
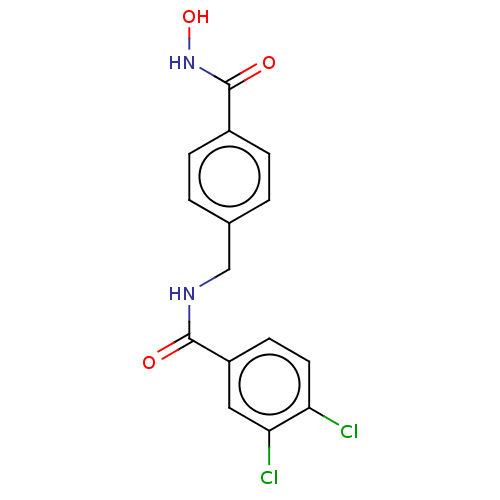
(CHEMBL4447715)Show InChI InChI=1S/C15H12Cl2N2O3/c16-12-6-5-11(7-13(12)17)14(20)18-8-9-1-3-10(4-2-9)15(21)19-22/h1-7,22H,8H2,(H,18,20)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 expressed in HEK293T/17 cells using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate ... |
J Med Chem 62: 8557-8577 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00946
BindingDB Entry DOI: 10.7270/Q2542RXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human HDAC6 (1 to 1215 residues) expressed in baculovirus infected sf9 insect cells using p53 (379 to... |
J Med Chem 62: 8557-8577 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00946
BindingDB Entry DOI: 10.7270/Q2542RXH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50602490
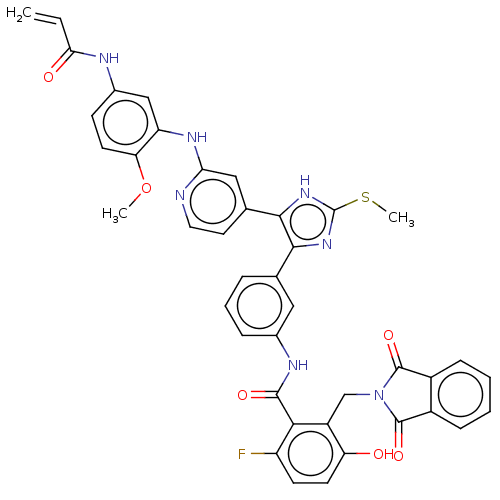
(CHEMBL5188399)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1cccc(NC(=O)c2c(F)ccc(O)c2CN2C(=O)c3ccccc3C2=O)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00848
BindingDB Entry DOI: 10.7270/Q2Q52TQZ |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human FGFR1 using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
| Assay Description
A commercial assay by Reaction Biology Corp. |
Cell Chem Biol 23: 1335-1340 (2016)
Article DOI: 10.1016/j.chembiol.2016.10.008
BindingDB Entry DOI: 10.7270/Q2WS8S22 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oplophorus-luciferin 2-monooxygenase catalytic subunit
(Oplophorus gracilirostris (Luminous shrimp)) | BDBM223182
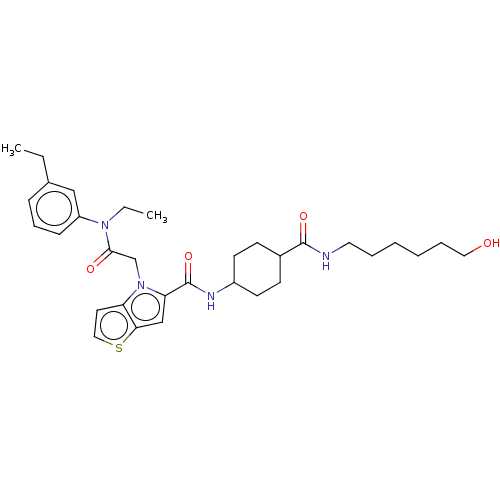
(1-(2-(Ethyl(3-ethylphenyl)amino)-2-oxoethyl)-N-(tr...)Show SMILES CCN(C(=O)Cn1c(cc2sccc12)C(=O)NC1CCC(CC1)C(=O)NCCCCCCO)c1cccc(CC)c1 |(54.27,-31.84,;54.27,-30.3,;52.94,-29.53,;52.94,-27.99,;51.6,-27.22,;54.27,-27.22,;54.27,-25.68,;55.52,-24.77,;55.04,-23.31,;53.5,-23.31,;52.25,-22.4,;51.01,-23.31,;51.48,-24.77,;53.02,-24.77,;56.98,-25.25,;57.3,-26.75,;58.13,-24.22,;59.59,-24.69,;59.91,-26.2,;61.37,-26.68,;62.52,-25.65,;62.2,-24.14,;60.73,-23.66,;63.98,-26.12,;65.13,-25.09,;64.3,-27.63,;65.77,-28.1,;66.09,-29.61,;67.55,-30.09,;67.87,-31.59,;69.34,-32.07,;69.66,-33.57,;71.12,-34.05,;51.6,-30.3,;50.27,-29.53,;48.94,-30.3,;48.94,-31.84,;50.27,-32.61,;50.27,-34.15,;51.6,-34.92,;51.6,-31.84,)| Show InChI InChI=1S/C32H44N4O4S/c1-3-23-10-9-11-26(20-23)35(4-2)30(38)22-36-27-16-19-41-29(27)21-28(36)32(40)34-25-14-12-24(13-15-25)31(39)33-17-7-5-6-8-18-37/h9-11,16,19-21,24-25,37H,3-8,12-15,17-18,22H2,1-2H3,(H,33,39)(H,34,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Promega Biosciences LLC
| Assay Description
NanoLuc enzyme was diluted to 0.4 ng/mL in CO2 independent media + 10% FBS to make the detection reagent. A 3× dilution series of each inhibitor was ... |
ACS Chem Biol 12: 1028-1037 (2017)
Article DOI: 10.1021/acschembio.6b01129
BindingDB Entry DOI: 10.7270/Q2SF2V1S |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM417049

(4-((6-chloro-3,4-dihydroquinolin-1(2H)-yl)methyl)-...)Show InChI InChI=1S/C17H17ClN2O2/c18-15-7-8-16-14(10-15)2-1-9-20(16)11-12-3-5-13(6-4-12)17(21)19-22/h3-8,10,22H,1-2,9,11H2,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human HDAC6 (1 to 1215 residues) expressed in baculovirus infected sf9 insect cells using p53 (379 to... |
J Med Chem 62: 8557-8577 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00946
BindingDB Entry DOI: 10.7270/Q2542RXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50571521
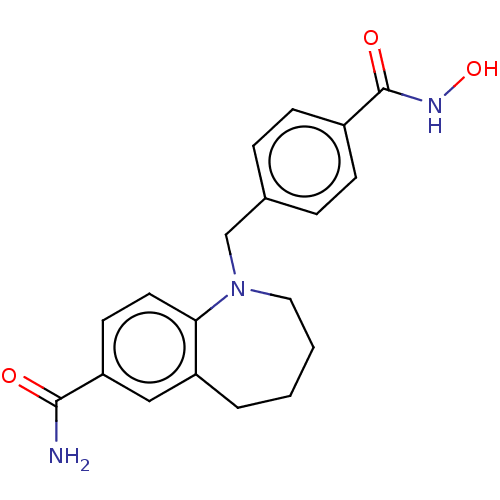
(CHEMBL4847663) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM110036
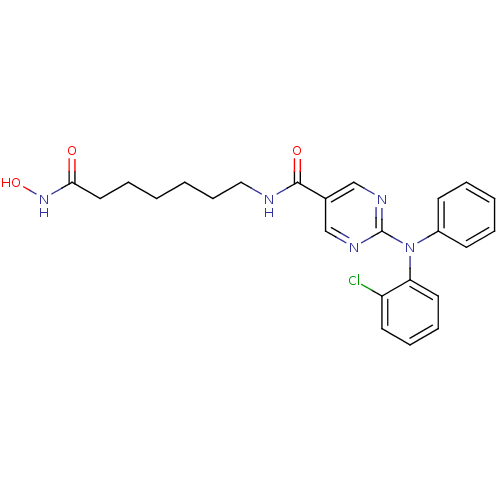
(US8609678, 2-((2-chlorophenyl)(phenyl)amino)-N-(7-...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1Cl Show InChI InChI=1S/C24H26ClN5O3/c25-20-12-7-8-13-21(20)30(19-10-4-3-5-11-19)24-27-16-18(17-28-24)23(32)26-15-9-2-1-6-14-22(31)29-33/h3-5,7-8,10-13,16-17,33H,1-2,6,9,14-15H2,(H,26,32)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length N-terminal GST-tagged HDAC6 expressed in baculovirus infected Sf9 insect cells assessed as decrease in re... |
J Med Chem 62: 8557-8577 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00946
BindingDB Entry DOI: 10.7270/Q2542RXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50571524
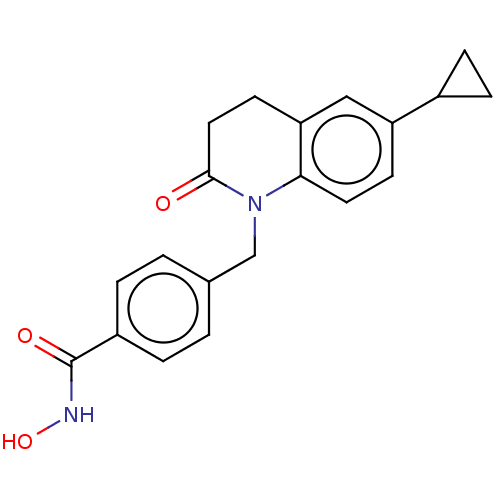
(CHEMBL4847122)Show SMILES ONC(=O)c1ccc(CN2C(=O)CCc3cc(ccc23)C2CC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50571522

(CHEMBL4859648) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM417049

(4-((6-chloro-3,4-dihydroquinolin-1(2H)-yl)methyl)-...)Show InChI InChI=1S/C17H17ClN2O2/c18-15-7-8-16-14(10-15)2-1-9-20(16)11-12-3-5-13(6-4-12)17(21)19-22/h3-8,10,22H,1-2,9,11H2,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length recombinant human HDAC1 expressed in baculovirus infected Sf9 insect cells using RHKKAc fluorogenic peptide as substrate pr... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50261816
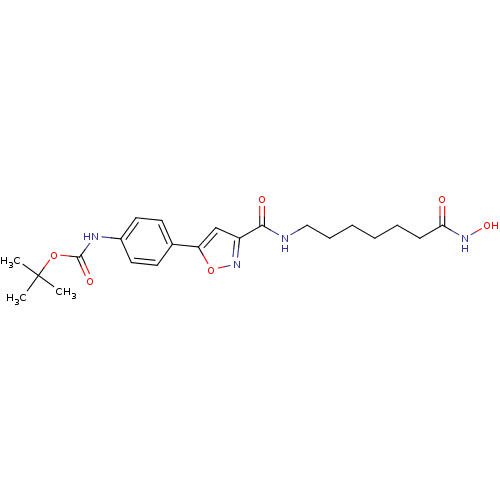
(CHEMBL511749 | tert-butyl 4-(3-((7-(hydroxyamino)-...)Show SMILES CC(C)(C)OC(=O)Nc1ccc(cc1)-c1cc(no1)C(=O)NCCCCCCC(=O)NO Show InChI InChI=1S/C22H30N4O6/c1-22(2,3)31-21(29)24-16-11-9-15(10-12-16)18-14-17(26-32-18)20(28)23-13-7-5-4-6-8-19(27)25-30/h9-12,14,30H,4-8,13H2,1-3H3,(H,23,28)(H,24,29)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length C-terminal His/FLAG tagged human HDAC1 (1 to 482 residues) expressed in baculovirus infected sf9 insect cells u... |
J Med Chem 62: 8557-8577 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00946
BindingDB Entry DOI: 10.7270/Q2542RXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM417070
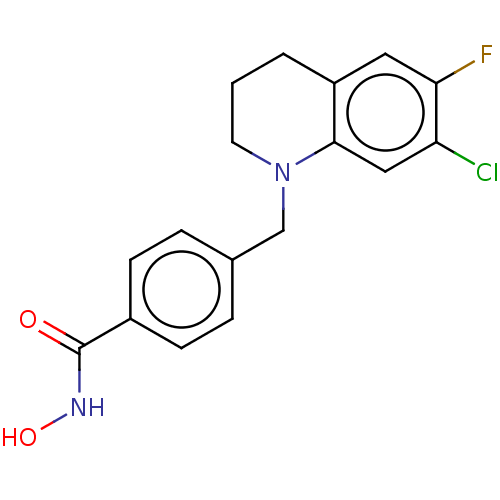
(US10456394, Example 7)Show InChI InChI=1S/C17H16ClFN2O2/c18-14-9-16-13(8-15(14)19)2-1-7-21(16)10-11-3-5-12(6-4-11)17(22)20-23/h3-6,8-9,23H,1-2,7,10H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length recombinant human HDAC1 expressed in baculovirus infected Sf9 insect cells using RHKKAc fluorogenic peptide as substrate pr... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3 [781-1124]
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
| Assay Description
In this ELISA-based kinase activity assay, a 96-well assay plate is coated with an artificial polypeptide serving as kinase substrate that contains t... |
Cell Chem Biol 23: 1335-1340 (2016)
Article DOI: 10.1016/j.chembiol.2016.10.008
BindingDB Entry DOI: 10.7270/Q2WS8S22 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM417068
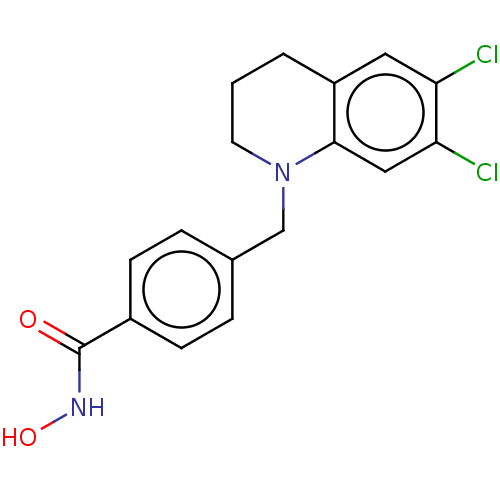
(US10456394, Example 6)Show InChI InChI=1S/C17H16Cl2N2O2/c18-14-8-13-2-1-7-21(16(13)9-15(14)19)10-11-3-5-12(6-4-11)17(22)20-23/h3-6,8-9,23H,1-2,7,10H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length recombinant human HDAC1 expressed in baculovirus infected Sf9 insect cells using RHKKAc fluorogenic peptide as substrate pr... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to FLT1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Oplophorus-luciferin 2-monooxygenase catalytic subunit
(Oplophorus gracilirostris (Luminous shrimp)) | BDBM223160
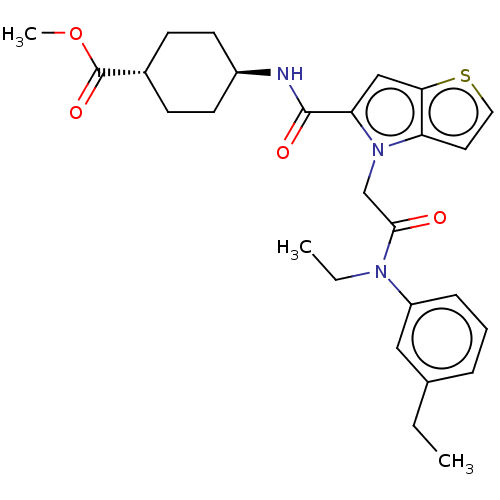
(Methyl trans-4-(4-(2-(ethyl(3-ethylphenyl)amino)-2...)Show SMILES CCN(C(=O)Cn1c(cc2sccc12)C(=O)N[C@H]1CC[C@@H](CC1)C(=O)OC)c1cccc(CC)c1 |r,wU:17.18,wD:20.25,(16.5,-21.06,;16.5,-19.52,;15.17,-18.75,;15.17,-17.21,;13.84,-16.44,;16.5,-16.44,;16.5,-14.9,;17.75,-14,;17.27,-12.53,;15.73,-12.53,;14.49,-11.63,;13.24,-12.53,;13.72,-14,;15.26,-14,;19.21,-14.48,;19.53,-15.98,;20.36,-13.45,;21.82,-13.92,;22.97,-12.89,;24.43,-13.37,;24.75,-14.87,;23.61,-15.9,;22.14,-15.43,;26.22,-15.35,;27.36,-14.32,;26.54,-16.86,;28,-17.33,;13.84,-19.52,;13.84,-21.06,;12.5,-21.83,;11.17,-21.06,;11.17,-19.52,;9.83,-18.75,;8.5,-19.52,;12.5,-18.75,)| Show InChI InChI=1S/C27H33N3O4S/c1-4-18-7-6-8-21(15-18)29(5-2)25(31)17-30-22-13-14-35-24(22)16-23(30)26(32)28-20-11-9-19(10-12-20)27(33)34-3/h6-8,13-16,19-20H,4-5,9-12,17H2,1-3H3,(H,28,32)/t19-,20- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Promega Biosciences LLC
| Assay Description
NanoLuc enzyme was diluted to 0.4 ng/mL in CO2 independent media + 10% FBS to make the detection reagent. A 3× dilution series of each inhibitor was ... |
ACS Chem Biol 12: 1028-1037 (2017)
Article DOI: 10.1021/acschembio.6b01129
BindingDB Entry DOI: 10.7270/Q2SF2V1S |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50592040
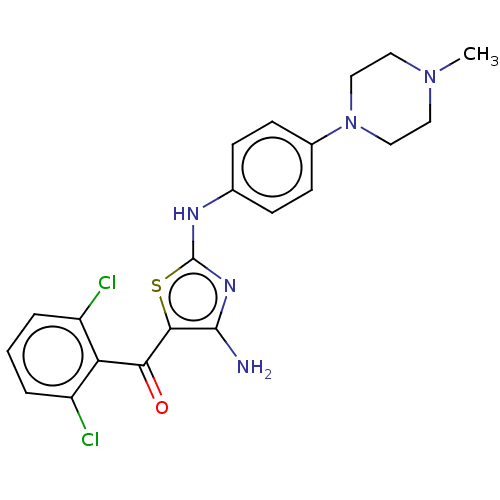
(CHEMBL1989043)Show SMILES CN1CCN(CC1)c1ccc(Nc2nc(N)c(s2)C(=O)c2c(Cl)cccc2Cl)cc1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114433
BindingDB Entry DOI: 10.7270/Q2BV7MMH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50398716

(CHEMBL2179618 | US10227295, Compound 5g | US940985...)Show InChI InChI=1S/C19H23N3O3/c1-2-3-13-22(19(24)20-17-7-5-4-6-8-17)14-15-9-11-16(12-10-15)18(23)21-25/h4-12,25H,2-3,13-14H2,1H3,(H,20,24)(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 expressed in HEK293T/17 cells using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate ... |
J Med Chem 62: 8557-8577 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00946
BindingDB Entry DOI: 10.7270/Q2542RXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50571520
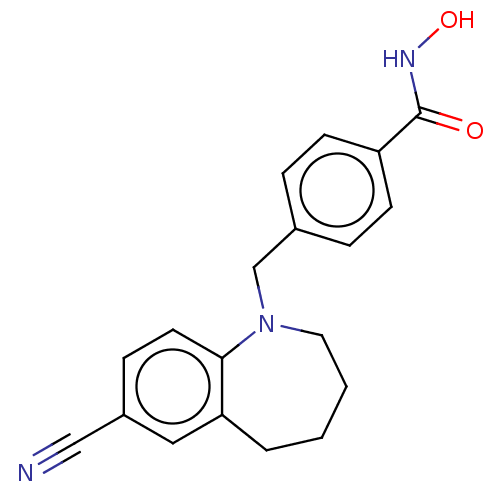
(CHEMBL4856920) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50439674
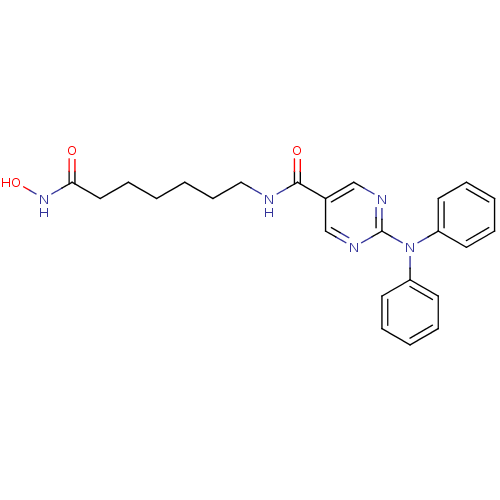
(RICOLINOSTAT | US10858323, Compound 2 | US11207431...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H27N5O3/c30-22(28-32)15-9-1-2-10-16-25-23(31)19-17-26-24(27-18-19)29(20-11-5-3-6-12-20)21-13-7-4-8-14-21/h3-8,11-14,17-18,32H,1-2,9-10,15-16H2,(H,25,31)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length N-terminal GST-tagged HDAC6 expressed in baculovirus infected Sf9 insect cells assessed as decrease in re... |
J Med Chem 62: 8557-8577 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00946
BindingDB Entry DOI: 10.7270/Q2542RXH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data