 Found 50 hits with Last Name = 'scott' and Initial = 'md'
Found 50 hits with Last Name = 'scott' and Initial = 'md' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375165
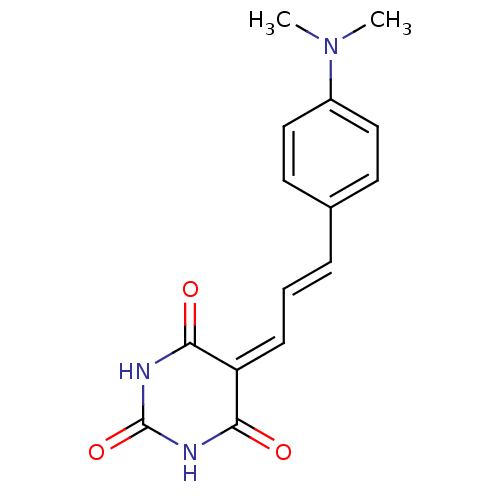
(CHEMBL271696)Show SMILES [#6]-[#7](-[#6])-c1ccc(\[#6]=[#6]\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-2=O)cc1 Show InChI InChI=1S/C15H15N3O3/c1-18(2)11-8-6-10(7-9-11)4-3-5-12-13(19)16-15(21)17-14(12)20/h3-9H,1-2H3,(H2,16,17,19,20,21)/b4-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375162
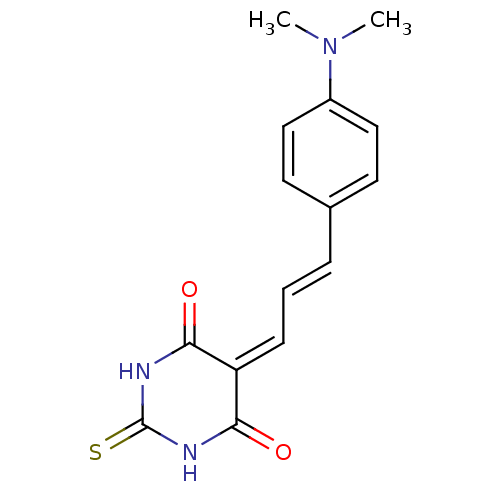
(CHEMBL257930)Show SMILES [#6]-[#7](-[#6])-c1ccc(\[#6]=[#6]\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=S)-[#7]-[#6]-2=O)cc1 Show InChI InChI=1S/C15H15N3O2S/c1-18(2)11-8-6-10(7-9-11)4-3-5-12-13(19)16-15(21)17-14(12)20/h3-9H,1-2H3,(H2,16,17,19,20,21)/b4-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375171
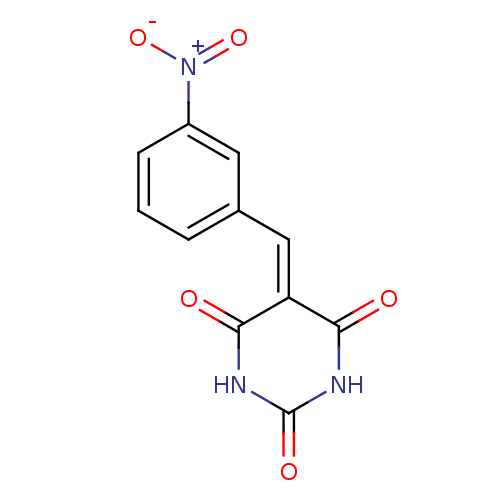
(CHEMBL255586)Show SMILES [#8-]-[#7+](=O)-c1cccc(\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-2=O)c1 Show InChI InChI=1S/C11H7N3O5/c15-9-8(10(16)13-11(17)12-9)5-6-2-1-3-7(4-6)14(18)19/h1-5H,(H2,12,13,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375169
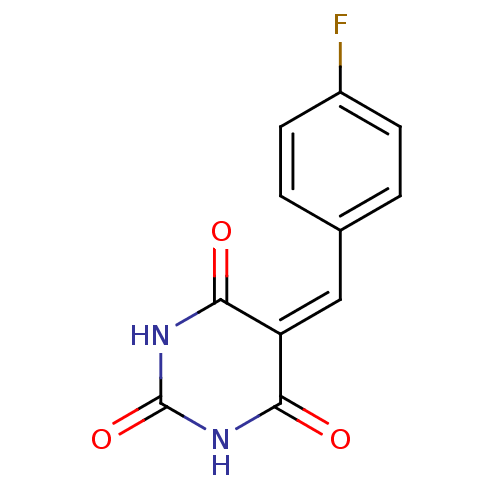
(CHEMBL270813)Show SMILES Fc1ccc(\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-2=O)cc1 Show InChI InChI=1S/C11H7FN2O3/c12-7-3-1-6(2-4-7)5-8-9(15)13-11(17)14-10(8)16/h1-5H,(H2,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375167
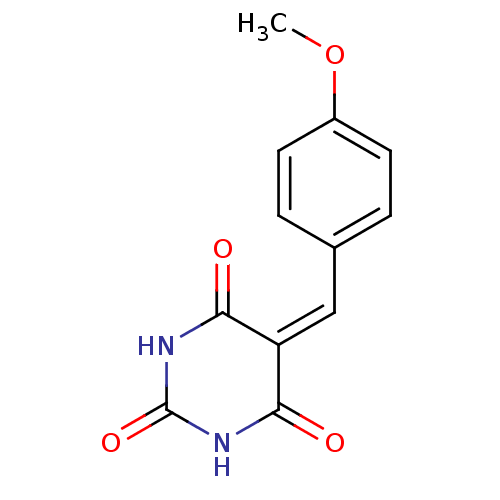
(CHEMBL270356)Show SMILES [#6]-[#8]-c1ccc(\[#6]=[#6]-2/[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-2=O)cc1 Show InChI InChI=1S/C12H10N2O4/c1-18-8-4-2-7(3-5-8)6-9-10(15)13-12(17)14-11(9)16/h2-6H,1H3,(H2,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375170

(CHEMBL255143)Show SMILES [#8]-[#6](=O)-c1ccc(\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-2=O)cc1 Show InChI InChI=1S/C12H8N2O5/c15-9-8(10(16)14-12(19)13-9)5-6-1-3-7(4-2-6)11(17)18/h1-5H,(H,17,18)(H2,13,14,15,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375182
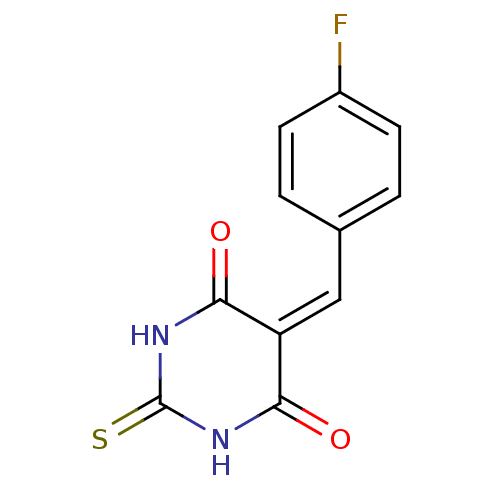
(CHEMBL270596)Show SMILES Fc1ccc(\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=S)-[#7]-[#6]-2=O)cc1 Show InChI InChI=1S/C11H7FN2O2S/c12-7-3-1-6(2-4-7)5-8-9(15)13-11(17)14-10(8)16/h1-5H,(H2,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375172
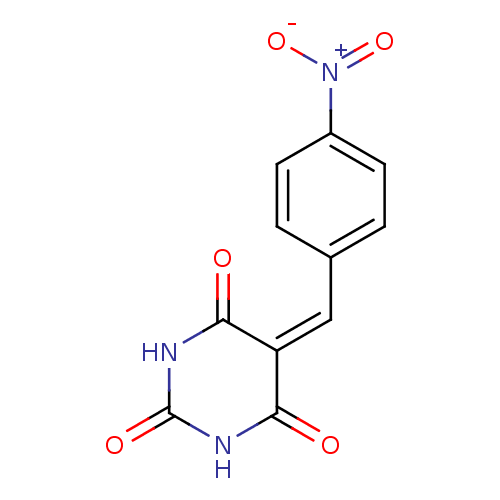
(CHEMBL404480)Show SMILES [#8-]-[#7+](=O)-c1ccc(\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-2=O)cc1 Show InChI InChI=1S/C11H7N3O5/c15-9-8(10(16)13-11(17)12-9)5-6-1-3-7(4-2-6)14(18)19/h1-5H,(H2,12,13,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375166
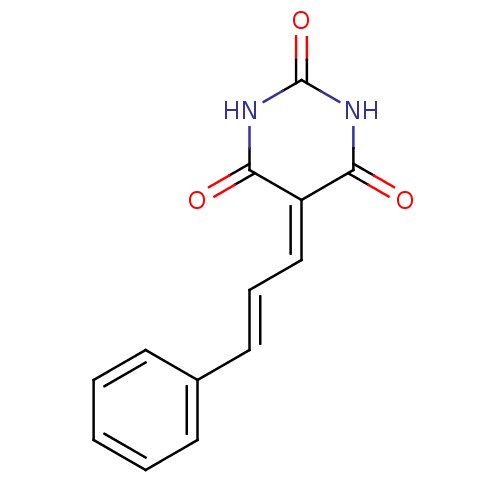
(CHEMBL270142)Show SMILES O=[#6]-1-[#7]-[#6](=O)\[#6](=[#6]\[#6]=[#6]\c2ccccc2)-[#6](=O)-[#7]-1 Show InChI InChI=1S/C13H10N2O3/c16-11-10(12(17)15-13(18)14-11)8-4-7-9-5-2-1-3-6-9/h1-8H,(H2,14,15,16,17,18)/b7-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375168
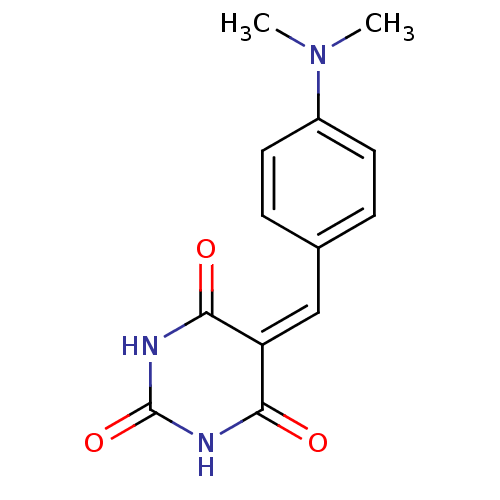
(CHEMBL258080)Show SMILES [#6]-[#7](-[#6])-c1ccc(\[#6]=[#6]-2/[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-2=O)cc1 Show InChI InChI=1S/C13H13N3O3/c1-16(2)9-5-3-8(4-6-9)7-10-11(17)14-13(19)15-12(10)18/h3-7H,1-2H3,(H2,14,15,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50237549
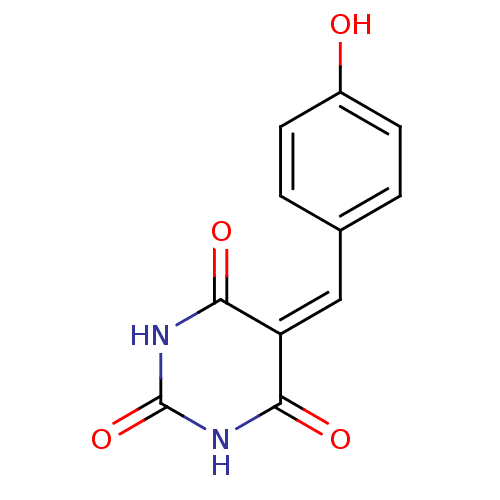
(5-(4-hydroxybenzylidene)pyrimidine-2,4,6(1H,3H,5H)...)Show SMILES [#8]-c1ccc(\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-2=O)cc1 Show InChI InChI=1S/C11H8N2O4/c14-7-3-1-6(2-4-7)5-8-9(15)12-11(17)13-10(8)16/h1-5,14H,(H2,12,13,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375163
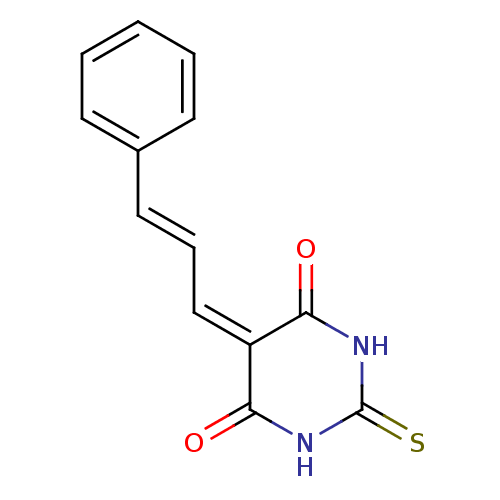
(CHEMBL402646)Show SMILES O=[#6]1-[#7]-[#6](=S)-[#7]-[#6](=O)\[#6]-1=[#6]/[#6]=[#6]/c1ccccc1 Show InChI InChI=1S/C13H10N2O2S/c16-11-10(12(17)15-13(18)14-11)8-4-7-9-5-2-1-3-6-9/h1-8H,(H2,14,15,16,17,18)/b7-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50237564
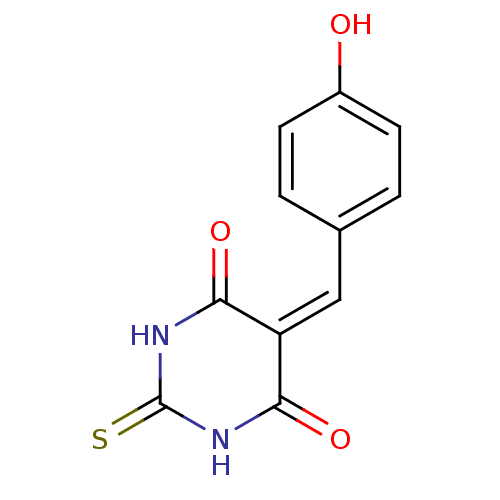
(5-(4-Hydroxybenzylidene)-2-thioxodihydropyrimidine...)Show SMILES [#8]-c1ccc(\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=S)-[#7]-[#6]-2=O)cc1 Show InChI InChI=1S/C11H8N2O3S/c14-7-3-1-6(2-4-7)5-8-9(15)12-11(17)13-10(8)16/h1-5,14H,(H2,12,13,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375188
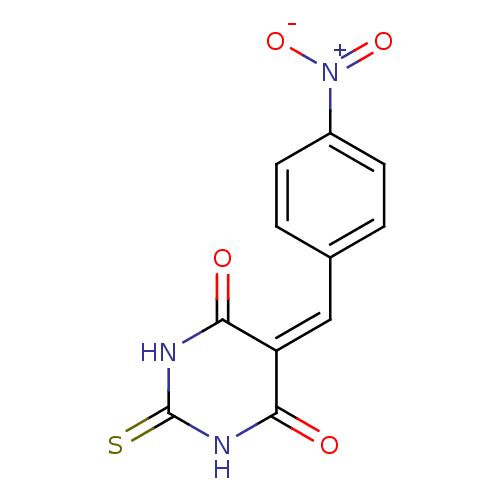
(CHEMBL408042)Show SMILES [#8-]-[#7+](=O)-c1ccc(\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=S)-[#7]-[#6]-2=O)cc1 Show InChI InChI=1S/C11H7N3O4S/c15-9-8(10(16)13-11(19)12-9)5-6-1-3-7(4-2-6)14(17)18/h1-5H,(H2,12,13,15,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375187
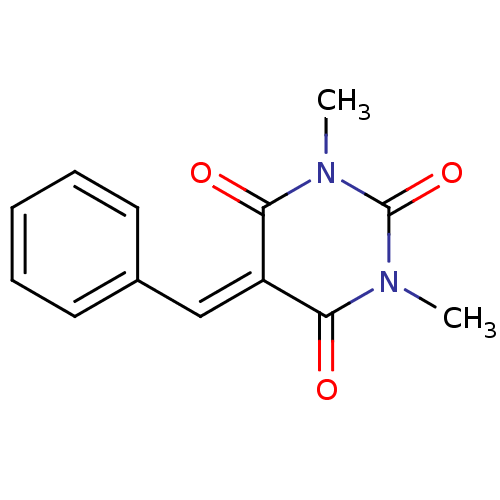
(CHEMBL403400)Show SMILES [#6]-[#7]-1-[#6](=O)-[#7](-[#6])-[#6](=O)\[#6](=[#6]/c2ccccc2)-[#6]-1=O Show InChI InChI=1S/C13H12N2O3/c1-14-11(16)10(12(17)15(2)13(14)18)8-9-6-4-3-5-7-9/h3-8H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375186
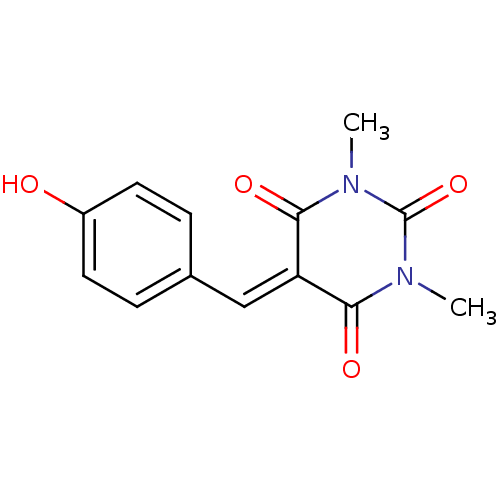
(CHEMBL273177)Show SMILES [#6]-[#7]-1-[#6](=O)-[#7](-[#6])-[#6](=O)\[#6](=[#6]/c2ccc(-[#8])cc2)-[#6]-1=O Show InChI InChI=1S/C13H12N2O4/c1-14-11(17)10(12(18)15(2)13(14)19)7-8-3-5-9(16)6-4-8/h3-7,16H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375185
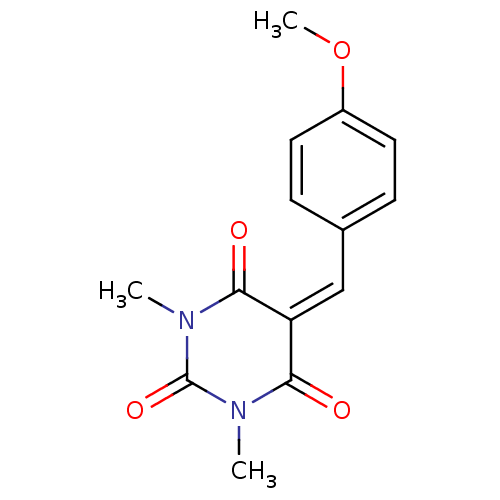
(CHEMBL256878)Show SMILES [#6]-[#8]-c1ccc(\[#6]=[#6]-2\[#6](=O)-[#7](-[#6])-[#6](=O)-[#7](-[#6])-[#6]-2=O)cc1 Show InChI InChI=1S/C14H14N2O4/c1-15-12(17)11(13(18)16(2)14(15)19)8-9-4-6-10(20-3)7-5-9/h4-8H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375184
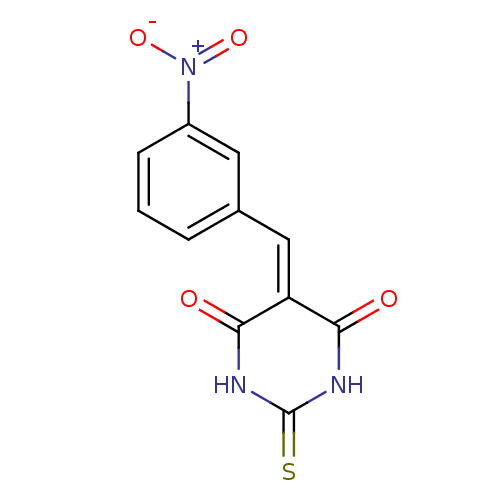
(CHEMBL408043)Show SMILES [#8-]-[#7+](=O)-c1cccc(\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=S)-[#7]-[#6]-2=O)c1 Show InChI InChI=1S/C11H7N3O4S/c15-9-8(10(16)13-11(19)12-9)5-6-2-1-3-7(4-6)14(17)18/h1-5H,(H2,12,13,15,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375183
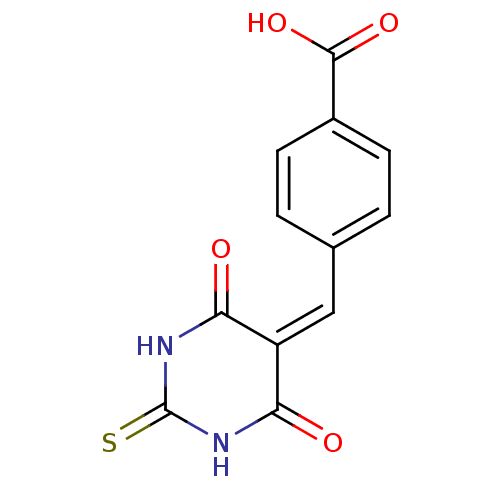
(CHEMBL439416)Show SMILES [#8]-[#6](=O)-c1ccc(\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=S)-[#7]-[#6]-2=O)cc1 Show InChI InChI=1S/C12H8N2O4S/c15-9-8(10(16)14-12(19)13-9)5-6-1-3-7(4-2-6)11(17)18/h1-5H,(H,17,18)(H2,13,14,15,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375181
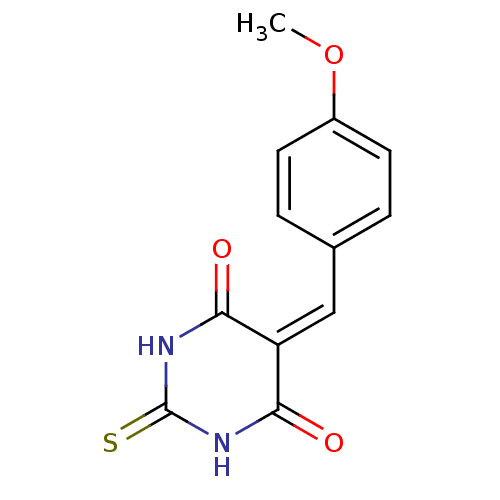
(CHEMBL271859)Show SMILES [#6]-[#8]-c1ccc(\[#6]=[#6]-2/[#6](=O)-[#7]-[#6](=S)-[#7]-[#6]-2=O)cc1 Show InChI InChI=1S/C12H10N2O3S/c1-17-8-4-2-7(3-5-8)6-9-10(15)13-12(18)14-11(9)16/h2-6H,1H3,(H2,13,14,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375180
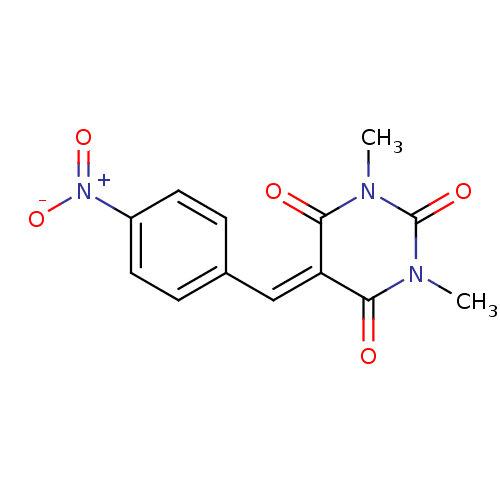
(CHEMBL256269)Show SMILES [#6]-[#7]-1-[#6](=O)-[#7](-[#6])-[#6](=O)\[#6](=[#6]/c2ccc(cc2)-[#7+](-[#8-])=O)-[#6]-1=O Show InChI InChI=1S/C13H11N3O5/c1-14-11(17)10(12(18)15(2)13(14)19)7-8-3-5-9(6-4-8)16(20)21/h3-7H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375179
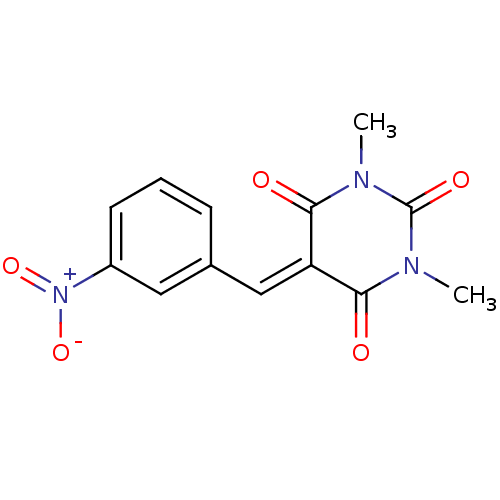
(CHEMBL257927)Show SMILES [#6]-[#7]-1-[#6](=O)-[#7](-[#6])-[#6](=O)\[#6](=[#6]/c2cccc(c2)-[#7+](-[#8-])=O)-[#6]-1=O Show InChI InChI=1S/C13H11N3O5/c1-14-11(17)10(12(18)15(2)13(14)19)7-8-4-3-5-9(6-8)16(20)21/h3-7H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375178
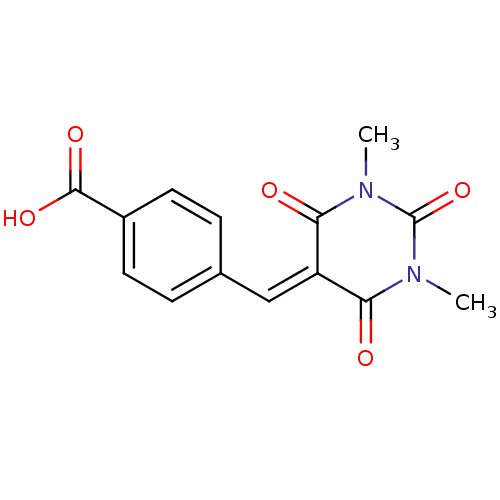
(CHEMBL404693)Show SMILES [#6]-[#7]-1-[#6](=O)-[#7](-[#6])-[#6](=O)\[#6](=[#6]/c2ccc(cc2)-[#6](-[#8])=O)-[#6]-1=O Show InChI InChI=1S/C14H12N2O5/c1-15-11(17)10(12(18)16(2)14(15)21)7-8-3-5-9(6-4-8)13(19)20/h3-7H,1-2H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375177
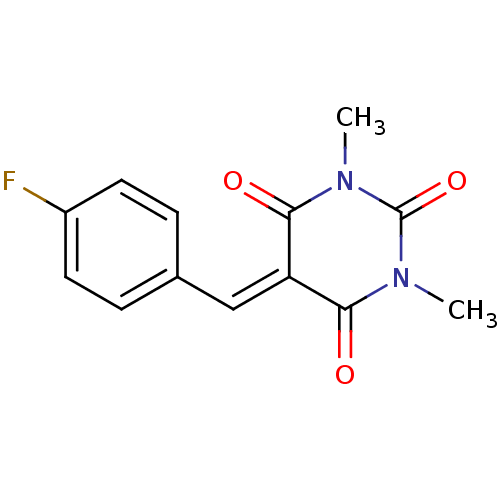
(CHEMBL403366)Show SMILES [#6]-[#7]-1-[#6](=O)-[#7](-[#6])-[#6](=O)\[#6](=[#6]/c2ccc(F)cc2)-[#6]-1=O Show InChI InChI=1S/C13H11FN2O3/c1-15-11(17)10(12(18)16(2)13(15)19)7-8-3-5-9(14)6-4-8/h3-7H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375176
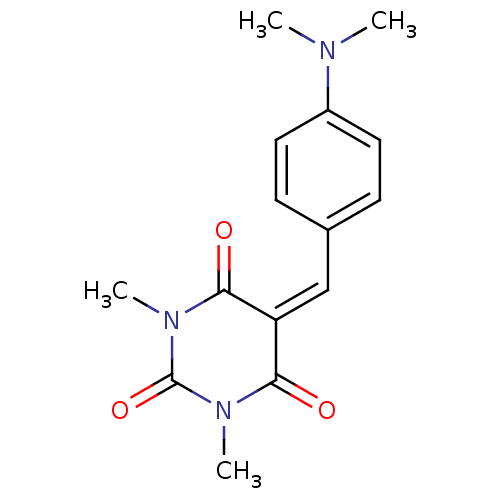
(CHEMBL404233)Show SMILES [#6]-[#7](-[#6])-c1ccc(\[#6]=[#6]-2\[#6](=O)-[#7](-[#6])-[#6](=O)-[#7](-[#6])-[#6]-2=O)cc1 Show InChI InChI=1S/C15H17N3O3/c1-16(2)11-7-5-10(6-8-11)9-12-13(19)17(3)15(21)18(4)14(12)20/h5-9H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375175
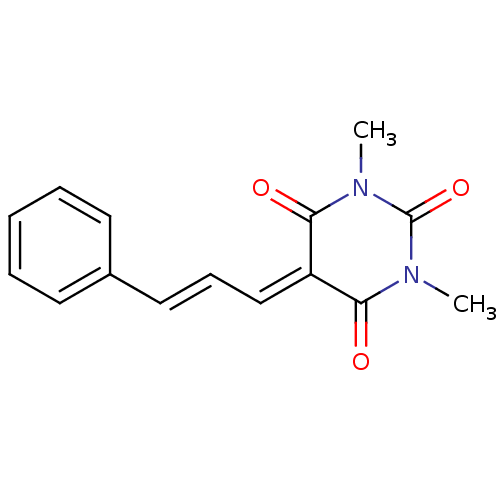
(CHEMBL256879)Show SMILES [#6]-[#7]-1-[#6](=O)-[#7](-[#6])-[#6](=O)\[#6](=[#6]/[#6]=[#6]/c2ccccc2)-[#6]-1=O Show InChI InChI=1S/C15H14N2O3/c1-16-13(18)12(14(19)17(2)15(16)20)10-6-9-11-7-4-3-5-8-11/h3-10H,1-2H3/b9-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375174
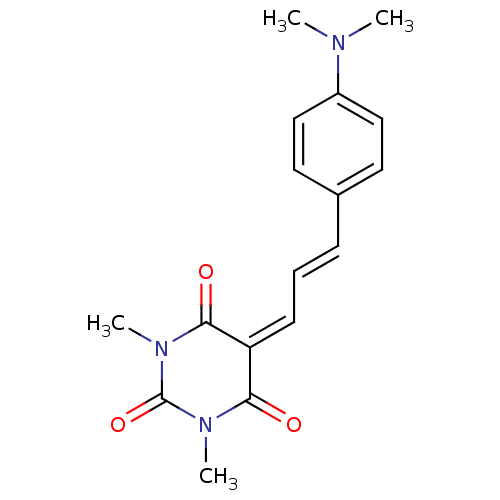
(CHEMBL258079)Show SMILES [#6]-[#7](-[#6])-c1ccc(\[#6]=[#6]\[#6]=[#6]-2\[#6](=O)-[#7](-[#6])-[#6](=O)-[#7](-[#6])-[#6]-2=O)cc1 Show InChI InChI=1S/C17H19N3O3/c1-18(2)13-10-8-12(9-11-13)6-5-7-14-15(21)19(3)17(23)20(4)16(14)22/h5-11H,1-4H3/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375173
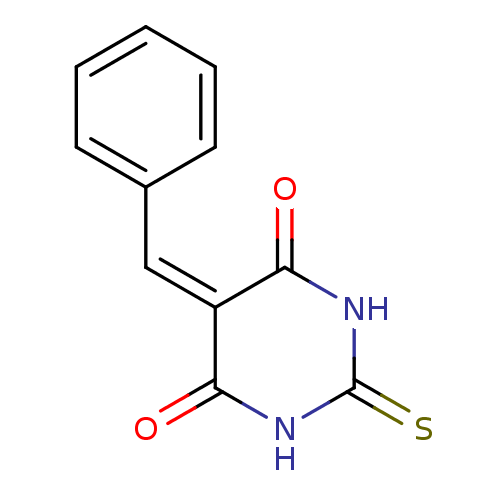
(CHEMBL256700)Show SMILES O=[#6]1-[#7]-[#6](=S)-[#7]-[#6](=O)\[#6]-1=[#6]\c1ccccc1 Show InChI InChI=1S/C11H8N2O2S/c14-9-8(10(15)13-11(16)12-9)6-7-4-2-1-3-5-7/h1-6H,(H2,12,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375164
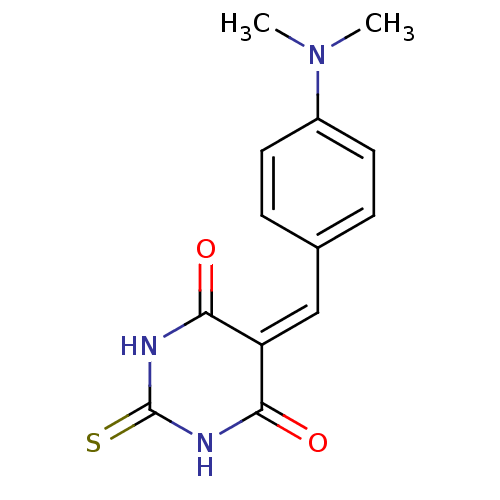
(CHEMBL269821)Show SMILES [#6]-[#7](-[#6])-c1ccc(\[#6]=[#6]-2/[#6](=O)-[#7]-[#6](=S)-[#7]-[#6]-2=O)cc1 Show InChI InChI=1S/C13H13N3O2S/c1-16(2)9-5-3-8(4-6-9)7-10-11(17)14-13(19)15-12(10)18/h3-7H,1-2H3,(H2,14,15,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50375161
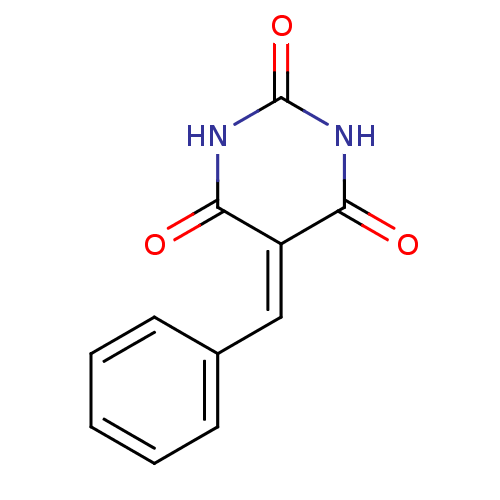
(CHEMBL272757)Show SMILES O=[#6]-1-[#7]-[#6](=O)\[#6](=[#6]/c2ccccc2)-[#6](=O)-[#7]-1 Show InChI InChI=1S/C11H8N2O3/c14-9-8(10(15)13-11(16)12-9)6-7-4-2-1-3-5-7/h1-6H,(H2,12,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North Dakota State University
Curated by ChEMBL
| Assay Description
Inhibition of human methionine aminopeptidase 1 |
Bioorg Med Chem Lett 18: 2373-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.066
BindingDB Entry DOI: 10.7270/Q2348M7J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 263 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US8895245 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7GBK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 263 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
S-Adenosyl-L-homocysteine (SAH) was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive c... |
US Patent US9333217 (2016)
BindingDB Entry DOI: 10.7270/Q2XP73TJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 283 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
S-Adenosyl-L-homocysteine (SAH) was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive c... |
US Patent US9333217 (2016)
BindingDB Entry DOI: 10.7270/Q2XP73TJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 283 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US8895245 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7GBK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US8895245 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7GBK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
S-Adenosyl-L-homocysteine (SAH) was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive c... |
US Patent US9333217 (2016)
BindingDB Entry DOI: 10.7270/Q2XP73TJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 467 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US8895245 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7GBK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 467 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
S-Adenosyl-L-homocysteine (SAH) was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive c... |
US Patent US9333217 (2016)
BindingDB Entry DOI: 10.7270/Q2XP73TJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM227458
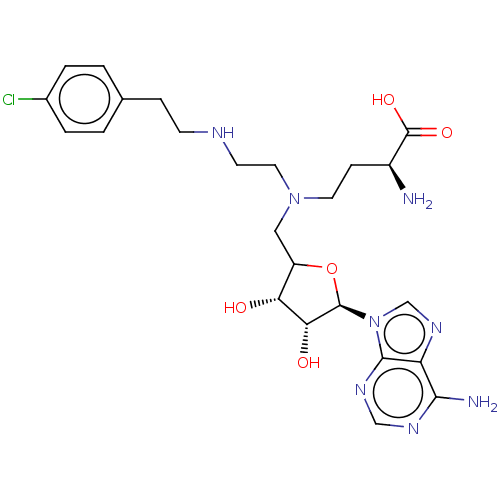
(US9333217, 75)Show SMILES N[C@@H](CCN(CCNCCc1ccc(Cl)cc1)CC1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H33ClN8O5/c25-15-3-1-14(2-4-15)5-7-28-8-10-32(9-6-16(26)24(36)37)11-17-19(34)20(35)23(38-17)33-13-31-18-21(27)29-12-30-22(18)33/h1-4,12-13,16-17,19-20,23,28,34-35H,5-11,26H2,(H,36,37)(H2,27,29,30)/t16-,17?,19+,20+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US9333217 (2016)
BindingDB Entry DOI: 10.7270/Q2XP73TJ |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM139690
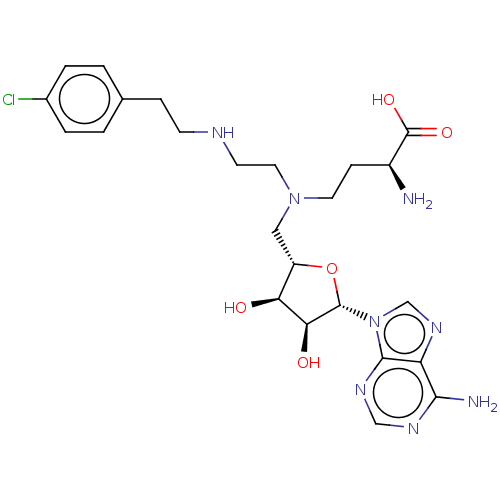
(US8895245, 75)Show SMILES N[C@@H](CCN(CCNCCc1ccc(Cl)cc1)C[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H33ClN8O5/c25-15-3-1-14(2-4-15)5-7-28-8-10-32(9-6-16(26)24(36)37)11-17-19(34)20(35)23(38-17)33-13-31-18-21(27)29-12-30-22(18)33/h1-4,12-13,16-17,19-20,23,28,34-35H,5-11,26H2,(H,36,37)(H2,27,29,30)/t16-,17-,19-,20-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US8895245 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7GBK |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM139690

(US8895245, 75)Show SMILES N[C@@H](CCN(CCNCCc1ccc(Cl)cc1)C[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H33ClN8O5/c25-15-3-1-14(2-4-15)5-7-28-8-10-32(9-6-16(26)24(36)37)11-17-19(34)20(35)23(38-17)33-13-31-18-21(27)29-12-30-22(18)33/h1-4,12-13,16-17,19-20,23,28,34-35H,5-11,26H2,(H,36,37)(H2,27,29,30)/t16-,17-,19-,20-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US8895245 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7GBK |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM227458

(US9333217, 75)Show SMILES N[C@@H](CCN(CCNCCc1ccc(Cl)cc1)CC1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H33ClN8O5/c25-15-3-1-14(2-4-15)5-7-28-8-10-32(9-6-16(26)24(36)37)11-17-19(34)20(35)23(38-17)33-13-31-18-21(27)29-12-30-22(18)33/h1-4,12-13,16-17,19-20,23,28,34-35H,5-11,26H2,(H,36,37)(H2,27,29,30)/t16-,17?,19+,20+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US9333217 (2016)
BindingDB Entry DOI: 10.7270/Q2XP73TJ |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
S-Adenosyl-L-homocysteine (SAH) was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive c... |
US Patent US9333217 (2016)
BindingDB Entry DOI: 10.7270/Q2XP73TJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US8895245 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7GBK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM139690

(US8895245, 75)Show SMILES N[C@@H](CCN(CCNCCc1ccc(Cl)cc1)C[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H33ClN8O5/c25-15-3-1-14(2-4-15)5-7-28-8-10-32(9-6-16(26)24(36)37)11-17-19(34)20(35)23(38-17)33-13-31-18-21(27)29-12-30-22(18)33/h1-4,12-13,16-17,19-20,23,28,34-35H,5-11,26H2,(H,36,37)(H2,27,29,30)/t16-,17-,19-,20-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.18E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US8895245 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7GBK |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM227458

(US9333217, 75)Show SMILES N[C@@H](CCN(CCNCCc1ccc(Cl)cc1)CC1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H33ClN8O5/c25-15-3-1-14(2-4-15)5-7-28-8-10-32(9-6-16(26)24(36)37)11-17-19(34)20(35)23(38-17)33-13-31-18-21(27)29-12-30-22(18)33/h1-4,12-13,16-17,19-20,23,28,34-35H,5-11,26H2,(H,36,37)(H2,27,29,30)/t16-,17?,19+,20+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.18E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US9333217 (2016)
BindingDB Entry DOI: 10.7270/Q2XP73TJ |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM139690

(US8895245, 75)Show SMILES N[C@@H](CCN(CCNCCc1ccc(Cl)cc1)C[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H33ClN8O5/c25-15-3-1-14(2-4-15)5-7-28-8-10-32(9-6-16(26)24(36)37)11-17-19(34)20(35)23(38-17)33-13-31-18-21(27)29-12-30-22(18)33/h1-4,12-13,16-17,19-20,23,28,34-35H,5-11,26H2,(H,36,37)(H2,27,29,30)/t16-,17-,19-,20-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.56E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US8895245 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7GBK |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM227458

(US9333217, 75)Show SMILES N[C@@H](CCN(CCNCCc1ccc(Cl)cc1)CC1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H33ClN8O5/c25-15-3-1-14(2-4-15)5-7-28-8-10-32(9-6-16(26)24(36)37)11-17-19(34)20(35)23(38-17)33-13-31-18-21(27)29-12-30-22(18)33/h1-4,12-13,16-17,19-20,23,28,34-35H,5-11,26H2,(H,36,37)(H2,27,29,30)/t16-,17?,19+,20+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.56E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US9333217 (2016)
BindingDB Entry DOI: 10.7270/Q2XP73TJ |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM227458

(US9333217, 75)Show SMILES N[C@@H](CCN(CCNCCc1ccc(Cl)cc1)CC1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H33ClN8O5/c25-15-3-1-14(2-4-15)5-7-28-8-10-32(9-6-16(26)24(36)37)11-17-19(34)20(35)23(38-17)33-13-31-18-21(27)29-12-30-22(18)33/h1-4,12-13,16-17,19-20,23,28,34-35H,5-11,26H2,(H,36,37)(H2,27,29,30)/t16-,17?,19+,20+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.95E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US9333217 (2016)
BindingDB Entry DOI: 10.7270/Q2XP73TJ |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM139690

(US8895245, 75)Show SMILES N[C@@H](CCN(CCNCCc1ccc(Cl)cc1)C[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H33ClN8O5/c25-15-3-1-14(2-4-15)5-7-28-8-10-32(9-6-16(26)24(36)37)11-17-19(34)20(35)23(38-17)33-13-31-18-21(27)29-12-30-22(18)33/h1-4,12-13,16-17,19-20,23,28,34-35H,5-11,26H2,(H,36,37)(H2,27,29,30)/t16-,17-,19-,20-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8.95E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US8895245 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7GBK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data