 Found 70 hits with Last Name = 'sznaidman' and Initial = 'ml'
Found 70 hits with Last Name = 'sznaidman' and Initial = 'ml' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50033666

(2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methox...)Show SMILES Nc1nc2n(COCCOP(O)(=O)OP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C8H13N5O9P2/c9-8-11-6-5(7(14)12-8)10-3-13(6)4-20-1-2-21-24(18,19)22-23(15,16)17/h3H,1-2,4H2,(H,18,19)(H2,15,16,17)(H3,9,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049967

(CHEMBL174603 | [4-(5-Amino-7-oxo-6,7-dihydro-[1,2,...)Show InChI InChI=1S/C9H15N6O5P/c10-9-11-7-6(8(16)12-9)13-14-15(7)5-20-3-1-2-4-21(17,18)19/h1-5H2,(H2,17,18,19)(H3,10,11,12,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049963
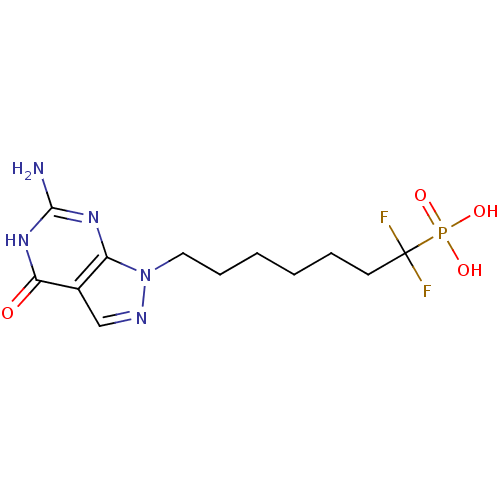
(CHEMBL368924 | [7-(6-Amino-4-oxo-4,5-dihydro-pyraz...)Show SMILES Nc1nc2n(CCCCCCC(F)(F)P(O)(O)=O)ncc2c(=O)[nH]1 Show InChI InChI=1S/C12H18F2N5O4P/c13-12(14,24(21,22)23)5-3-1-2-4-6-19-9-8(7-16-19)10(20)18-11(15)17-9/h7H,1-6H2,(H2,21,22,23)(H3,15,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049969
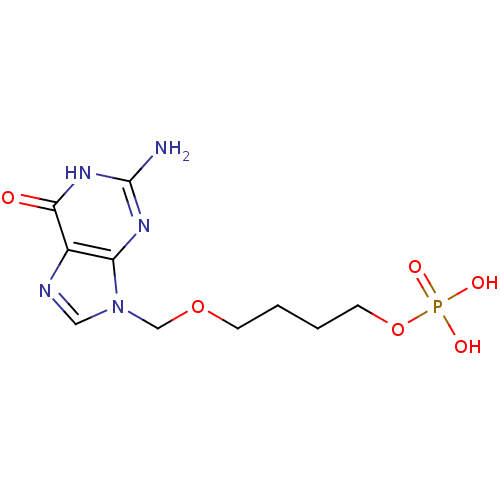
(CHEMBL172316 | Phosphoric acid mono-[4-(2-amino-6-...)Show InChI InChI=1S/C10H16N5O6P/c11-10-13-8-7(9(16)14-10)12-5-15(8)6-20-3-1-2-4-21-22(17,18)19/h5H,1-4,6H2,(H2,17,18,19)(H3,11,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049966
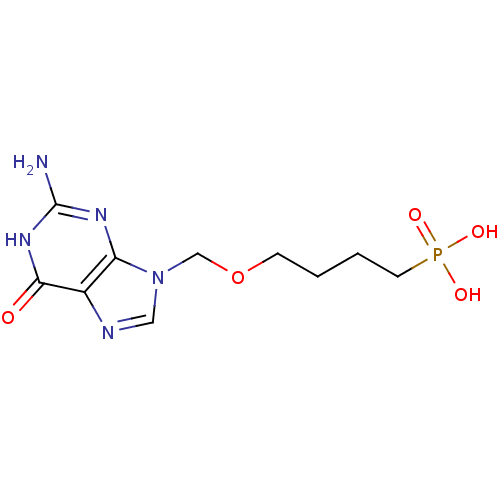
(CHEMBL177948 | [4-(2-Amino-6-oxo-1,6-dihydro-purin...)Show InChI InChI=1S/C10H16N5O5P/c11-10-13-8-7(9(16)14-10)12-5-15(8)6-20-3-1-2-4-21(17,18)19/h5H,1-4,6H2,(H2,17,18,19)(H3,11,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049971

(CHEMBL177190 | [7-(2-Amino-6-oxo-1,6-dihydro-purin...)Show SMILES Nc1nc2n(CCCCCCC(F)(F)P(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C12H18F2N5O4P/c13-12(14,24(21,22)23)5-3-1-2-4-6-19-7-16-8-9(19)17-11(15)18-10(8)20/h7H,1-6H2,(H2,21,22,23)(H3,15,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049954

(CHEMBL369052 | [5-(5-Amino-7-oxo-6,7-dihydro-[1,2,...)Show InChI InChI=1S/C9H15N6O4P/c10-9-11-7-6(8(16)12-9)13-14-15(7)4-2-1-3-5-20(17,18)19/h1-5H2,(H2,17,18,19)(H3,10,11,12,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049959
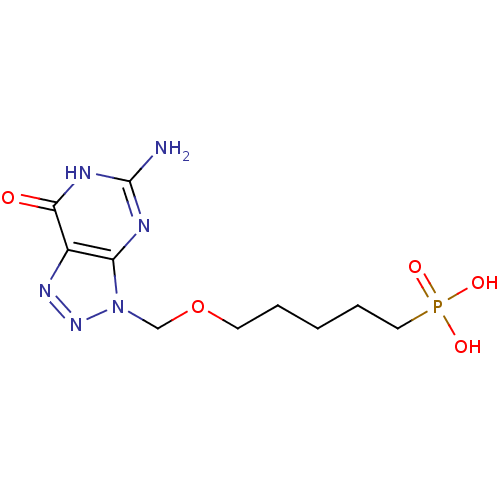
(CHEMBL173142 | [5-(5-Amino-7-oxo-6,7-dihydro-[1,2,...)Show InChI InChI=1S/C10H17N6O5P/c11-10-12-8-7(9(17)13-10)14-15-16(8)6-21-4-2-1-3-5-22(18,19)20/h1-6H2,(H2,18,19,20)(H3,11,12,13,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049972

(CHEMBL176448 | [5-(2-Amino-6-oxo-1,6-dihydro-purin...)Show InChI InChI=1S/C11H18N5O5P/c12-11-14-9-8(10(17)15-11)13-6-16(9)7-21-4-2-1-3-5-22(18,19)20/h6H,1-5,7H2,(H2,18,19,20)(H3,12,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049957
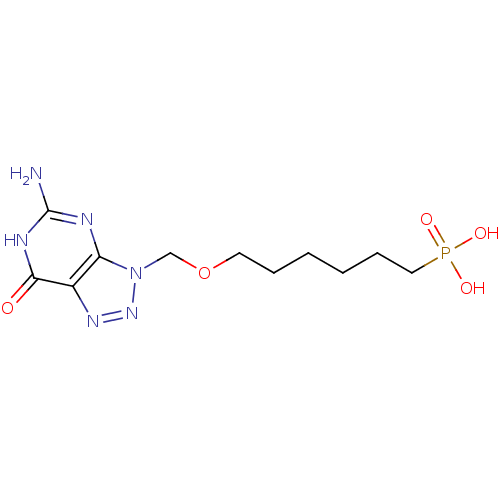
(CHEMBL366963 | [6-(5-Amino-7-oxo-6,7-dihydro-[1,2,...)Show InChI InChI=1S/C11H19N6O5P/c12-11-13-9-8(10(18)14-11)15-16-17(9)7-22-5-3-1-2-4-6-23(19,20)21/h1-7H2,(H2,19,20,21)(H3,12,13,14,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049968

(CHEMBL177945 | [6-(2-Amino-6-oxo-1,6-dihydro-purin...)Show InChI InChI=1S/C12H20N5O5P/c13-12-15-10-9(11(18)16-12)14-7-17(10)8-22-5-3-1-2-4-6-23(19,20)21/h7H,1-6,8H2,(H2,19,20,21)(H3,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049955
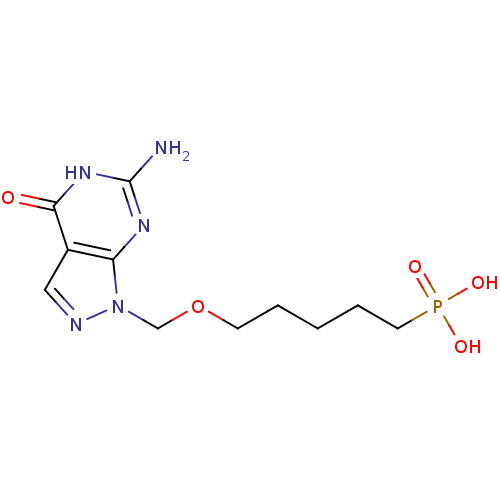
(CHEMBL175362 | [5-(6-Amino-4-oxo-4,5-dihydro-pyraz...)Show InChI InChI=1S/C11H18N5O5P/c12-11-14-9-8(10(17)15-11)6-13-16(9)7-21-4-2-1-3-5-22(18,19)20/h6H,1-5,7H2,(H2,18,19,20)(H3,12,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049960
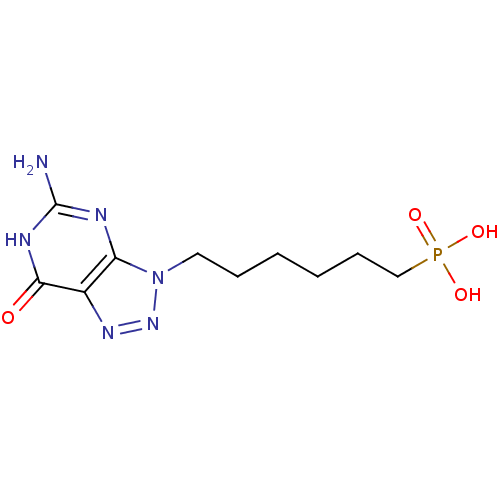
(CHEMBL368064 | [6-(5-Amino-7-oxo-6,7-dihydro-[1,2,...)Show InChI InChI=1S/C10H17N6O4P/c11-10-12-8-7(9(17)13-10)14-15-16(8)5-3-1-2-4-6-21(18,19)20/h1-6H2,(H2,18,19,20)(H3,11,12,13,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049961
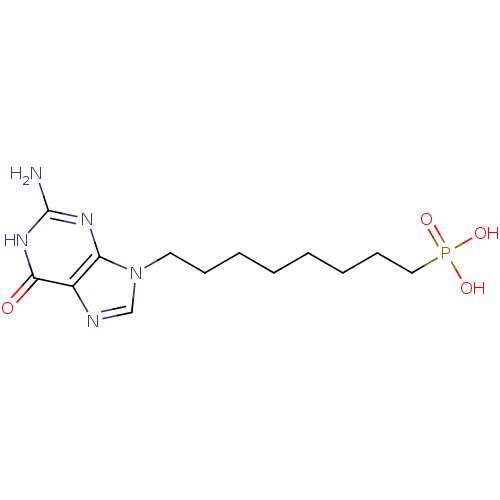
(CHEMBL354409 | [8-(2-Amino-6-oxo-1,6-dihydro-purin...)Show InChI InChI=1S/C13H22N5O4P/c14-13-16-11-10(12(19)17-13)15-9-18(11)7-5-3-1-2-4-6-8-23(20,21)22/h9H,1-8H2,(H2,20,21,22)(H3,14,16,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049962
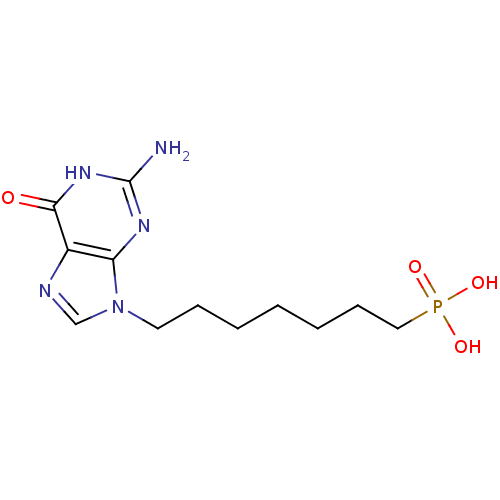
(CHEMBL172844 | [7-(2-Amino-6-oxo-1,6-dihydro-purin...)Show InChI InChI=1S/C12H20N5O4P/c13-12-15-10-9(11(18)16-12)14-8-17(10)6-4-2-1-3-5-7-22(19,20)21/h8H,1-7H2,(H2,19,20,21)(H3,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049958
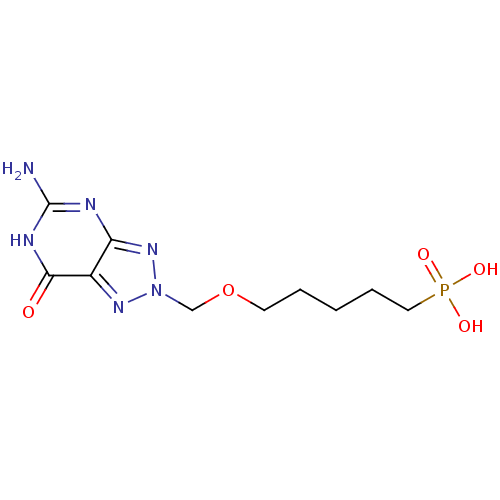
(CHEMBL172306 | [5-(5-Amino-7-oxo-6,7-dihydro-[1,2,...)Show InChI InChI=1S/C10H17N6O5P/c11-10-12-8-7(9(17)13-10)14-16(15-8)6-21-4-2-1-3-5-22(18,19)20/h1-6H2,(H2,18,19,20)(H3,11,12,13,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049970
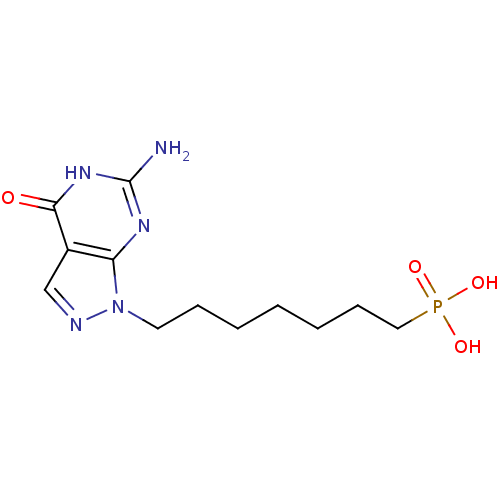
(CHEMBL176480 | TCMDC-137339 | [7-(6-Amino-4-oxo-4,...)Show InChI InChI=1S/C12H20N5O4P/c13-12-15-10-9(11(18)16-12)8-14-17(10)6-4-2-1-3-5-7-22(19,20)21/h8H,1-7H2,(H2,19,20,21)(H3,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049964
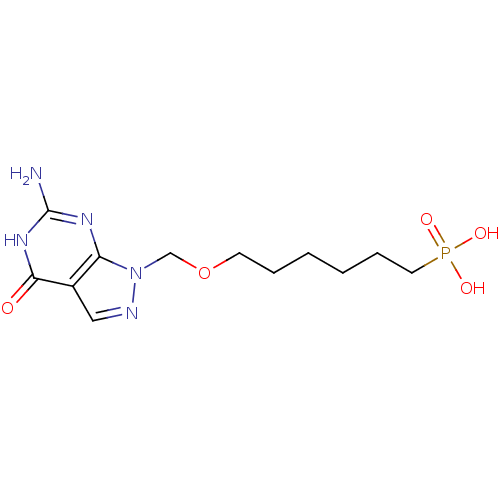
(CHEMBL173083 | [6-(6-Amino-4-oxo-4,5-dihydro-pyraz...)Show InChI InChI=1S/C12H20N5O5P/c13-12-15-10-9(11(18)16-12)7-14-17(10)8-22-5-3-1-2-4-6-23(19,20)21/h7H,1-6,8H2,(H2,19,20,21)(H3,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049956
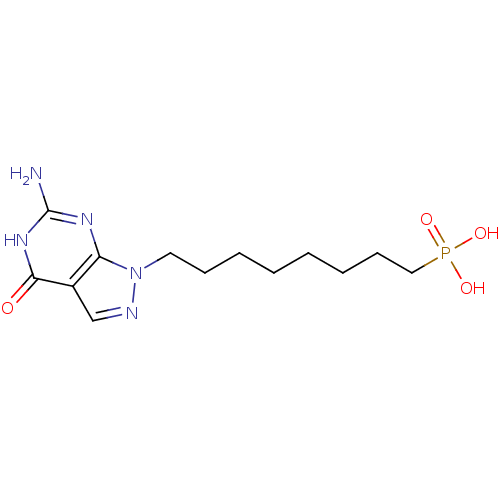
(CHEMBL176649 | [8-(6-Amino-4-oxo-4,5-dihydro-pyraz...)Show InChI InChI=1S/C13H22N5O4P/c14-13-16-11-10(12(19)17-13)9-15-18(11)7-5-3-1-2-4-6-8-23(20,21)22/h9H,1-8H2,(H2,20,21,22)(H3,14,16,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049965
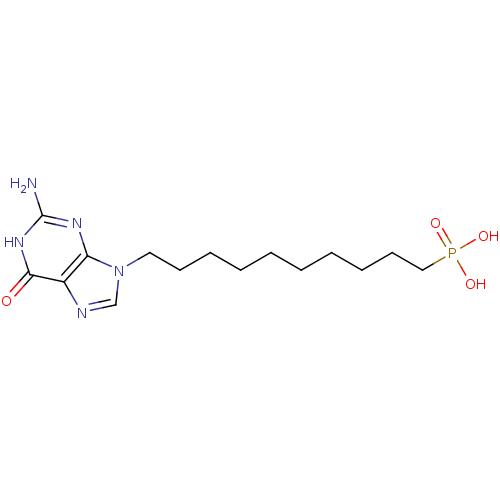
(CHEMBL176447 | [10-(2-Amino-6-oxo-1,6-dihydro-puri...)Show InChI InChI=1S/C15H26N5O4P/c16-15-18-13-12(14(21)19-15)17-11-20(13)9-7-5-3-1-2-4-6-8-10-25(22,23)24/h11H,1-10H2,(H2,22,23,24)(H3,16,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50049973
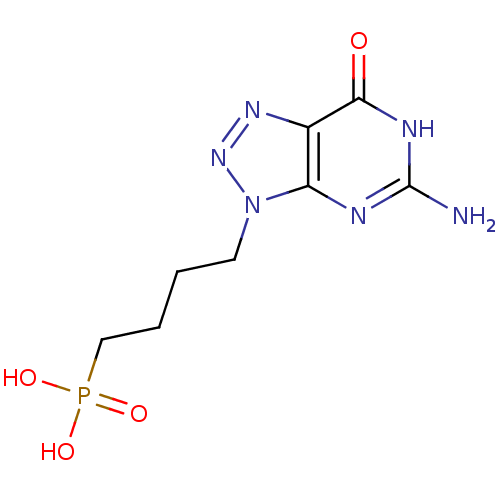
(CHEMBL175305 | [4-(5-Amino-7-oxo-6,7-dihydro-[1,2,...)Show InChI InChI=1S/C8H13N6O4P/c9-8-10-6-5(7(15)11-8)12-13-14(6)3-1-2-4-19(16,17)18/h1-4H2,(H2,16,17,18)(H3,9,10,11,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. |
J Med Chem 39: 949-56 (1996)
Article DOI: 10.1021/jm950736k
BindingDB Entry DOI: 10.7270/Q2SN082H |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28661
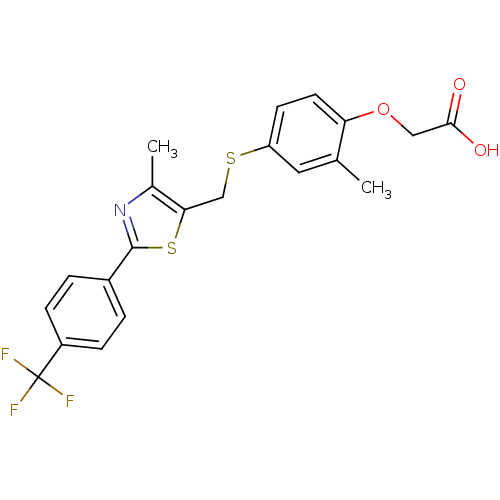
(2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO3S2/c1-12-9-16(7-8-17(12)28-10-19(26)27)29-11-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127222
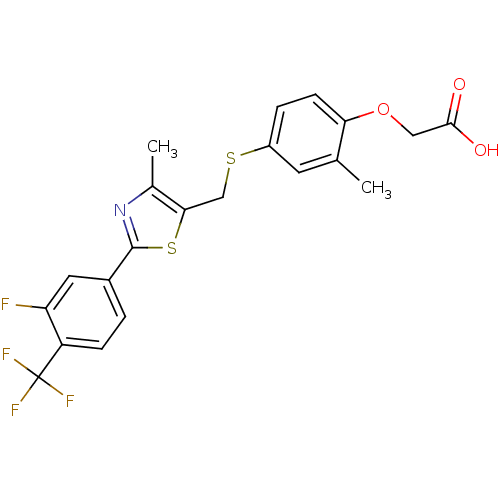
(CHEMBL38508 | GW-0742 | {4-[2-(3-Fluoro-4-trifluor...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(c(F)c1)C(F)(F)F Show InChI InChI=1S/C21H17F4NO3S2/c1-11-7-14(4-6-17(11)29-9-19(27)28)30-10-18-12(2)26-20(31-18)13-3-5-15(16(22)8-13)21(23,24)25/h3-8H,9-10H2,1-2H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127221
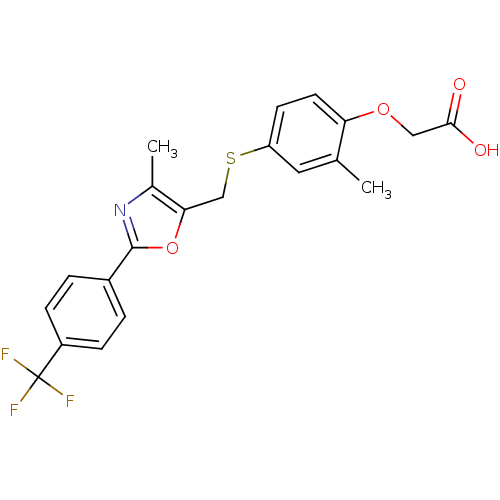
(CHEMBL289887 | {2-Methyl-4-[4-methyl-2-(4-trifluor...)Show SMILES Cc1nc(oc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO4S/c1-12-9-16(7-8-17(12)28-10-19(26)27)30-11-18-13(2)25-20(29-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximum transcriptional activation of human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127223
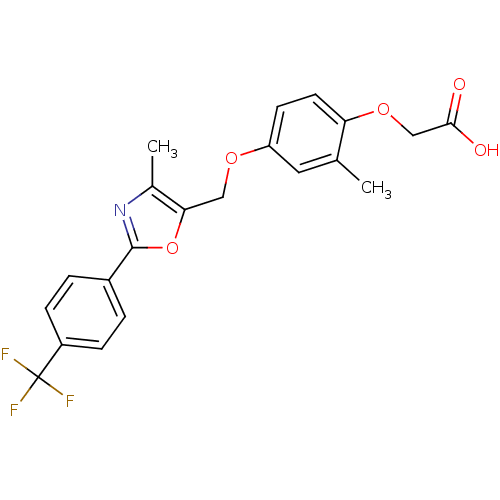
(CHEMBL37013 | {2-Methyl-4-[4-methyl-2-(4-trifluoro...)Show SMILES Cc1nc(oc1COc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO5/c1-12-9-16(7-8-17(12)29-11-19(26)27)28-10-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127215
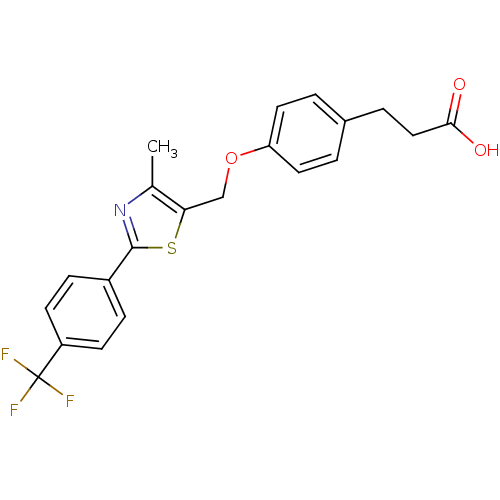
(3-{4-[4-Methyl-2-(4-trifluoromethyl-phenyl)-thiazo...)Show SMILES Cc1nc(sc1COc1ccc(CCC(O)=O)cc1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO3S/c1-13-18(12-28-17-9-2-14(3-10-17)4-11-19(26)27)29-20(25-13)15-5-7-16(8-6-15)21(22,23)24/h2-3,5-10H,4,11-12H2,1H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127218
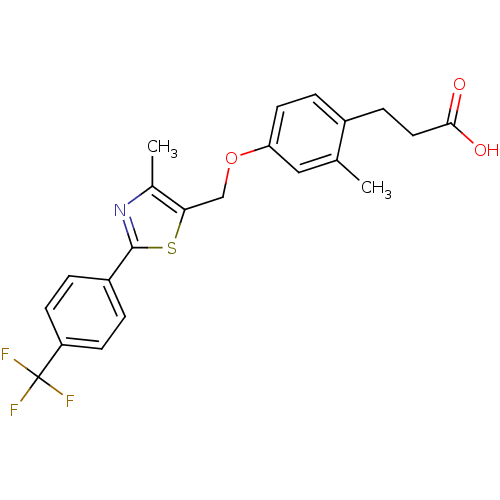
(3-{2-Methyl-4-[4-methyl-2-(4-trifluoromethyl-pheny...)Show SMILES Cc1nc(sc1COc1ccc(CCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H20F3NO3S/c1-13-11-18(9-5-15(13)6-10-20(27)28)29-12-19-14(2)26-21(30-19)16-3-7-17(8-4-16)22(23,24)25/h3-5,7-9,11H,6,10,12H2,1-2H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127220
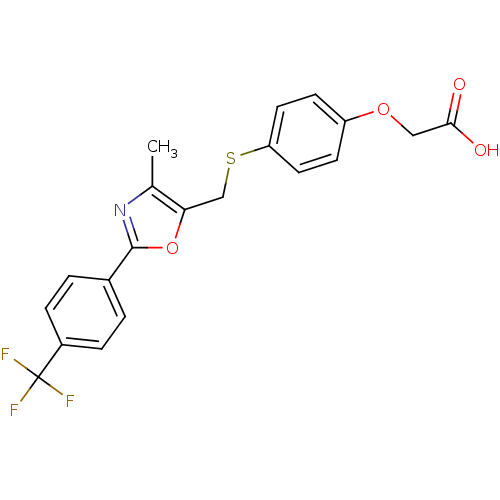
(CHEMBL37495 | {4-[4-Methyl-2-(4-trifluoromethyl-ph...)Show SMILES Cc1nc(oc1CSc1ccc(OCC(O)=O)cc1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C20H16F3NO4S/c1-12-17(11-29-16-8-6-15(7-9-16)27-10-18(25)26)28-19(24-12)13-2-4-14(5-3-13)20(21,22)23/h2-9H,10-11H2,1H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127224
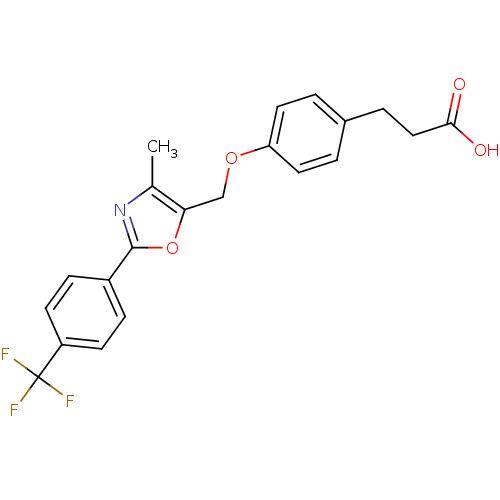
(3-{4-[4-Methyl-2-(4-trifluoromethyl-phenyl)-oxazol...)Show SMILES Cc1nc(oc1COc1ccc(CCC(O)=O)cc1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO4/c1-13-18(12-28-17-9-2-14(3-10-17)4-11-19(26)27)29-20(25-13)15-5-7-16(8-6-15)21(22,23)24/h2-3,5-10H,4,11-12H2,1H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127216
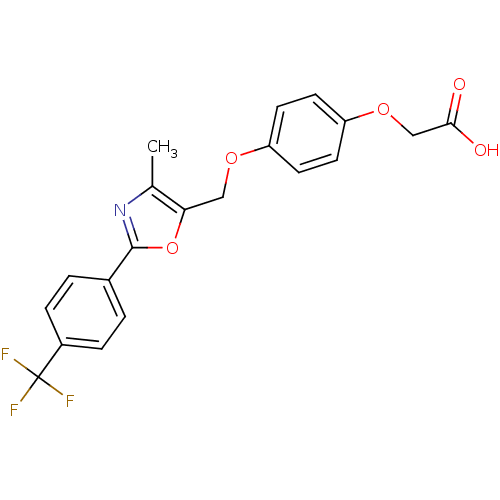
(CHEMBL416794 | {4-[4-Methyl-2-(4-trifluoromethyl-p...)Show SMILES Cc1nc(oc1COc1ccc(OCC(O)=O)cc1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C20H16F3NO5/c1-12-17(10-27-15-6-8-16(9-7-15)28-11-18(25)26)29-19(24-12)13-2-4-14(5-3-13)20(21,22)23/h2-9H,10-11H2,1H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127217

((E)-3-{2-Methyl-4-[4-methyl-2-(4-trifluoromethyl-p...)Show SMILES Cc1nc(sc1COc1ccc(\C=C\C(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H18F3NO3S/c1-13-11-18(9-5-15(13)6-10-20(27)28)29-12-19-14(2)26-21(30-19)16-3-7-17(8-4-16)22(23,24)25/h3-11H,12H2,1-2H3,(H,27,28)/b10-6+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127219
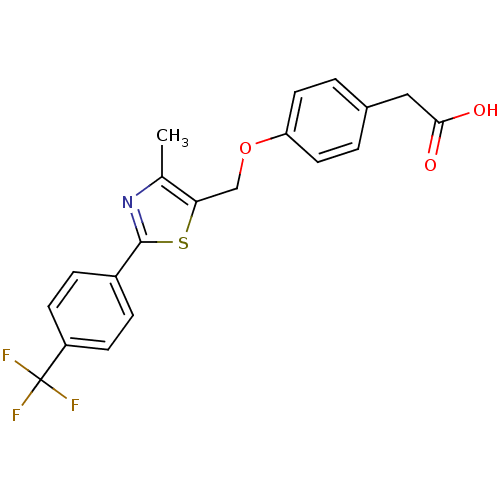
(CHEMBL39129 | {4-[4-Methyl-2-(4-trifluoromethyl-ph...)Show SMILES Cc1nc(sc1COc1ccc(CC(O)=O)cc1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C20H16F3NO3S/c1-12-17(11-27-16-8-2-13(3-9-16)10-18(25)26)28-19(24-12)14-4-6-15(7-5-14)20(21,22)23/h2-9H,10-11H2,1H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
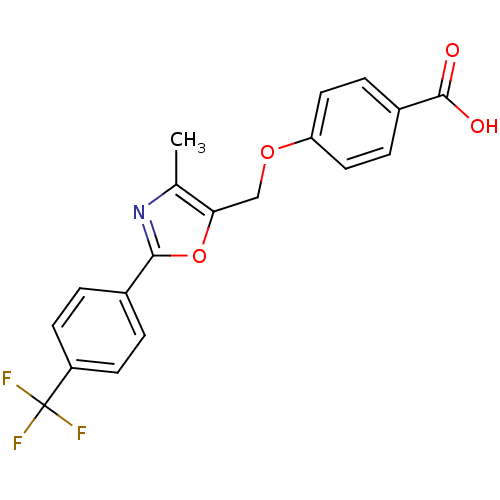
(Homo sapiens (Human)) | BDBM50127225
(4-[4-Methyl-2-(4-trifluoromethyl-phenyl)-oxazol-5-...)Show SMILES Cc1nc(oc1COc1ccc(cc1)C(O)=O)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C19H14F3NO4/c1-11-16(10-26-15-8-4-13(5-9-15)18(24)25)27-17(23-11)12-2-6-14(7-3-12)19(20,21)22/h2-9H,10H2,1H3,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127223

(CHEMBL37013 | {2-Methyl-4-[4-methyl-2-(4-trifluoro...)Show SMILES Cc1nc(oc1COc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO5/c1-12-9-16(7-8-17(12)29-11-19(26)27)28-10-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127225

(4-[4-Methyl-2-(4-trifluoromethyl-phenyl)-oxazol-5-...)Show SMILES Cc1nc(oc1COc1ccc(cc1)C(O)=O)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C19H14F3NO4/c1-11-16(10-26-15-8-4-13(5-9-15)18(24)25)27-17(23-11)12-2-6-14(7-3-12)19(20,21)22/h2-9H,10H2,1H3,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximum transcriptional activation of human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127216

(CHEMBL416794 | {4-[4-Methyl-2-(4-trifluoromethyl-p...)Show SMILES Cc1nc(oc1COc1ccc(OCC(O)=O)cc1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C20H16F3NO5/c1-12-17(10-27-15-6-8-16(9-7-15)28-11-18(25)26)29-19(24-12)13-2-4-14(5-3-13)20(21,22)23/h2-9H,10-11H2,1H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 470 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximum transcriptional activation of human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127217

((E)-3-{2-Methyl-4-[4-methyl-2-(4-trifluoromethyl-p...)Show SMILES Cc1nc(sc1COc1ccc(\C=C\C(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H18F3NO3S/c1-13-11-18(9-5-15(13)6-10-20(27)28)29-12-19-14(2)26-21(30-19)16-3-7-17(8-4-16)22(23,24)25/h3-11H,12H2,1-2H3,(H,27,28)/b10-6+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximum transcriptional activation of human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127222

(CHEMBL38508 | GW-0742 | {4-[2-(3-Fluoro-4-trifluor...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(c(F)c1)C(F)(F)F Show InChI InChI=1S/C21H17F4NO3S2/c1-11-7-14(4-6-17(11)29-9-19(27)28)30-10-18-12(2)26-20(31-18)13-3-5-15(16(22)8-13)21(23,24)25/h3-8H,9-10H2,1-2H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximum transcriptional activation of human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127219

(CHEMBL39129 | {4-[4-Methyl-2-(4-trifluoromethyl-ph...)Show SMILES Cc1nc(sc1COc1ccc(CC(O)=O)cc1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C20H16F3NO3S/c1-12-17(11-27-16-8-2-13(3-9-16)10-18(25)26)28-19(24-12)14-4-6-15(7-5-14)20(21,22)23/h2-9H,10-11H2,1H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximum transcriptional activation of human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127215

(3-{4-[4-Methyl-2-(4-trifluoromethyl-phenyl)-thiazo...)Show SMILES Cc1nc(sc1COc1ccc(CCC(O)=O)cc1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO3S/c1-13-18(12-28-17-9-2-14(3-10-17)4-11-19(26)27)29-20(25-13)15-5-7-16(8-6-15)21(22,23)24/h2-3,5-10H,4,11-12H2,1H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximum transcriptional activation of human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28661

(2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO3S2/c1-12-9-16(7-8-17(12)28-10-19(26)27)29-11-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximum transcriptional activation of human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127224

(3-{4-[4-Methyl-2-(4-trifluoromethyl-phenyl)-oxazol...)Show SMILES Cc1nc(oc1COc1ccc(CCC(O)=O)cc1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO4/c1-13-18(12-28-17-9-2-14(3-10-17)4-11-19(26)27)29-20(25-13)15-5-7-16(8-6-15)21(22,23)24/h2-3,5-10H,4,11-12H2,1H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximum transcriptional activation of human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127220

(CHEMBL37495 | {4-[4-Methyl-2-(4-trifluoromethyl-ph...)Show SMILES Cc1nc(oc1CSc1ccc(OCC(O)=O)cc1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C20H16F3NO4S/c1-12-17(11-29-16-8-6-15(7-9-16)27-10-18(25)26)28-19(24-12)13-2-4-14(5-3-13)20(21,22)23/h2-9H,10-11H2,1H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximum transcriptional activation of human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127221

(CHEMBL289887 | {2-Methyl-4-[4-methyl-2-(4-trifluor...)Show SMILES Cc1nc(oc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO4S/c1-12-9-16(7-8-17(12)28-10-19(26)27)30-11-18-13(2)25-20(29-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximum transcriptional activation of human PPAR delta receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50127216

(CHEMBL416794 | {4-[4-Methyl-2-(4-trifluoromethyl-p...)Show SMILES Cc1nc(oc1COc1ccc(OCC(O)=O)cc1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C20H16F3NO5/c1-12-17(10-27-15-6-8-16(9-7-15)28-11-18(25)26)29-19(24-12)13-2-4-14(5-3-13)20(21,22)23/h2-9H,10-11H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximum transcriptional activation of human PPAR gamma receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM28661

(2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO3S2/c1-12-9-16(7-8-17(12)28-10-19(26)27)29-11-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Activity against murine PPAR gamma receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50127218

(3-{2-Methyl-4-[4-methyl-2-(4-trifluoromethyl-pheny...)Show SMILES Cc1nc(sc1COc1ccc(CCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H20F3NO3S/c1-13-11-18(9-5-15(13)6-10-20(27)28)29-12-19-14(2)26-21(30-19)16-3-7-17(8-4-16)22(23,24)25/h3-5,7-9,11H,6,10,12H2,1-2H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximum transcriptional activation of human PPAR alpha receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50127218

(3-{2-Methyl-4-[4-methyl-2-(4-trifluoromethyl-pheny...)Show SMILES Cc1nc(sc1COc1ccc(CCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H20F3NO3S/c1-13-11-18(9-5-15(13)6-10-20(27)28)29-12-19-14(2)26-21(30-19)16-3-7-17(8-4-16)22(23,24)25/h3-5,7-9,11H,6,10,12H2,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximum transcriptional activation of human PPAR gamma receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50127219

(CHEMBL39129 | {4-[4-Methyl-2-(4-trifluoromethyl-ph...)Show SMILES Cc1nc(sc1COc1ccc(CC(O)=O)cc1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C20H16F3NO3S/c1-12-17(11-27-16-8-2-13(3-9-16)10-18(25)26)28-19(24-12)14-4-6-15(7-5-14)20(21,22)23/h2-9H,10-11H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximum transcriptional activation of human PPAR gamma receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28661

(2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO3S2/c1-12-9-16(7-8-17(12)28-10-19(26)27)29-11-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 850 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximum transcriptional activation of human PPAR gamma receptor |
Bioorg Med Chem Lett 13: 1517-21 (2003)
BindingDB Entry DOI: 10.7270/Q2VQ322G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data