 Found 201 hits with Last Name = 'martin' and Initial = 'ms'
Found 201 hits with Last Name = 'martin' and Initial = 'ms' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8146

(N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...)Show InChI InChI=1S/C18H16N6/c1-12-8-13(2)10-14(9-12)22-18-19-7-5-16(23-18)15-11-21-24-17(15)4-3-6-20-24/h3-11H,1-2H3,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8136

(N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{pyrazolo[1...)Show InChI InChI=1S/C18H14N6O2/c1-2-15-13(11-21-24(15)20-6-1)14-5-7-19-18(23-14)22-12-3-4-16-17(10-12)26-9-8-25-16/h1-7,10-11H,8-9H2,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM8128

(N-(3-methoxyphenyl)-4-{pyrazolo[1,5-a]pyridazin-3-...)Show InChI InChI=1S/C17H14N6O/c1-24-13-5-2-4-12(10-13)21-17-18-9-7-15(22-17)14-11-20-23-16(14)6-3-8-19-23/h2-11H,1H3,(H,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM8137

(N-(3,4-difluorophenyl)-4-{pyrazolo[1,5-a]pyridazin...)Show InChI InChI=1S/C16H10F2N6/c17-12-4-3-10(8-13(12)18)22-16-19-7-5-14(23-16)11-9-21-24-15(11)2-1-6-20-24/h1-9H,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM8146

(N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...)Show InChI InChI=1S/C18H16N6/c1-12-8-13(2)10-14(9-12)22-18-19-7-5-16(23-18)15-11-21-24-17(15)4-3-6-20-24/h3-11H,1-2H3,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM8126
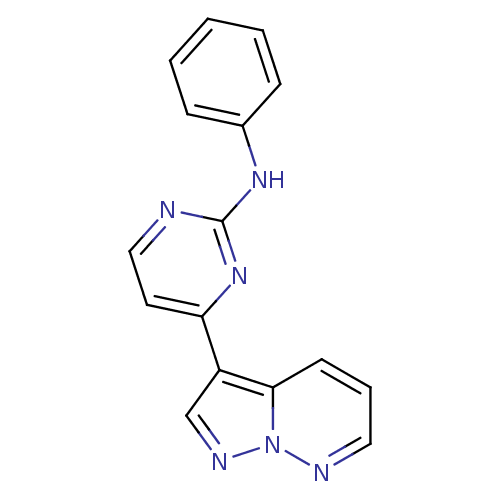
(N-phenyl-4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidi...)Show InChI InChI=1S/C16H12N6/c1-2-5-12(6-3-1)20-16-17-10-8-14(21-16)13-11-19-22-15(13)7-4-9-18-22/h1-11H,(H,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50422364
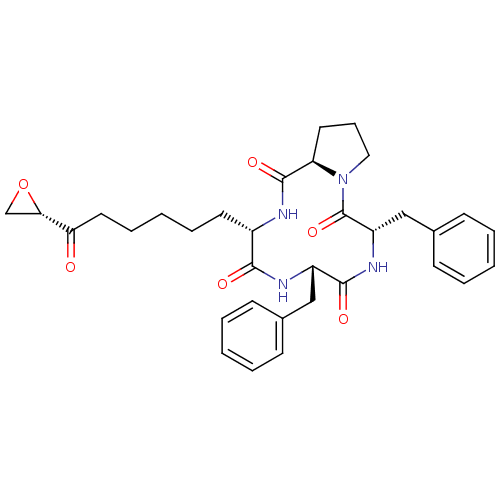
(TRAPOXIN B)Show SMILES O=C(CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H]1CO1 Show InChI InChI=1S/C33H40N4O6/c38-28(29-21-43-29)17-9-3-8-15-24-30(39)35-25(19-22-11-4-1-5-12-22)31(40)36-26(20-23-13-6-2-7-14-23)33(42)37-18-10-16-27(37)32(41)34-24/h1-2,4-7,11-14,24-27,29H,3,8-10,15-21H2,(H,34,41)(H,35,39)(H,36,40)/t24-,25-,26-,27+,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM8136

(N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{pyrazolo[1...)Show InChI InChI=1S/C18H14N6O2/c1-2-15-13(11-21-24(15)20-6-1)14-5-7-19-18(23-14)22-12-3-4-16-17(10-12)26-9-8-25-16/h1-7,10-11H,8-9H2,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8137

(N-(3,4-difluorophenyl)-4-{pyrazolo[1,5-a]pyridazin...)Show InChI InChI=1S/C16H10F2N6/c17-12-4-3-10(8-13(12)18)22-16-19-7-5-14(23-16)11-9-21-24-15(11)2-1-6-20-24/h1-9H,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8128

(N-(3-methoxyphenyl)-4-{pyrazolo[1,5-a]pyridazin-3-...)Show InChI InChI=1S/C17H14N6O/c1-24-13-5-2-4-12(10-13)21-17-18-9-7-15(22-17)14-11-20-23-16(14)6-3-8-19-23/h2-11H,1H3,(H,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474360
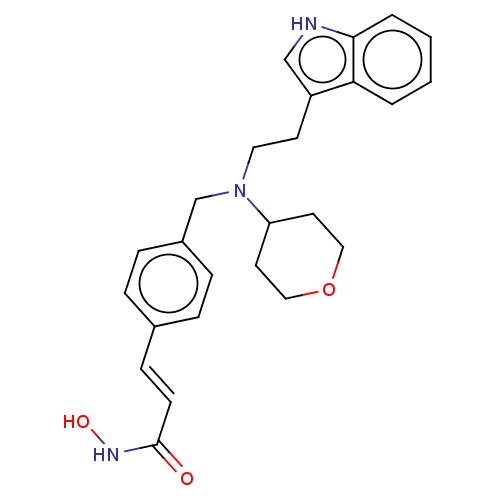
(CHEMBL357231)Show SMILES ONC(=O)\C=C\c1ccc(CN(CCc2c[nH]c3ccccc23)C2CCOCC2)cc1 Show InChI InChI=1S/C25H29N3O3/c29-25(27-30)10-9-19-5-7-20(8-6-19)18-28(22-12-15-31-16-13-22)14-11-21-17-26-24-4-2-1-3-23(21)24/h1-10,17,22,26,30H,11-16,18H2,(H,27,29)/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50506295
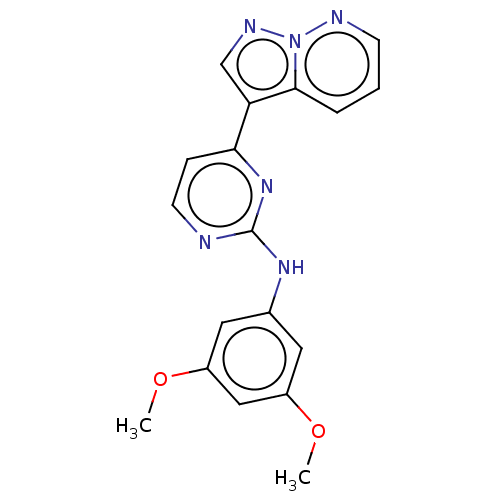
(GW801372X)Show InChI InChI=1S/C18H16N6O2/c1-25-13-8-12(9-14(10-13)26-2)22-18-19-7-5-16(23-18)15-11-21-24-17(15)4-3-6-20-24/h3-11H,1-2H3,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474337

(CHEMBL141502)Show SMILES COc1ccc2[nH]cc(CCNCc3ccc(\C=C\C(=O)NO)cc3)c2c1 Show InChI InChI=1S/C21H23N3O3/c1-27-18-7-8-20-19(12-18)17(14-23-20)10-11-22-13-16-4-2-15(3-5-16)6-9-21(25)24-26/h2-9,12,14,22-23,26H,10-11,13H2,1H3,(H,24,25)/b9-6+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474333

(CHEMBL140013)Show SMILES ONC(=O)\C=C\c1ccc(CNCCc2c[nH]c3ccc(F)cc23)cc1 Show InChI InChI=1S/C20H20FN3O2/c21-17-6-7-19-18(11-17)16(13-23-19)9-10-22-12-15-3-1-14(2-4-15)5-8-20(25)24-26/h1-8,11,13,22-23,26H,9-10,12H2,(H,24,25)/b8-5+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474351

(CHEMBL140014)Show InChI InChI=1S/C20H20N2O3/c23-20(22-24)10-9-15-5-7-16(8-6-15)13-21-12-11-17-14-25-19-4-2-1-3-18(17)19/h1-10,14,21,24H,11-13H2,(H,22,23)/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50506295

(GW801372X)Show InChI InChI=1S/C18H16N6O2/c1-25-13-8-12(9-14(10-13)26-2)22-18-19-7-5-16(23-18)15-11-21-24-17(15)4-3-6-20-24/h3-11H,1-2H3,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50018011
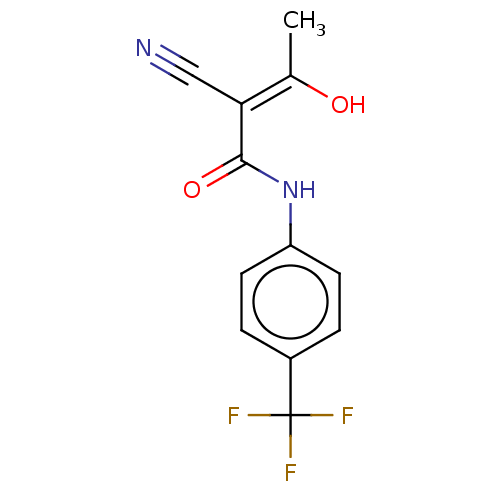
(Aubagio | CHEBI:68540 | HMR-1726 | TERIFLUNOMIDE)Show InChI InChI=1S/C12H9F3N2O2/c1-7(18)10(6-16)11(19)17-9-4-2-8(3-5-9)12(13,14)15/h2-5,18H,1H3,(H,17,19)/b10-7- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His6-tagged rat DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assay |
J Med Chem 63: 4929-4956 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00311
BindingDB Entry DOI: 10.7270/Q2M90D6Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50506295

(GW801372X)Show InChI InChI=1S/C18H16N6O2/c1-25-13-8-12(9-14(10-13)26-2)22-18-19-7-5-16(23-18)15-11-21-24-17(15)4-3-6-20-24/h3-11H,1-2H3,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474359

(CHEMBL143255)Show SMILES ONC(=O)\C=C\c1ccc(CN(CCc2c[nH]c3ccccc23)C2CCCCC2)cc1 Show InChI InChI=1S/C26H31N3O2/c30-26(28-31)15-14-20-10-12-21(13-11-20)19-29(23-6-2-1-3-7-23)17-16-22-18-27-25-9-5-4-8-24(22)25/h4-5,8-15,18,23,27,31H,1-3,6-7,16-17,19H2,(H,28,30)/b15-14+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8126

(N-phenyl-4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidi...)Show InChI InChI=1S/C16H12N6/c1-2-5-12(6-3-1)20-16-17-10-8-14(21-16)13-11-19-22-15(13)7-4-9-18-22/h1-11H,(H,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474346

(CHEMBL140566)Show SMILES CC(NCCc1c[nH]c2ccccc12)c1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15(17-9-6-16(7-10-17)8-11-21(25)24-26)22-13-12-18-14-23-20-5-3-2-4-19(18)20/h2-11,14-15,22-23,26H,12-13H2,1H3,(H,24,25)/b11-8+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM323704
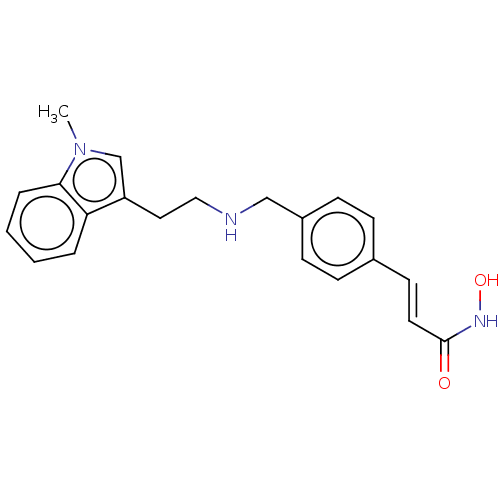
(US10188756, Compound CN107)Show InChI InChI=1S/C21H23N3O2/c1-24-15-18(19-4-2-3-5-20(19)24)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)23-26/h2-11,15,22,26H,12-14H2,1H3,(H,23,25)/b11-10+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50134227
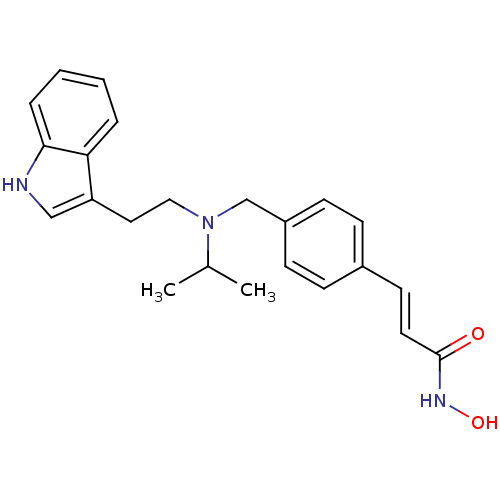
((E)-N-Hydroxy-3-[4-({[2-(1H-indol-3-yl)-ethyl]-iso...)Show SMILES CC(C)N(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C23H27N3O2/c1-17(2)26(14-13-20-15-24-22-6-4-3-5-21(20)22)16-19-9-7-18(8-10-19)11-12-23(27)25-28/h3-12,15,17,24,28H,13-14,16H2,1-2H3,(H,25,27)/b12-11+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474361

(CHEMBL348256)Show SMILES OCCN(CCc1cnc2ccccc2c1)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C23H25N3O3/c27-14-13-26(12-11-20-15-21-3-1-2-4-22(21)24-16-20)17-19-7-5-18(6-8-19)9-10-23(28)25-29/h1-10,15-16,27,29H,11-14,17H2,(H,25,28)/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474348

(CHEMBL140088)Show SMILES CC(Cc1c[nH]c2ccccc12)NCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15(12-18-14-23-20-5-3-2-4-19(18)20)22-13-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,14-15,22-23,26H,12-13H2,1H3,(H,24,25)/b11-10+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474338
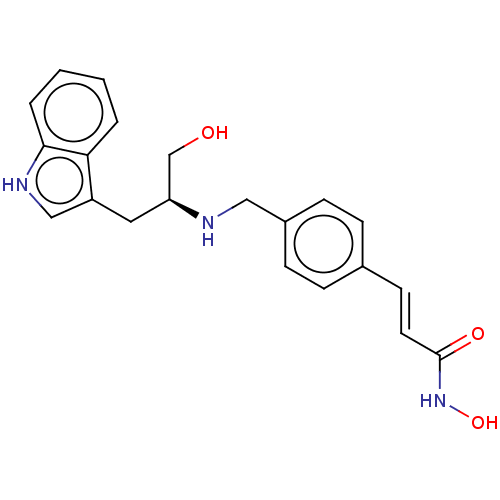
(CHEMBL343823)Show SMILES OC[C@H](Cc1c[nH]c2ccccc12)NCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O3/c25-14-18(11-17-13-23-20-4-2-1-3-19(17)20)22-12-16-7-5-15(6-8-16)9-10-21(26)24-27/h1-10,13,18,22-23,25,27H,11-12,14H2,(H,24,26)/b10-9+/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
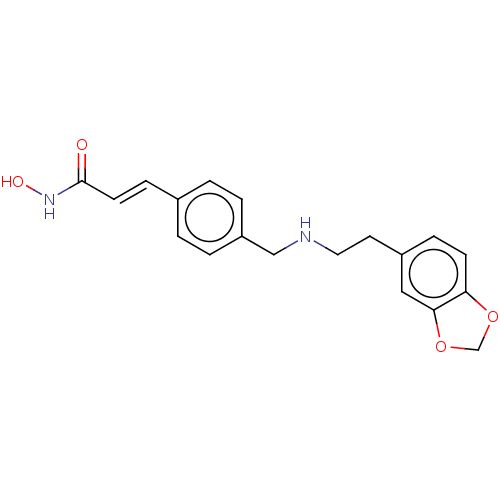
(Homo sapiens (Human)) | BDBM50474357
(CHEMBL343068)Show InChI InChI=1S/C19H20N2O4/c22-19(21-23)8-6-14-1-3-16(4-2-14)12-20-10-9-15-5-7-17-18(11-15)25-13-24-17/h1-8,11,20,23H,9-10,12-13H2,(H,21,22)/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
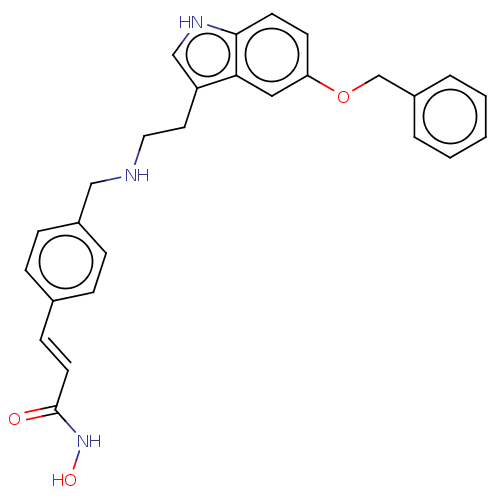
(Homo sapiens (Human)) | BDBM50474353
(CHEMBL141087)Show SMILES ONC(=O)\C=C\c1ccc(CNCCc2c[nH]c3ccc(OCc4ccccc4)cc23)cc1 Show InChI InChI=1S/C27H27N3O3/c31-27(30-32)13-10-20-6-8-21(9-7-20)17-28-15-14-23-18-29-26-12-11-24(16-25(23)26)33-19-22-4-2-1-3-5-22/h1-13,16,18,28-29,32H,14-15,17,19H2,(H,30,31)/b13-10+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
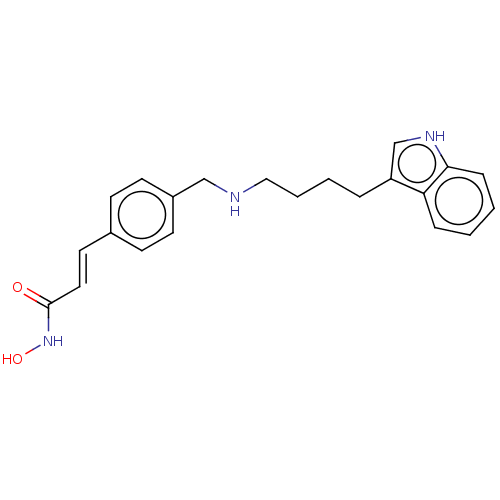
(Homo sapiens (Human)) | BDBM50474362
(CHEMBL143283)Show SMILES ONC(=O)\C=C\c1ccc(CNCCCCc2c[nH]c3ccccc23)cc1 Show InChI InChI=1S/C22H25N3O2/c26-22(25-27)13-12-17-8-10-18(11-9-17)15-23-14-4-3-5-19-16-24-21-7-2-1-6-20(19)21/h1-2,6-13,16,23-24,27H,3-5,14-15H2,(H,25,26)/b13-12+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
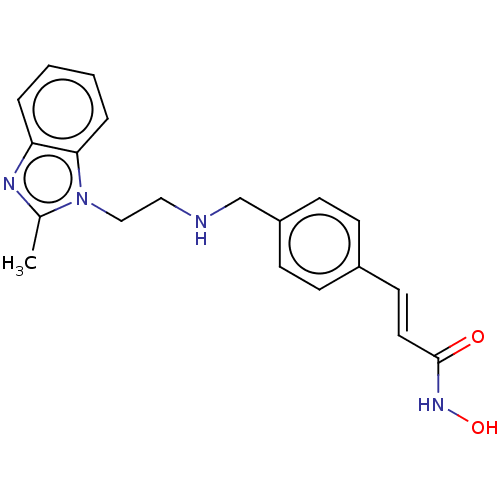
(Homo sapiens (Human)) | BDBM50474334
(CHEMBL142931)Show InChI InChI=1S/C20H22N4O2/c1-15-22-18-4-2-3-5-19(18)24(15)13-12-21-14-17-8-6-16(7-9-17)10-11-20(25)23-26/h2-11,21,26H,12-14H2,1H3,(H,23,25)/b11-10+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8197

(4-{6-methyl-2-phenylpyrazolo[1,5-a]pyridazin-3-yl}...)Show SMILES Cc1ccc2c(c(nn2n1)-c1ccccc1)-c1ccnc(Nc2cccc(c2)C(F)(F)F)n1 Show InChI InChI=1S/C24H17F3N6/c1-15-10-11-20-21(22(32-33(20)31-15)16-6-3-2-4-7-16)19-12-13-28-23(30-19)29-18-9-5-8-17(14-18)24(25,26)27/h2-14H,1H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474364
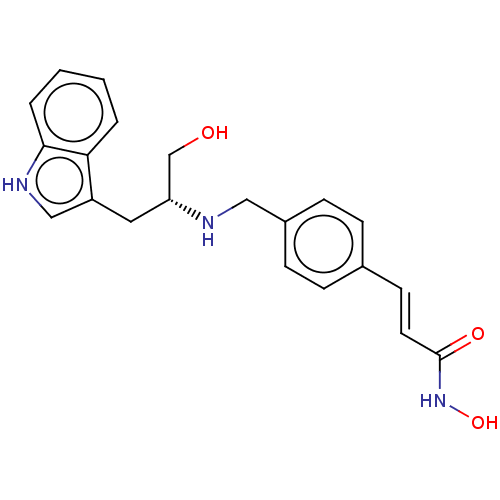
(CHEMBL358059)Show SMILES OC[C@@H](Cc1c[nH]c2ccccc12)NCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O3/c25-14-18(11-17-13-23-20-4-2-1-3-19(17)20)22-12-16-7-5-15(6-8-16)9-10-21(26)24-27/h1-10,13,18,22-23,25,27H,11-12,14H2,(H,24,26)/b10-9+/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474355
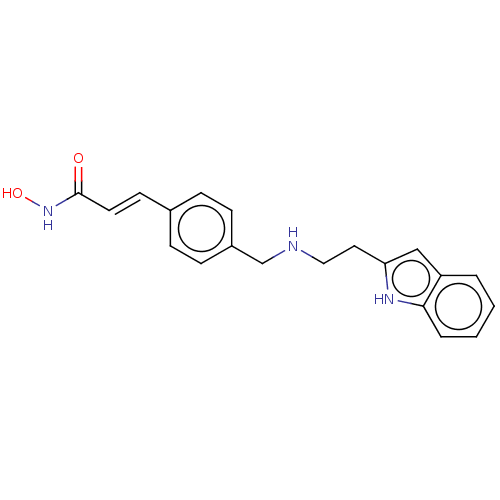
(CHEMBL142120)Show InChI InChI=1S/C20H21N3O2/c24-20(23-25)10-9-15-5-7-16(8-6-15)14-21-12-11-18-13-17-3-1-2-4-19(17)22-18/h1-10,13,21-22,25H,11-12,14H2,(H,23,24)/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25458

((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...)Show SMILES [H][C@@]12CC=C(c3cccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |t:3,23| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of CYP17 (unknown origin) |
ACS Med Chem Lett 7: 708-13 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00137
BindingDB Entry DOI: 10.7270/Q22V2KN5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8152
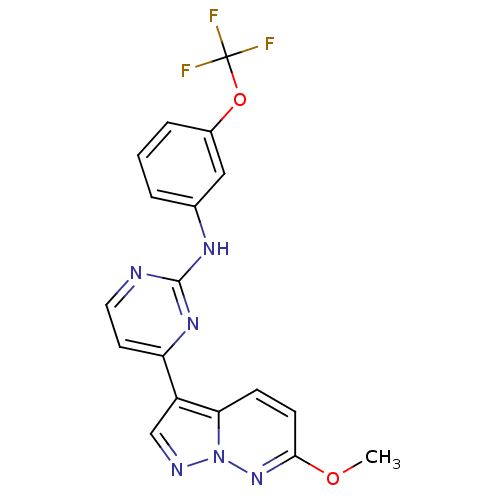
(4-{6-methoxypyrazolo[1,5-a]pyridazin-3-yl}-N-[3-(t...)Show SMILES COc1ccc2c(cnn2n1)-c1ccnc(Nc2cccc(OC(F)(F)F)c2)n1 Show InChI InChI=1S/C18H13F3N6O2/c1-28-16-6-5-15-13(10-23-27(15)26-16)14-7-8-22-17(25-14)24-11-3-2-4-12(9-11)29-18(19,20)21/h2-10H,1H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8133

(N-(4-tert-butylphenyl)-4-{pyrazolo[1,5-a]pyridazin...)Show SMILES CC(C)(C)c1ccc(Nc2nccc(n2)-c2cnn3ncccc23)cc1 Show InChI InChI=1S/C20H20N6/c1-20(2,3)14-6-8-15(9-7-14)24-19-21-12-10-17(25-19)16-13-23-26-18(16)5-4-11-22-26/h4-13H,1-3H3,(H,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8189

(N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{2-[3-(trif...)Show SMILES FC(F)(F)c1cccc(c1)-c1nn2ncccc2c1-c1ccnc(Nc2ccc3OCCOc3c2)n1 Show InChI InChI=1S/C25H17F3N6O2/c26-25(27,28)16-4-1-3-15(13-16)23-22(19-5-2-9-30-34(19)33-23)18-8-10-29-24(32-18)31-17-6-7-20-21(14-17)36-12-11-35-20/h1-10,13-14H,11-12H2,(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474347
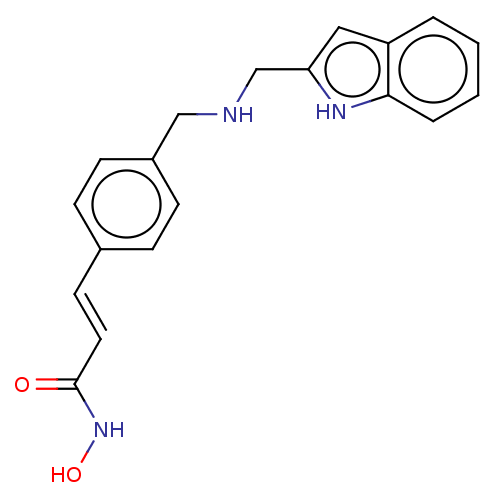
(CHEMBL335812)Show InChI InChI=1S/C19H19N3O2/c23-19(22-24)10-9-14-5-7-15(8-6-14)12-20-13-17-11-16-3-1-2-4-18(16)21-17/h1-11,20-21,24H,12-13H2,(H,22,23)/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474363
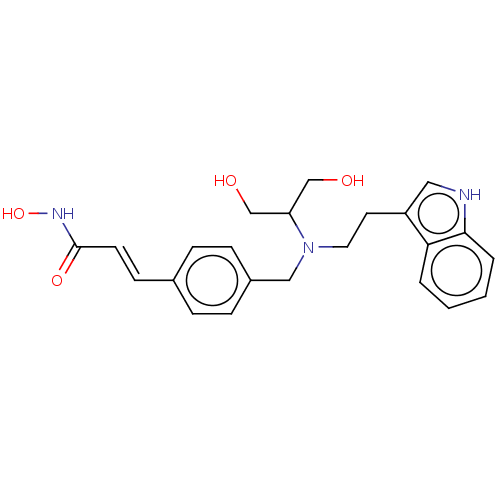
(CHEMBL343091)Show SMILES OCC(CO)N(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C23H27N3O4/c27-15-20(16-28)26(12-11-19-13-24-22-4-2-1-3-21(19)22)14-18-7-5-17(6-8-18)9-10-23(29)25-30/h1-10,13,20,24,27-28,30H,11-12,14-16H2,(H,25,29)/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474344
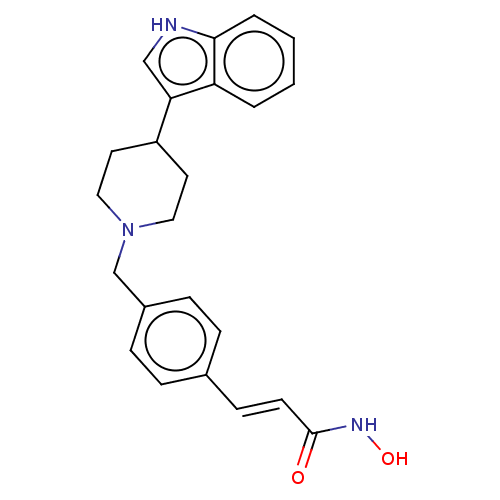
(CHEMBL140811)Show SMILES ONC(=O)\C=C\c1ccc(CN2CCC(CC2)c2c[nH]c3ccccc23)cc1 Show InChI InChI=1S/C23H25N3O2/c27-23(25-28)10-9-17-5-7-18(8-6-17)16-26-13-11-19(12-14-26)21-15-24-22-4-2-1-3-20(21)22/h1-10,15,19,24,28H,11-14,16H2,(H,25,27)/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474358
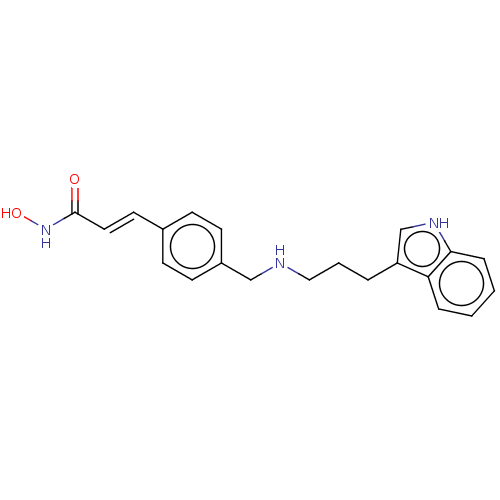
(CHEMBL139836)Show SMILES ONC(=O)\C=C\c1ccc(CNCCCc2c[nH]c3ccccc23)cc1 Show InChI InChI=1S/C21H23N3O2/c25-21(24-26)12-11-16-7-9-17(10-8-16)14-22-13-3-4-18-15-23-20-6-2-1-5-19(18)20/h1-2,5-12,15,22-23,26H,3-4,13-14H2,(H,24,25)/b12-11+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474354
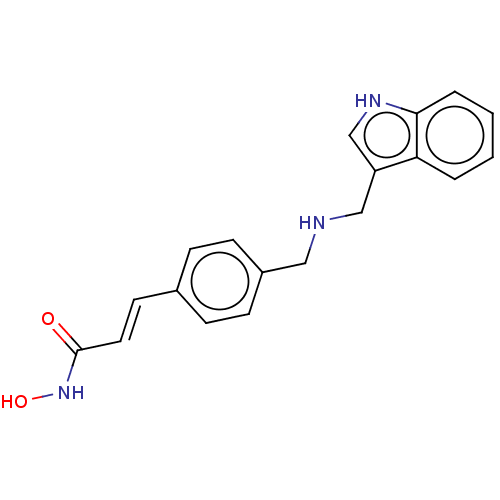
(CHEMBL140530)Show InChI InChI=1S/C19H19N3O2/c23-19(22-24)10-9-14-5-7-15(8-6-14)11-20-12-16-13-21-18-4-2-1-3-17(16)18/h1-10,13,20-21,24H,11-12H2,(H,22,23)/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50074715
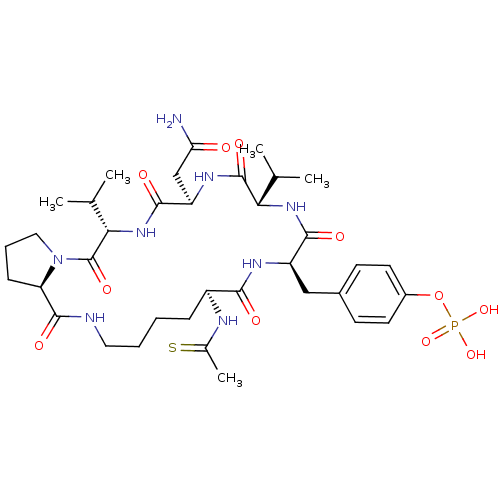
(CHEMBL433805 | Phosphoric acid mono-[4-((5S,8R,11S...)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](Cc2ccc(OP(O)(O)=O)cc2)NC(=O)[C@H](CCCCNC(=O)[C@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](CC(N)=O)NC1=O)C(C)C)NC(C)=S Show InChI InChI=1S/C36H55N8O11PS/c1-19(2)29-35(50)41-26(18-28(37)45)33(48)43-30(20(3)4)36(51)44-16-8-10-27(44)34(49)38-15-7-6-9-24(39-21(5)57)31(46)40-25(32(47)42-29)17-22-11-13-23(14-12-22)55-56(52,53)54/h11-14,19-20,24-27,29-30H,6-10,15-18H2,1-5H3,(H2,37,45)(H,38,49)(H,39,57)(H,40,46)(H,41,50)(H,42,47)(H,43,48)(H2,52,53,54)/t24-,25+,26+,27+,29-,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA. |
J Med Chem 42: 971-80 (1999)
Article DOI: 10.1021/jm9811007
BindingDB Entry DOI: 10.7270/Q28S4P35 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM8128

(N-(3-methoxyphenyl)-4-{pyrazolo[1,5-a]pyridazin-3-...)Show InChI InChI=1S/C17H14N6O/c1-24-13-5-2-4-12(10-13)21-17-18-9-7-15(22-17)14-11-20-23-16(14)6-3-8-19-23/h2-11H,1H3,(H,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... |
J Med Chem 63: 756-783 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01741
BindingDB Entry DOI: 10.7270/Q2PR809G |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50134232
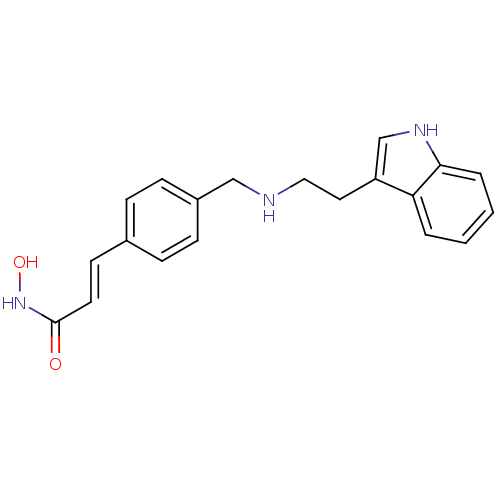
((E)-N-Hydroxy-3-(4-{[2-(1H-indol-3-yl)-ethylamino]...)Show InChI InChI=1S/C20H21N3O2/c24-20(23-25)10-9-15-5-7-16(8-6-15)13-21-12-11-17-14-22-19-4-2-1-3-18(17)19/h1-10,14,21-22,25H,11-13H2,(H,23,24)/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474340
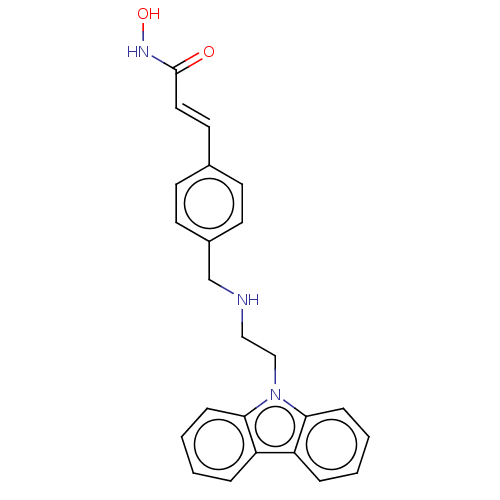
(CHEMBL341601)Show SMILES ONC(=O)\C=C\c1ccc(CNCCn2c3ccccc3c3ccccc23)cc1 Show InChI InChI=1S/C24H23N3O2/c28-24(26-29)14-13-18-9-11-19(12-10-18)17-25-15-16-27-22-7-3-1-5-20(22)21-6-2-4-8-23(21)27/h1-14,25,29H,15-17H2,(H,26,28)/b14-13+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474343
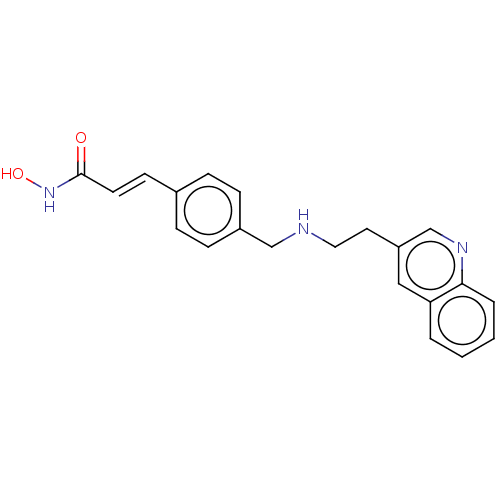
(CHEMBL139663)Show InChI InChI=1S/C21H21N3O2/c25-21(24-26)10-9-16-5-7-17(8-6-16)14-22-12-11-18-13-19-3-1-2-4-20(19)23-15-18/h1-10,13,15,22,26H,11-12,14H2,(H,24,25)/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50474349
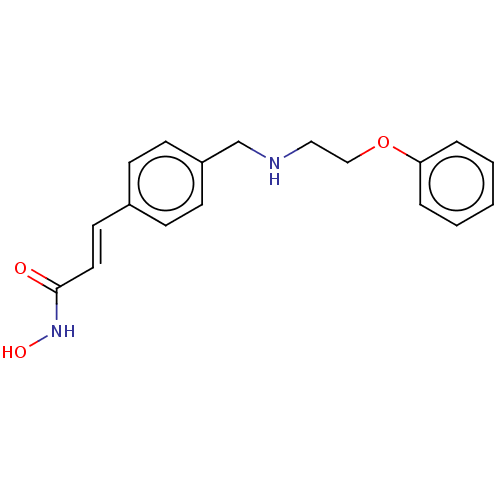
(CHEMBL140899)Show InChI InChI=1S/C18H20N2O3/c21-18(20-22)11-10-15-6-8-16(9-7-15)14-19-12-13-23-17-4-2-1-3-5-17/h1-11,19,22H,12-14H2,(H,20,21)/b11-10+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data