

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127171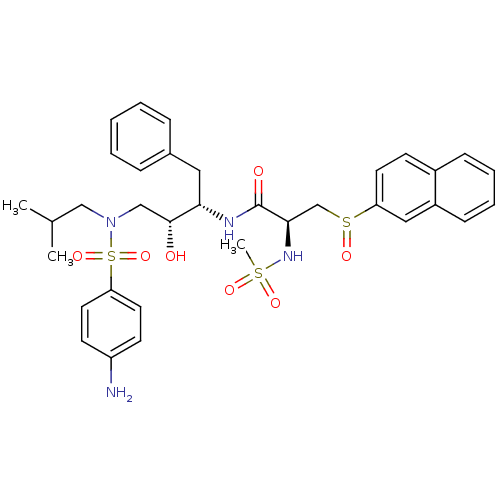 (CHEMBL299578 | N-{3-[(4-Amino-benzenesulfonyl)-iso...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM810 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50165949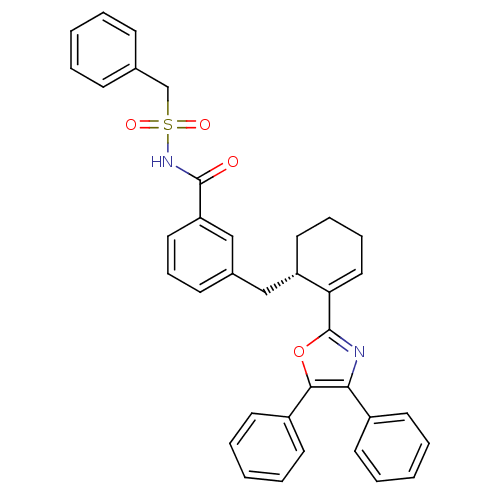 (C-Phenyl-N-{3-[2-((S)-5-phenyl-4-phenyl-oxazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127172 (4-Amino-N-[3-benzyl-2-hydroxy-6-methanesulfonylami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50165945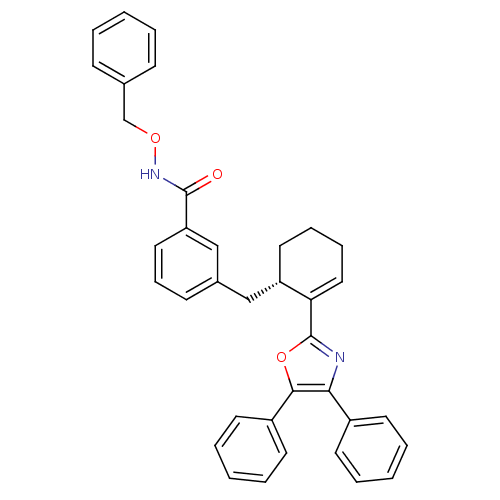 (CHEMBL371394 | N-Benzyloxy-3-[(S)-2-(4,5-diphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Rattus norvegicus) | BDBM50165949 (C-Phenyl-N-{3-[2-((S)-5-phenyl-4-phenyl-oxazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50165948 (6-{(S)-2-[(Benzofuran-2-carbonyl)-amino]-5-benzylo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Rattus norvegicus) | BDBM50165948 (6-{(S)-2-[(Benzofuran-2-carbonyl)-amino]-5-benzylo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50165946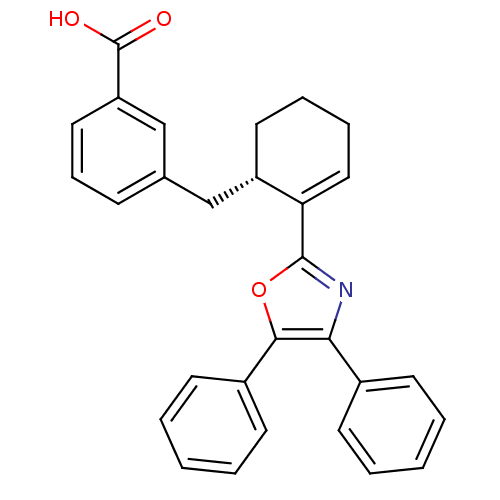 (3-[(S)-2-(4,5-Diphenyl-oxazol-2-yl)-cyclohex-2-eny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50165951 (6-{(S)-2-[(Benzofuran-2-carbonyl)-amino]-6-benzylo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50175277 (CHEMBL3809675) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant human SphK1 expressed in baculovirus infected sf9 cells using D-erythro-sphingosine as substrate in presence of [gamma-33P]... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50165943 ((S)-2-{(S)-5-Benzyloxycarbonylamino-2-[(1H-indole-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50165944 (CHEMBL189378 | {3-[(S)-2-(4,5-Diphenyl-oxazol-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50136234 (CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-Iloprost binding to human prostanoid IP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50165950 (CHEMBL192303 | {(S)-4-[(S)-1-(Benzyl-methyl-carbam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50165944 (CHEMBL189378 | {3-[(S)-2-(4,5-Diphenyl-oxazol-2-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-Iloprost binding to human prostanoid IP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50165947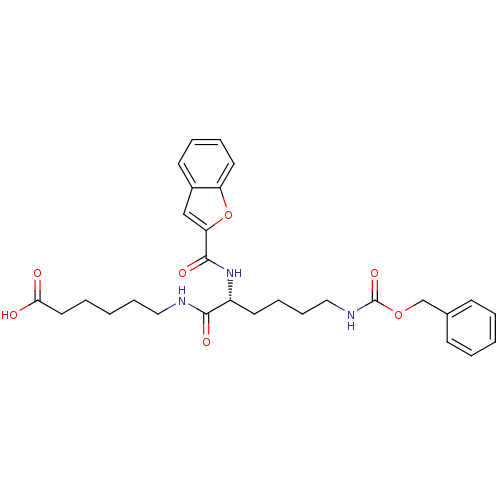 (6-{(R)-2-[(Benzofuran-2-carbonyl)-amino]-6-benzylo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50165946 (3-[(S)-2-(4,5-Diphenyl-oxazol-2-yl)-cyclohex-2-eny...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-Iloprost binding to human prostanoid IP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50136234 (CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibtion of [3H]-PGD-2 binding to human prostanoid DP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50136234 (CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-SQ-29,548 binding to human prostanoid TP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50136234 (CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP3 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50165943 ((S)-2-{(S)-5-Benzyloxycarbonylamino-2-[(1H-indole-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP1 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50136234 (CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP1 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50165945 (CHEMBL371394 | N-Benzyloxy-3-[(S)-2-(4,5-diphenyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGF-2 binding to human prostanoid FP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50165943 ((S)-2-{(S)-5-Benzyloxycarbonylamino-2-[(1H-indole-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-SQ-29,548 binding to human prostanoid TP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50136234 (CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP2 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50165943 ((S)-2-{(S)-5-Benzyloxycarbonylamino-2-[(1H-indole-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP3 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50165943 ((S)-2-{(S)-5-Benzyloxycarbonylamino-2-[(1H-indole-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGF-2 binding to human prostanoid FP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50165943 ((S)-2-{(S)-5-Benzyloxycarbonylamino-2-[(1H-indole-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibtion of [3H]-PGD-2 binding to human prostanoid DP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50165945 (CHEMBL371394 | N-Benzyloxy-3-[(S)-2-(4,5-diphenyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-SQ-29,548 binding to human prostanoid TP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50165945 (CHEMBL371394 | N-Benzyloxy-3-[(S)-2-(4,5-diphenyl-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibtion of [3H]-PGD-2 binding to human prostanoid DP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50165943 ((S)-2-{(S)-5-Benzyloxycarbonylamino-2-[(1H-indole-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP2 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50136234 (CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGF-2 binding to human prostanoid FP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50165943 ((S)-2-{(S)-5-Benzyloxycarbonylamino-2-[(1H-indole-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-Iloprost binding to human prostanoid IP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50136234 (CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM174110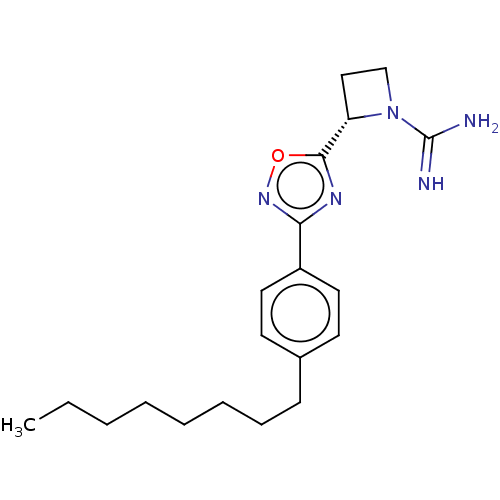 ((S)-2-(3-(4-octylphenyl)-1,2,4- oxadiazol-5-yl)aze...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant human SphK2 expressed in baculovirus infected sf9 cells using D-erythro-sphingosine as substrate after 20 mins in presence ... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50165946 (3-[(S)-2-(4,5-Diphenyl-oxazol-2-yl)-cyclohex-2-eny...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP1 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50165949 (C-Phenyl-N-{3-[2-((S)-5-phenyl-4-phenyl-oxazol-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGF-2 binding to human prostanoid FP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50165946 (3-[(S)-2-(4,5-Diphenyl-oxazol-2-yl)-cyclohex-2-eny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP2 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50165949 (C-Phenyl-N-{3-[2-((S)-5-phenyl-4-phenyl-oxazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP2 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50165949 (C-Phenyl-N-{3-[2-((S)-5-phenyl-4-phenyl-oxazol-2-y...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP3 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50165945 (CHEMBL371394 | N-Benzyloxy-3-[(S)-2-(4,5-diphenyl-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP3 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50165946 (3-[(S)-2-(4,5-Diphenyl-oxazol-2-yl)-cyclohex-2-eny...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibtion of [3H]-PGD-2 binding to human prostanoid DP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50165949 (C-Phenyl-N-{3-[2-((S)-5-phenyl-4-phenyl-oxazol-2-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-Iloprost binding to human prostanoid IP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50165949 (C-Phenyl-N-{3-[2-((S)-5-phenyl-4-phenyl-oxazol-2-y...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP1 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50165945 (CHEMBL371394 | N-Benzyloxy-3-[(S)-2-(4,5-diphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP2 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50165946 (3-[(S)-2-(4,5-Diphenyl-oxazol-2-yl)-cyclohex-2-eny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-SQ-29,548 binding to human prostanoid TP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50165945 (CHEMBL371394 | N-Benzyloxy-3-[(S)-2-(4,5-diphenyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-Iloprost binding to human prostanoid IP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1091 total ) | Next | Last >> |