

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470598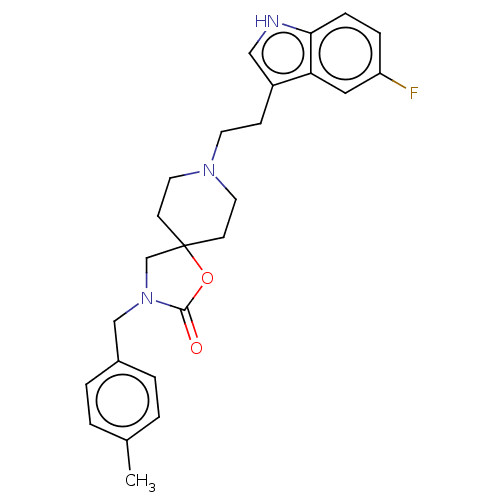 (CHEMBL126050) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50040516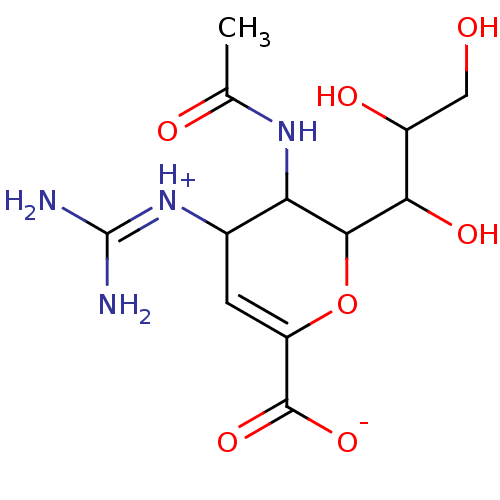 (3-(acetylamino)-4-{[amino(iminio)methyl]amino}-2-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description inhibition of Influenza A Sialidase | J Med Chem 37: 616-24 (1994) Checked by Author BindingDB Entry DOI: 10.7270/Q27P901J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470590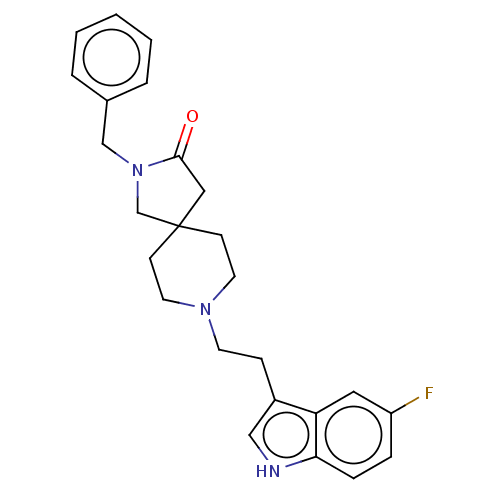 (CHEMBL341357) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470604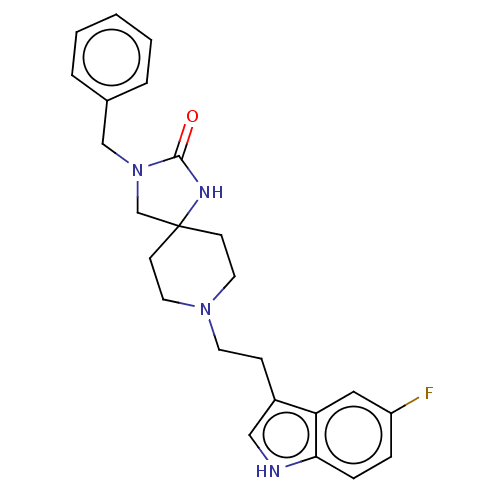 (CHEMBL338825) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470591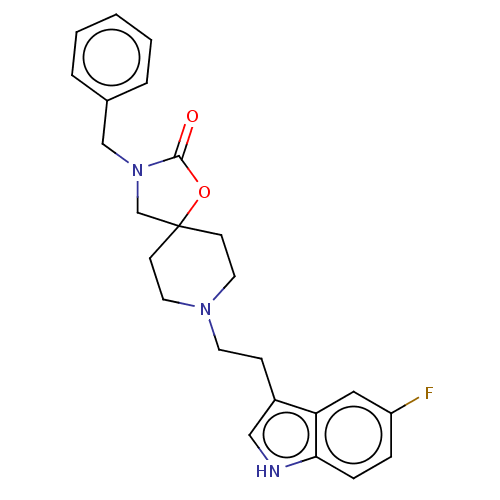 (CHEMBL124208) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470596 (CHEMBL125310) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470583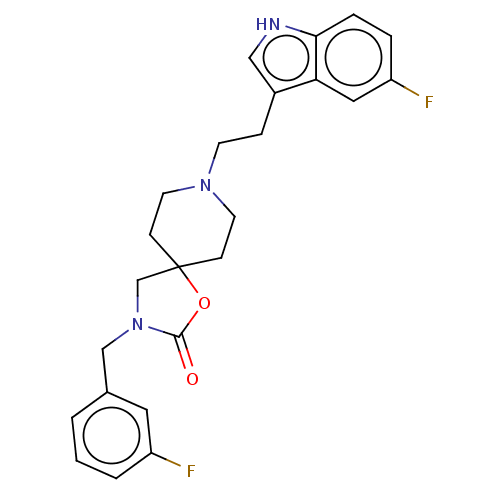 (CHEMBL125696) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470587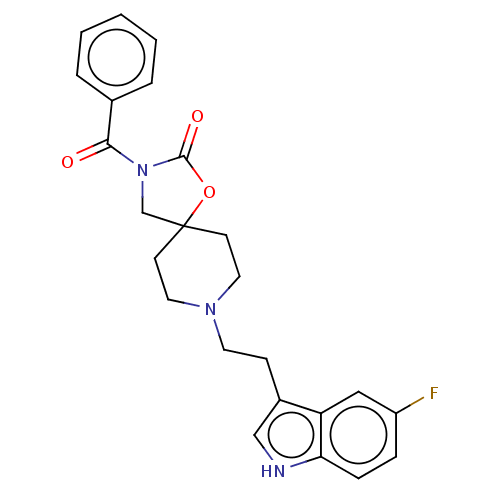 (CHEMBL124648) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50470591 (CHEMBL124208) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for binding affinity against Tachykinin receptor 2 from guinea pig trachea | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470605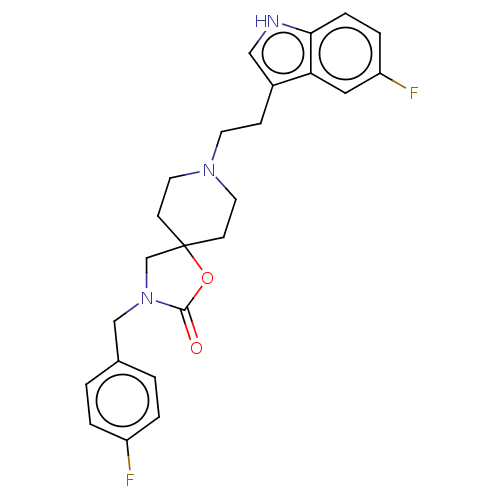 (CHEMBL122169) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470603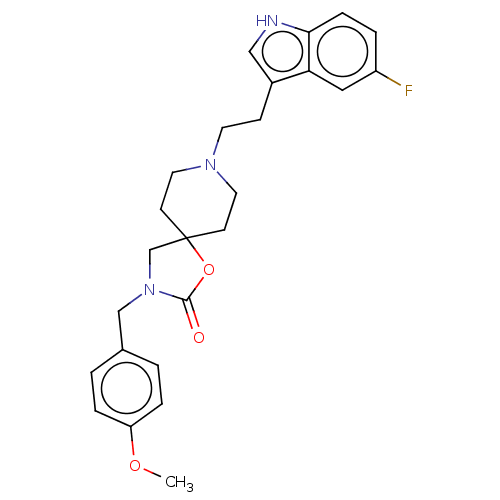 (CHEMBL124013) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470582 (CHEMBL126043) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470599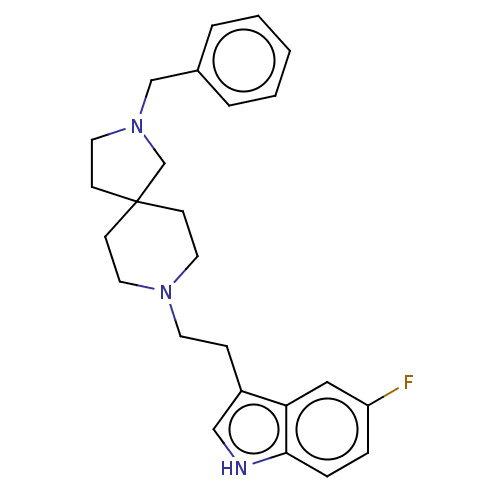 (CHEMBL330766) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470595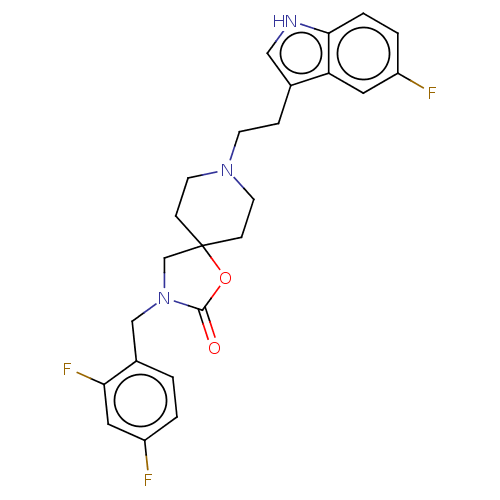 (CHEMBL122019) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50470591 (CHEMBL124208) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for binding affinity against Tachykinin receptor 2 from human expressed in CHO cells | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470592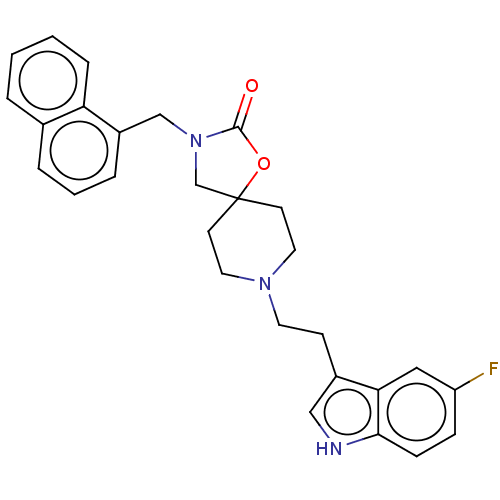 (CHEMBL339311) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470597 (CHEMBL330826) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470581 (CHEMBL341008) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470606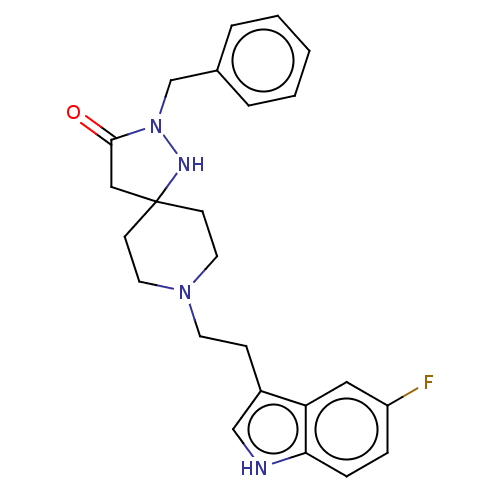 (CHEMBL446099) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470584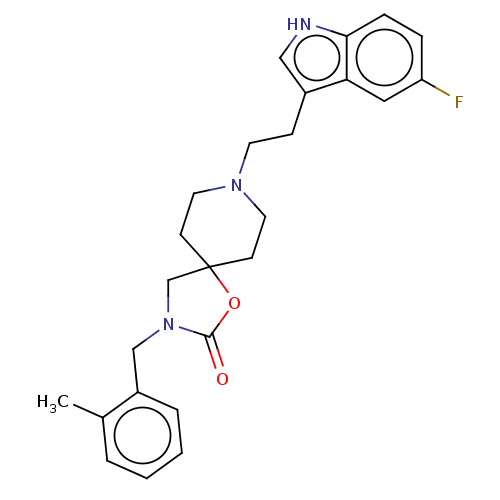 (CHEMBL125001) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470585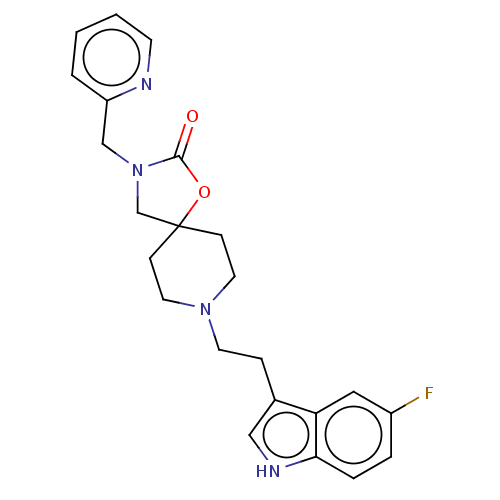 (CHEMBL415518) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470607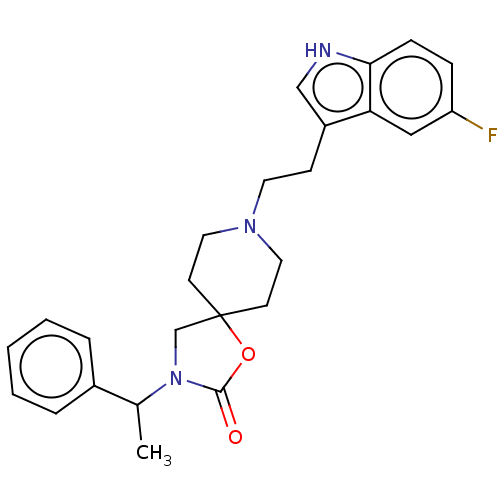 (CHEMBL124624) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470601 (CHEMBL123731) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470608 (CHEMBL338030) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50040515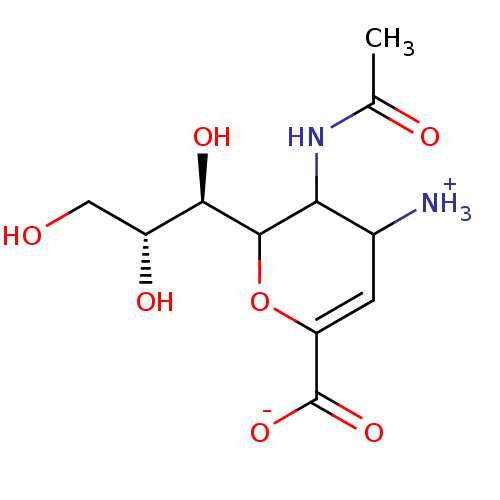 (3-(acetylamino)-4-ammonio-2-[(1R,2R)-1,2,3-trihydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description inhibition of Influenza A Sialidase | J Med Chem 37: 616-24 (1994) Checked by Author BindingDB Entry DOI: 10.7270/Q27P901J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92766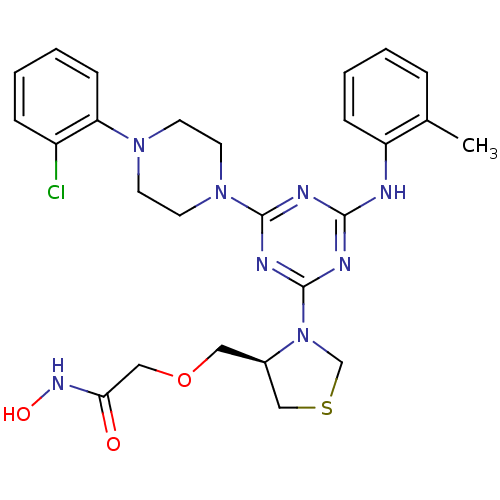 (PDF inhibitor, compound 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Binding assay using PDF inhibitors. | Biochemistry 50: 6642-54 (2011) Article DOI: 10.1021/bi200655g BindingDB Entry DOI: 10.7270/Q2MS3RBV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470589 (CHEMBL331011) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470594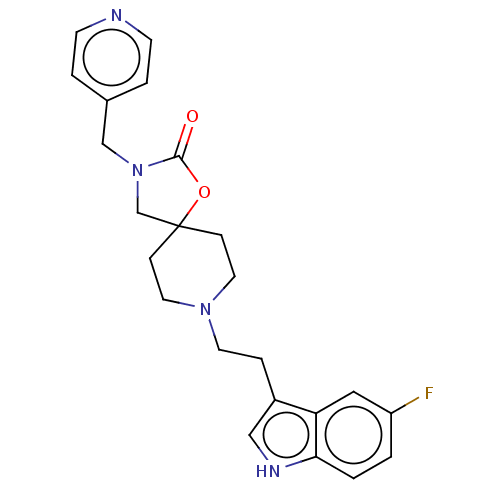 (CHEMBL340405) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470600 (CHEMBL435619) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470602 (CHEMBL340747) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92761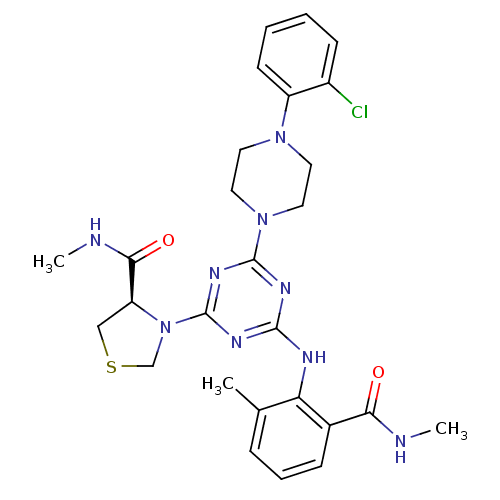 (PDF inhibitor, compound 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Binding assay using PDF inhibitors. | Biochemistry 50: 6642-54 (2011) Article DOI: 10.1021/bi200655g BindingDB Entry DOI: 10.7270/Q2MS3RBV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470586 (CHEMBL435610) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470593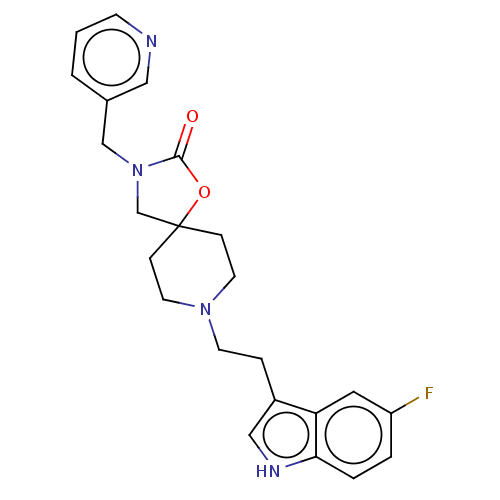 (CHEMBL126158) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92764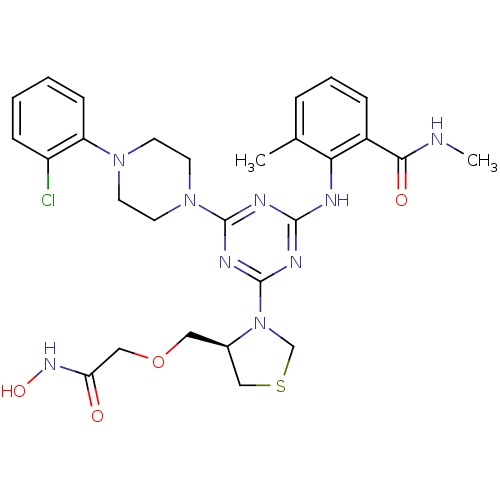 (PDF inhibitor, compound 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 213 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Binding assay using PDF inhibitors. | Biochemistry 50: 6642-54 (2011) Article DOI: 10.1021/bi200655g BindingDB Entry DOI: 10.7270/Q2MS3RBV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptide deformylase (Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92760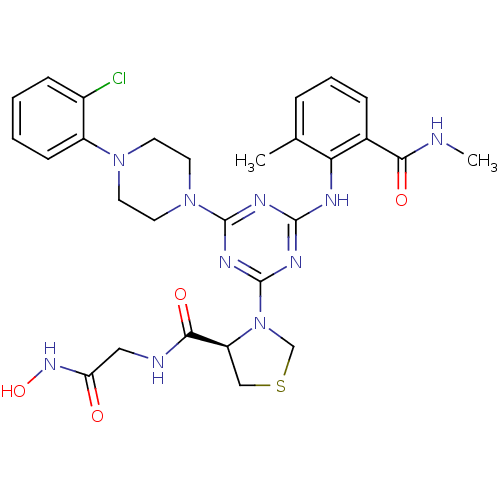 (PDF inhibitor, compound 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 334 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Binding assay using PDF inhibitors. | Biochemistry 50: 6642-54 (2011) Article DOI: 10.1021/bi200655g BindingDB Entry DOI: 10.7270/Q2MS3RBV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8885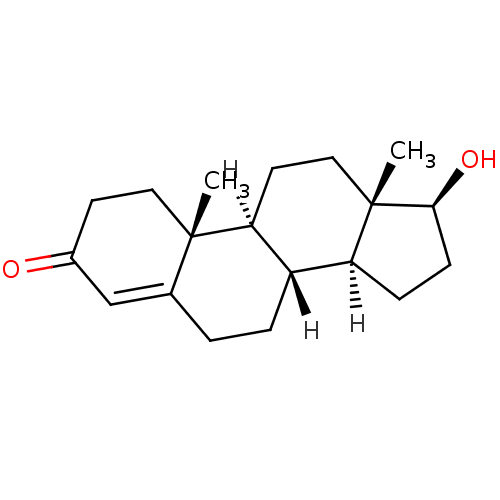 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human placental aromatase Cytochrome P450 19A1 | J Med Chem 26: 50-4 (1983) BindingDB Entry DOI: 10.7270/Q2V125CG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant (Ki) for Cytochrome P450 19A1 | J Med Chem 28: 200-4 (1985) BindingDB Entry DOI: 10.7270/Q2WM1FK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92765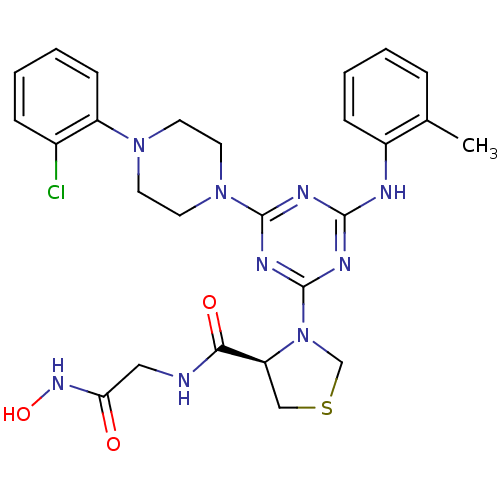 (PDF inhibitor, compound 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Binding assay using PDF inhibitors. | Biochemistry 50: 6642-54 (2011) Article DOI: 10.1021/bi200655g BindingDB Entry DOI: 10.7270/Q2MS3RBV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50015985 (3-Ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant (Ki) for Cytochrome P450 19A1 | J Med Chem 28: 200-4 (1985) BindingDB Entry DOI: 10.7270/Q2WM1FK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470588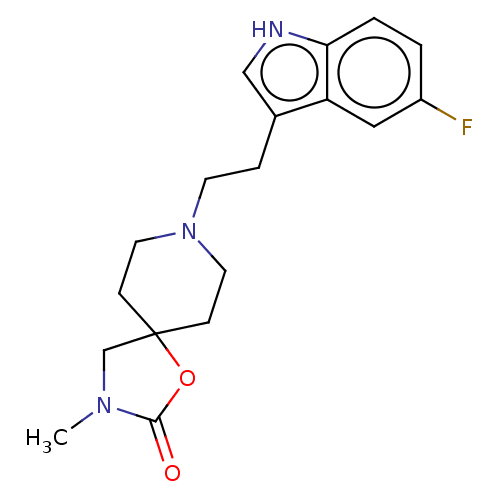 (CHEMBL340548) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50032861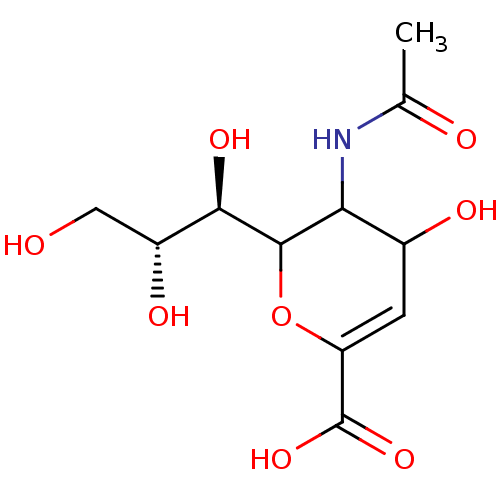 (5-((R)-Acetylamino)-4-hydroxy-6-((1R,2R)-1,2,3-tri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description inhibition of Influenza A Sialidase | J Med Chem 37: 616-24 (1994) Checked by Author BindingDB Entry DOI: 10.7270/Q27P901J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92759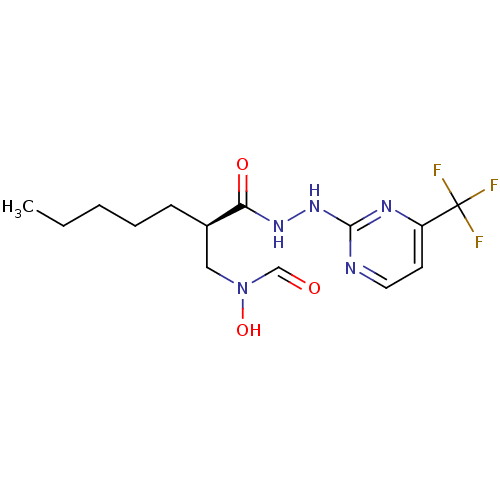 (PDF inhibitor, compound 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Binding assay using PDF inhibitors. | Biochemistry 50: 6642-54 (2011) Article DOI: 10.1021/bi200655g BindingDB Entry DOI: 10.7270/Q2MS3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50470591 (CHEMBL124208) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for binding affinity against Tachykinin receptor 1 from rabbit cortex | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50470591 (CHEMBL124208) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for binding affinity against Tachykinin receptor 1 from guinea pig trachea | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92762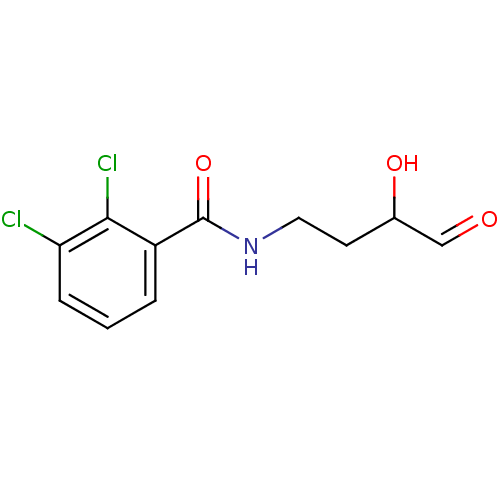 (PDF inhibitor, compound 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Binding assay using PDF inhibitors. | Biochemistry 50: 6642-54 (2011) Article DOI: 10.1021/bi200655g BindingDB Entry DOI: 10.7270/Q2MS3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92763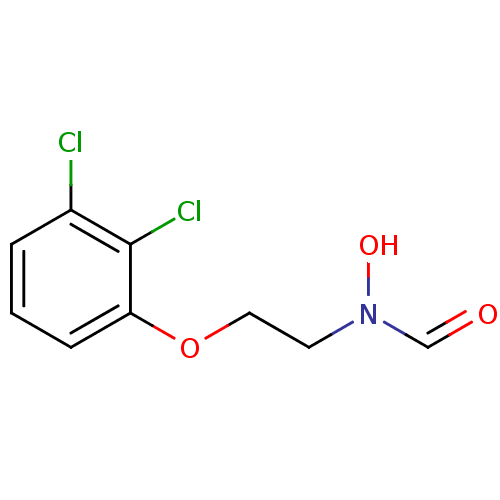 (PDF inhibitor, compound 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Binding assay using PDF inhibitors. | Biochemistry 50: 6642-54 (2011) Article DOI: 10.1021/bi200655g BindingDB Entry DOI: 10.7270/Q2MS3RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (GUINEA PIG) | BDBM50470591 (CHEMBL124208) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for binding affinity against Tachykinin receptor 3 from guinea pig cerebral cortex | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4942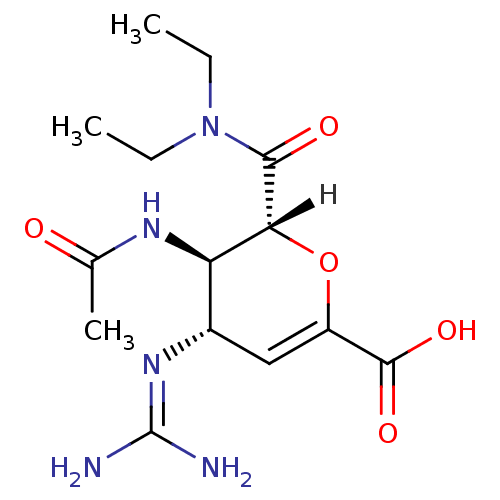 ((2R,3R,4S)-4-carbamimidamido-2-(diethylcarbamoyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4942 ((2R,3R,4S)-4-carbamimidamido-2-(diethylcarbamoyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against influenza A virus sialidase | Bioorg Med Chem Lett 7: 2239-2242 (1997) Article DOI: 10.1016/S0960-894X(97)00399-5 BindingDB Entry DOI: 10.7270/Q2RX9CKB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 446 total ) | Next | Last >> |