 Found 14642 hits with Last Name = 'ye' and Initial = 'n'
Found 14642 hits with Last Name = 'ye' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50184069
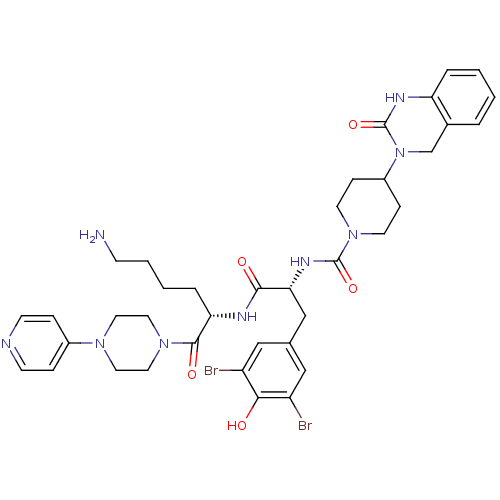
(CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...)Show SMILES NCCCC[C@H](NC(=O)[C@@H](Cc1cc(Br)c(O)c(Br)c1)NC(=O)N1CCC(CC1)N1Cc2ccccc2NC1=O)C(=O)N1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C38H47Br2N9O5/c39-29-21-25(22-30(40)34(29)50)23-33(45-37(53)48-15-10-28(11-16-48)49-24-26-5-1-2-6-31(26)44-38(49)54)35(51)43-32(7-3-4-12-41)36(52)47-19-17-46(18-20-47)27-8-13-42-14-9-27/h1-2,5-6,8-9,13-14,21-22,28,32-33,50H,3-4,7,10-12,15-20,23-24,41H2,(H,43,51)(H,44,54)(H,45,53)/t32-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from human cloned CLR/RAMP1 receptor expressed in E10 cells |
Bioorg Med Chem Lett 16: 2595-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.051
BindingDB Entry DOI: 10.7270/Q2HT2NX8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342639
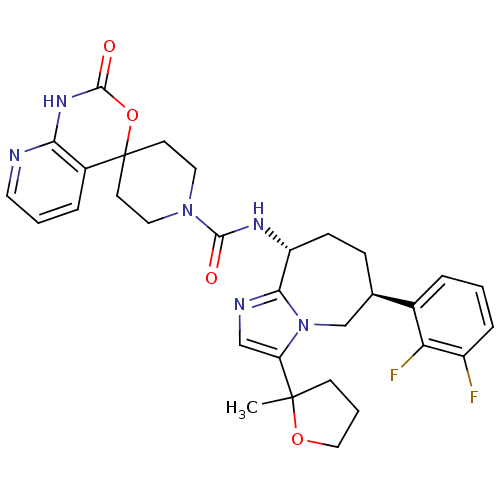
(CHEMBL1770729 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES CC1(CCCO1)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C31H34F2N6O4/c1-30(10-4-16-42-30)24-17-35-27-23(9-8-19(18-39(24)27)20-5-2-7-22(32)25(20)33)36-28(40)38-14-11-31(12-15-38)21-6-3-13-34-26(21)37-29(41)43-31/h2-3,5-7,13,17,19,23H,4,8-12,14-16,18H2,1H3,(H,36,40)(H,34,37,41)/t19-,23-,30?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342638
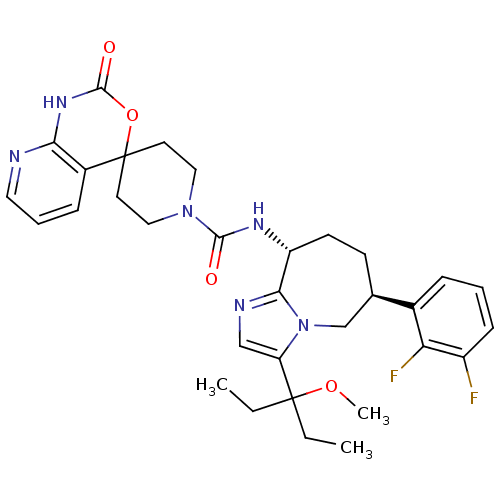
(CHEMBL1770728 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES CCC(CC)(OC)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C32H38F2N6O4/c1-4-31(5-2,43-3)25-18-36-28-24(12-11-20(19-40(25)28)21-8-6-10-23(33)26(21)34)37-29(41)39-16-13-32(14-17-39)22-9-7-15-35-27(22)38-30(42)44-32/h6-10,15,18,20,24H,4-5,11-14,16-17,19H2,1-3H3,(H,37,41)(H,35,38,42)/t20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342625
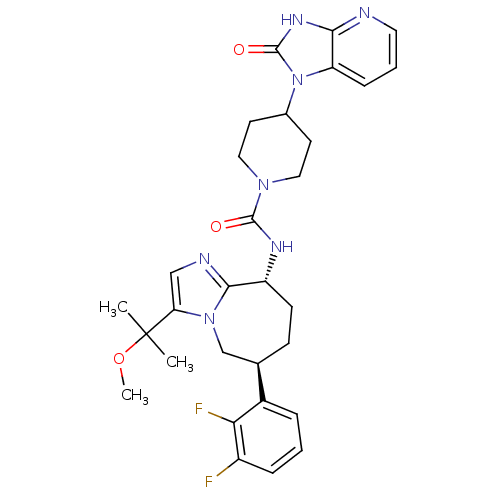
(CHEMBL1770715 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES COC(C)(C)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC(CC1)n1c2cccnc2[nH]c1=O |r| Show InChI InChI=1S/C30H35F2N7O3/c1-30(2,42-3)24-16-34-27-22(10-9-18(17-38(24)27)20-6-4-7-21(31)25(20)32)35-28(40)37-14-11-19(12-15-37)39-23-8-5-13-33-26(23)36-29(39)41/h4-8,13,16,18-19,22H,9-12,14-15,17H2,1-3H3,(H,35,40)(H,33,36,41)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM347461
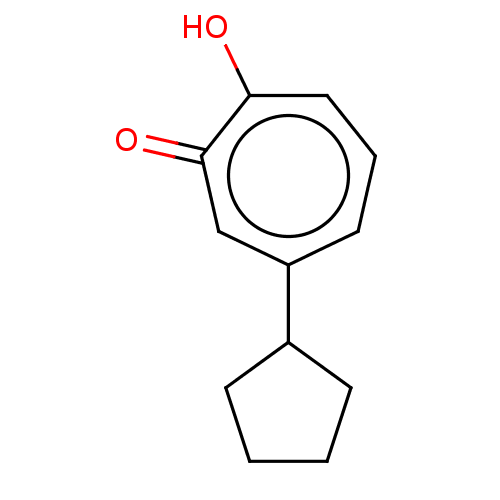
(US9790158, 12)Show InChI InChI=1S/C12H14O2/c13-11-7-3-6-10(8-12(11)14)9-4-1-2-5-9/h3,6-9H,1-2,4-5H2,(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342636
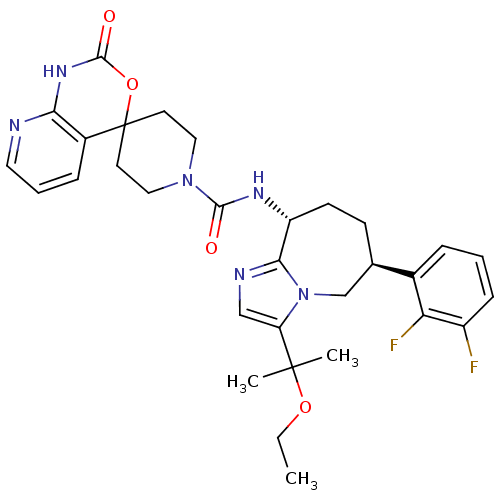
(CHEMBL1770726 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES CCOC(C)(C)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C31H36F2N6O4/c1-4-42-30(2,3)24-17-35-27-23(11-10-19(18-39(24)27)20-7-5-9-22(32)25(20)33)36-28(40)38-15-12-31(13-16-38)21-8-6-14-34-26(21)37-29(41)43-31/h5-9,14,17,19,23H,4,10-13,15-16,18H2,1-3H3,(H,36,40)(H,34,37,41)/t19-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342634
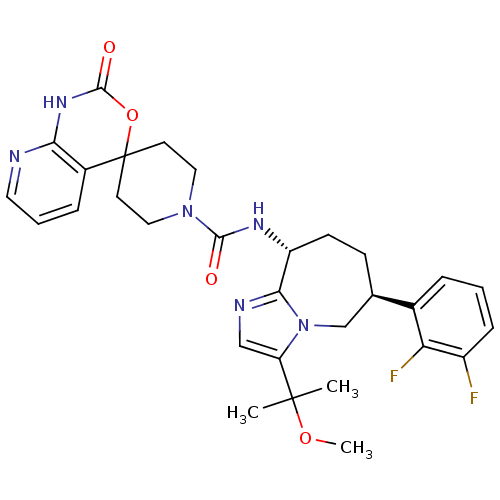
(CHEMBL1770724 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES COC(C)(C)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C30H34F2N6O4/c1-29(2,41-3)23-16-34-26-22(10-9-18(17-38(23)26)19-6-4-8-21(31)24(19)32)35-27(39)37-14-11-30(12-15-37)20-7-5-13-33-25(20)36-28(40)42-30/h4-8,13,16,18,22H,9-12,14-15,17H2,1-3H3,(H,35,39)(H,33,36,40)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50492541
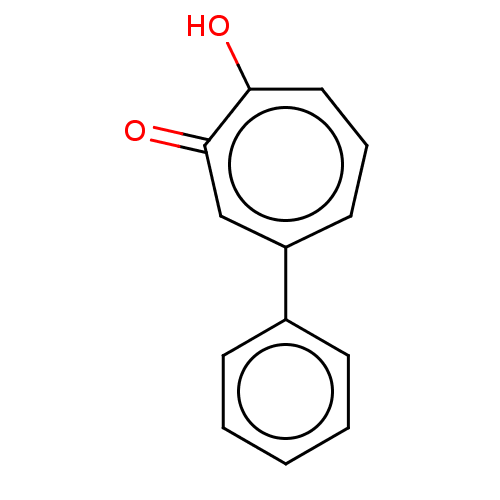
(CHEMBL2408242)Show InChI InChI=1S/C13H10O2/c14-12-8-4-7-11(9-13(12)15)10-5-2-1-3-6-10/h1-9H,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342630
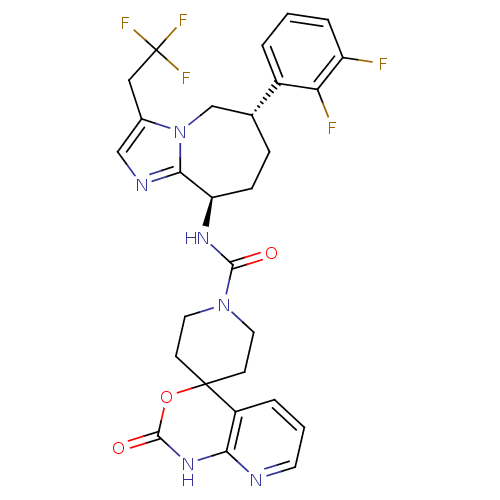
(CHEMBL1770720 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC4(CC3)OC(=O)Nc3ncccc43)c3ncc(CC(F)(F)F)n3C2)c1F |r| Show InChI InChI=1S/C28H27F5N6O3/c29-20-5-1-3-18(22(20)30)16-6-7-21(24-35-14-17(39(24)15-16)13-28(31,32)33)36-25(40)38-11-8-27(9-12-38)19-4-2-10-34-23(19)37-26(41)42-27/h1-5,10,14,16,21H,6-9,11-13,15H2,(H,36,40)(H,34,37,41)/t16-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50220136
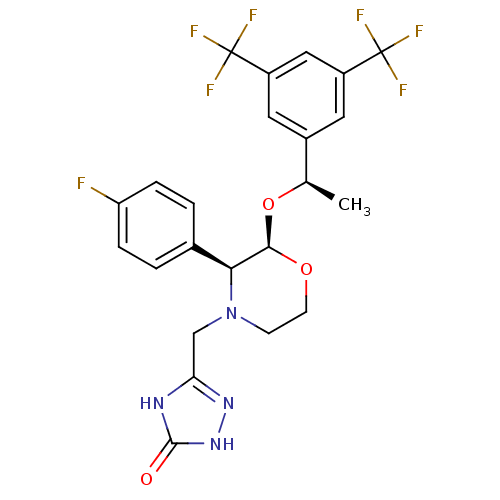
(3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...)Show SMILES C[C@@H](O[C@H]1OCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H21F7N4O3/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)37-20-19(13-2-4-17(24)5-3-13)34(6-7-36-20)11-18-31-21(35)33-32-18/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H2,31,32,33,35)/t12-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Binding affinity to NK1 receptor (unknown origin) |
Bioorg Med Chem Lett 24: 510-4 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.033
BindingDB Entry DOI: 10.7270/Q2KS6T1G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50492540

(CHEMBL2408243)Show InChI InChI=1S/C14H12O3/c1-17-12-7-5-10(6-8-12)11-3-2-4-13(15)14(16)9-11/h2-9H,1H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at dopamine D2 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01192
BindingDB Entry DOI: 10.7270/Q2XD15CH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM348884
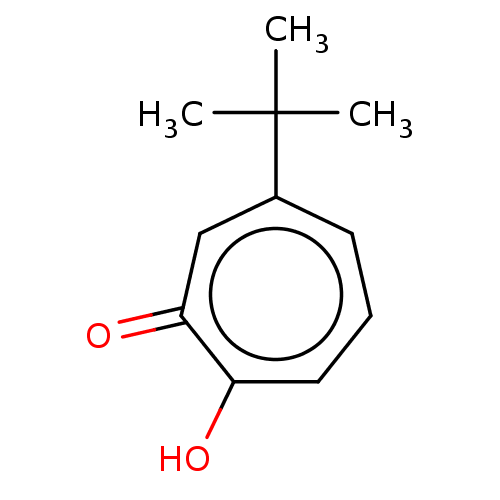
(US9790158, 10)Show InChI InChI=1S/C11H14O2/c1-11(2,3)8-5-4-6-9(12)10(13)7-8/h4-7H,1-3H3,(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030815
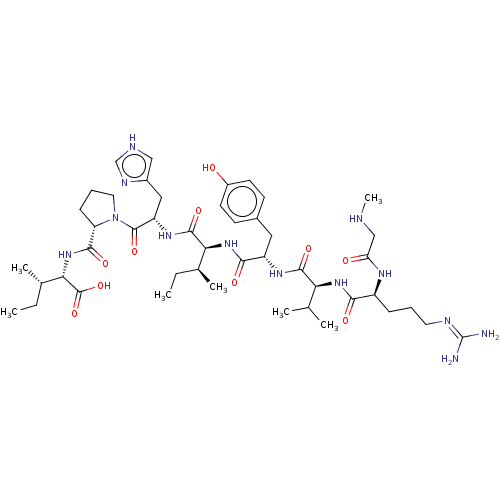
(CHEMBL404594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)[C@@H](C)CC)C(O)=O |wU:8.7,25.27,wD:41.44,4.4,15.16,29.31,45.48,2.2,62.65,(8.44,-18.06,;9.36,-17.24,;9.05,-15.73,;7.88,-15.34,;10.19,-14.7,;9.88,-13.19,;11.03,-12.16,;12.2,-12.55,;10.71,-10.66,;11.74,-9.51,;10.97,-8.18,;9.46,-8.5,;9.33,-10.02,;7.99,-10.79,;7.99,-12.02,;6.66,-10.02,;5.32,-10.79,;5.32,-12.33,;4.07,-13.22,;4.55,-14.68,;6.09,-14.68,;6.56,-13.21,;6.66,-8.48,;5.33,-7.71,;4.43,-8.22,;5.33,-6.17,;3.99,-5.39,;4,-3.85,;5.06,-3.24,;2.66,-3.08,;1.33,-3.85,;-.01,-3.08,;-1.34,-3.84,;-2.67,-3.07,;-2.67,-1.53,;-3.73,-.91,;-1.33,-.76,;,-1.54,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4.01,.77,;5.35,1.53,;5.36,3.07,;4.29,3.69,;6.69,3.84,;6.7,5.37,;8.04,6.14,;8.04,7.68,;9.38,8.44,;9.39,9.99,;10.45,10.6,;8.32,10.61,;8.03,3.06,;8.02,1.52,;6.94,.91,;9.35,.74,;9.34,-.8,;10.22,-1.32,;2.68,1.54,;1.61,.93,;2.69,2.78,;6.66,-5.4,;7.73,-6.01,;6.66,-3.86,;7.73,-3.24,;11.66,-15.17,;11.91,-16.38,;12.58,-14.35,)| Show InChI InChI=1S/C46H73N13O10/c1-8-26(5)37(43(66)55-33(21-29-22-50-24-52-29)44(67)59-19-11-13-34(59)41(64)58-38(45(68)69)27(6)9-2)57-40(63)32(20-28-14-16-30(60)17-15-28)54-42(65)36(25(3)4)56-39(62)31(53-35(61)23-49-7)12-10-18-51-46(47)48/h14-17,22,24-27,31-34,36-38,49,60H,8-13,18-21,23H2,1-7H3,(H,50,52)(H,53,61)(H,54,65)(H,55,66)(H,56,62)(H,57,63)(H,58,64)(H,68,69)(H4,47,48,51)/t26-,27-,31-,32-,33-,34-,36-,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Binding affinity to AT2 receptor (unknown origin) |
ACS Med Chem Lett 5: 1129-32 (2014)
Article DOI: 10.1021/ml500278g
BindingDB Entry DOI: 10.7270/Q2BV7J7R |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342612
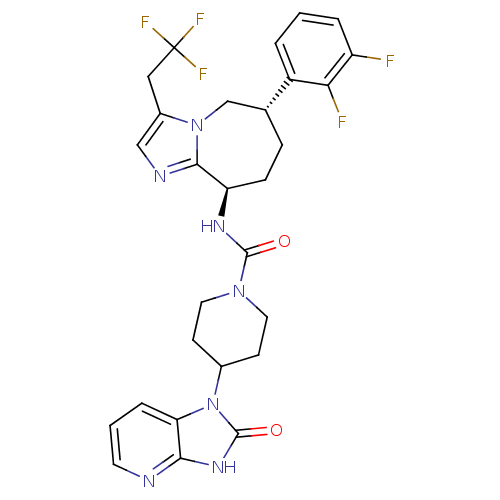
(CHEMBL1770557 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)c3ncc(CC(F)(F)F)n3C2)c1F |r| Show InChI InChI=1S/C28H28F5N7O2/c29-20-4-1-3-19(23(20)30)16-6-7-21(25-35-14-18(39(25)15-16)13-28(31,32)33)36-26(41)38-11-8-17(9-12-38)40-22-5-2-10-34-24(22)37-27(40)42/h1-5,10,14,16-17,21H,6-9,11-13,15H2,(H,36,41)(H,34,37,42)/t16-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342633
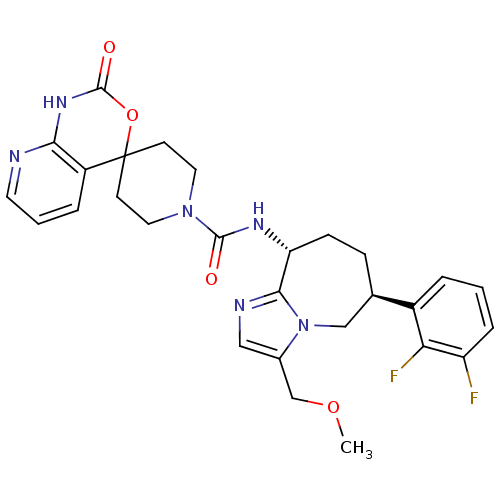
(CHEMBL1770723 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES COCc1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C28H30F2N6O4/c1-39-16-18-14-32-25-22(8-7-17(15-36(18)25)19-4-2-6-21(29)23(19)30)33-26(37)35-12-9-28(10-13-35)20-5-3-11-31-24(20)34-27(38)40-28/h2-6,11,14,17,22H,7-10,12-13,15-16H2,1H3,(H,33,37)(H,31,34,38)/t17-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85177
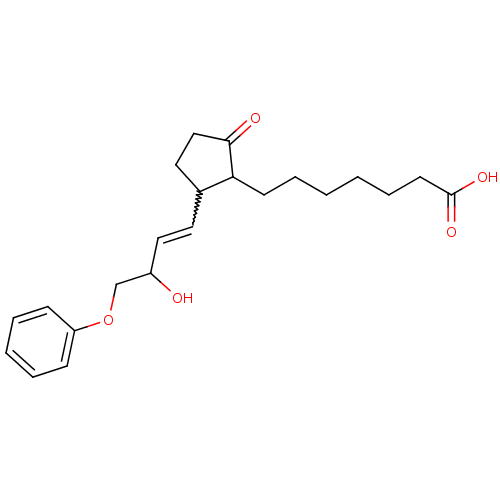
(CAS_80558-61-8 | M&B-28767 | NSC_119139)Show SMILES OC(COc1ccccc1)C=CC1CCC(=O)C1CCCCCCC(O)=O |w:11.12| Show InChI InChI=1S/C22H30O5/c23-18(16-27-19-8-4-3-5-9-19)14-12-17-13-15-21(24)20(17)10-6-1-2-7-11-22(25)26/h3-5,8-9,12,14,17-18,20,23H,1-2,6-7,10-11,13,15-16H2,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50370684
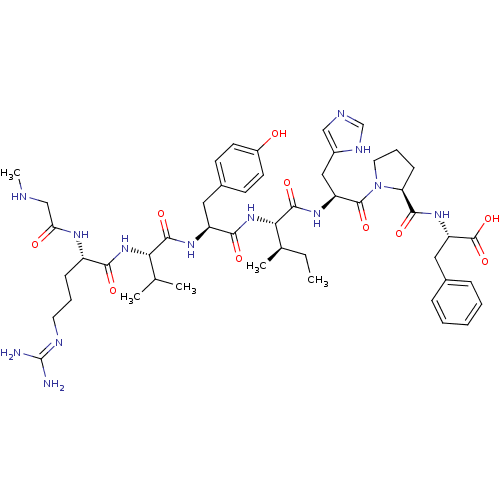
(CHEMBL1791349)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C49H71N13O10/c1-6-29(4)41(46(69)58-36(24-32-25-53-27-55-32)47(70)62-21-11-15-38(62)44(67)59-37(48(71)72)23-30-12-8-7-9-13-30)61-43(66)35(22-31-16-18-33(63)19-17-31)57-45(68)40(28(2)3)60-42(65)34(56-39(64)26-52-5)14-10-20-54-49(50)51/h7-9,12-13,16-19,25,27-29,34-38,40-41,52,63H,6,10-11,14-15,20-24,26H2,1-5H3,(H,53,55)(H,56,64)(H,57,68)(H,58,69)(H,59,67)(H,60,65)(H,61,66)(H,71,72)(H4,50,51,54)/t29-,34+,35+,36+,37+,38+,40+,41+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II receptor, type 1 in rat liver membrane using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 2 hr at ... |
J Med Chem 48: 6620-31 (2005)
Article DOI: 10.1021/jm050280z
BindingDB Entry DOI: 10.7270/Q2HX1DG6 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342637
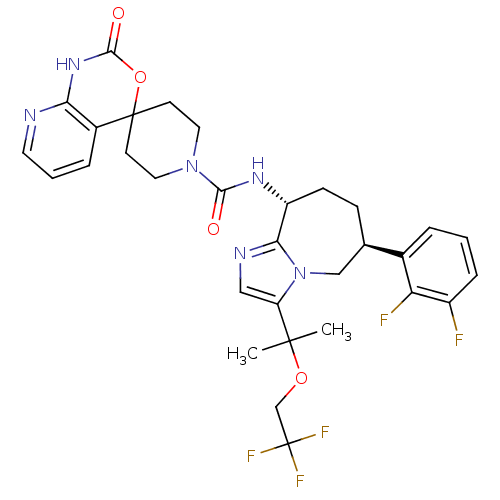
(CHEMBL1770727 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES CC(C)(OCC(F)(F)F)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C31H33F5N6O4/c1-29(2,45-17-31(34,35)36)23-15-38-26-22(9-8-18(16-42(23)26)19-5-3-7-21(32)24(19)33)39-27(43)41-13-10-30(11-14-41)20-6-4-12-37-25(20)40-28(44)46-30/h3-7,12,15,18,22H,8-11,13-14,16-17H2,1-2H3,(H,39,43)(H,37,40,44)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370684

(CHEMBL1791349)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C49H71N13O10/c1-6-29(4)41(46(69)58-36(24-32-25-53-27-55-32)47(70)62-21-11-15-38(62)44(67)59-37(48(71)72)23-30-12-8-7-9-13-30)61-43(66)35(22-31-16-18-33(63)19-17-31)57-45(68)40(28(2)3)60-42(65)34(56-39(64)26-52-5)14-10-20-54-49(50)51/h7-9,12-13,16-19,25,27-29,34-38,40-41,52,63H,6,10-11,14-15,20-24,26H2,1-5H3,(H,53,55)(H,56,64)(H,57,68)(H,58,69)(H,59,67)(H,60,65)(H,61,66)(H,71,72)(H4,50,51,54)/t29-,34+,35+,36+,37+,38+,40+,41+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II receptor, type 2 in pig uterus myometrium using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 1.5 h... |
J Med Chem 48: 6620-31 (2005)
Article DOI: 10.1021/jm050280z
BindingDB Entry DOI: 10.7270/Q2HX1DG6 |
More data for this
Ligand-Target Pair | |
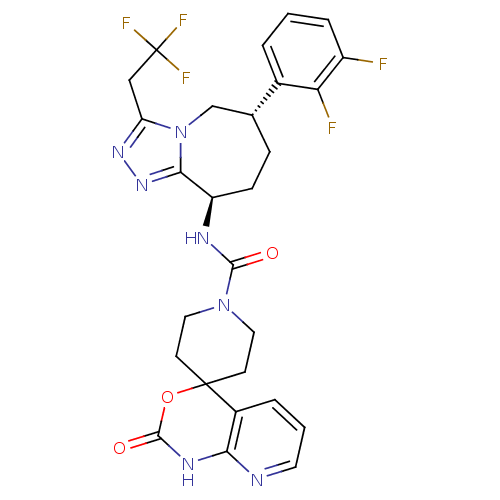
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342632
(CHEMBL1770722 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC4(CC3)OC(=O)Nc3ncccc43)c3nnc(CC(F)(F)F)n3C2)c1F |r| Show InChI InChI=1S/C27H26F5N7O3/c28-18-5-1-3-16(21(18)29)15-6-7-19(23-37-36-20(39(23)14-15)13-27(30,31)32)34-24(40)38-11-8-26(9-12-38)17-4-2-10-33-22(17)35-25(41)42-26/h1-5,10,15,19H,6-9,11-14H2,(H,34,40)(H,33,35,41)/t15-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50224426
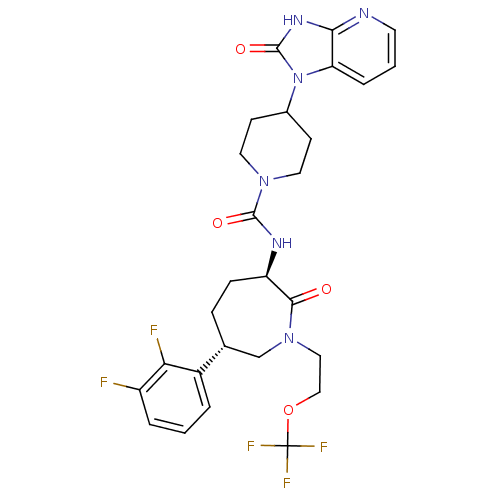
(CHEMBL238276 | N-{(3R,6S)-6-(2,3-difluorophenyl)-2...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)C(=O)N(CCOC(F)(F)F)C2)c1F Show InChI InChI=1S/C27H29F5N6O4/c28-19-4-1-3-18(22(19)29)16-6-7-20(24(39)37(15-16)13-14-42-27(30,31)32)34-25(40)36-11-8-17(9-12-36)38-21-5-2-10-33-23(21)35-26(38)41/h1-5,10,16-17,20H,6-9,11-15H2,(H,34,40)(H,33,35,41)/t16-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGPR from human CL receptor expressed in HEK293 cells |
J Med Chem 50: 5564-7 (2007)
Article DOI: 10.1021/jm070668p
BindingDB Entry DOI: 10.7270/Q28C9W02 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50048302

(4-[(R)-5-(4-Phenyl-3,6-dihydro-2H-pyridin-1-ylmeth...)Show SMILES Oc1ccc(cc1)C1=CCC[C@@H](CN2CCC(=CC2)c2ccccc2)C1 |c:17,t:8| Show InChI InChI=1S/C24H27NO/c26-24-11-9-21(10-12-24)23-8-4-5-19(17-23)18-25-15-13-22(14-16-25)20-6-2-1-3-7-20/h1-3,6-13,19,26H,4-5,14-18H2/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human Dopamine receptor D2L evaluated using [3H]-N-0437 |
J Med Chem 38: 5007-14 (1996)
BindingDB Entry DOI: 10.7270/Q2VQ31SG |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50291261
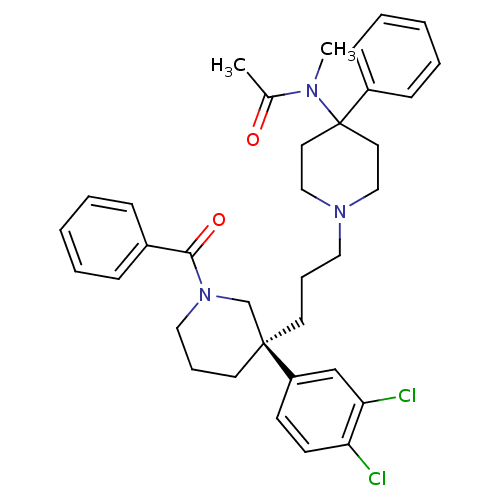
((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...)Show SMILES CN(C(C)=O)C1(CCN(CCC[C@@]2(CCCN(C2)C(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 |r| Show InChI InChI=1S/C35H41Cl2N3O2/c1-27(41)38(2)35(29-13-7-4-8-14-29)19-23-39(24-20-35)21-9-17-34(30-15-16-31(36)32(37)25-30)18-10-22-40(26-34)33(42)28-11-5-3-6-12-28/h3-8,11-16,25H,9-10,17-24,26H2,1-2H3/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor (unknown origin) |
Bioorg Med Chem Lett 24: 510-4 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.033
BindingDB Entry DOI: 10.7270/Q2KS6T1G |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50559185
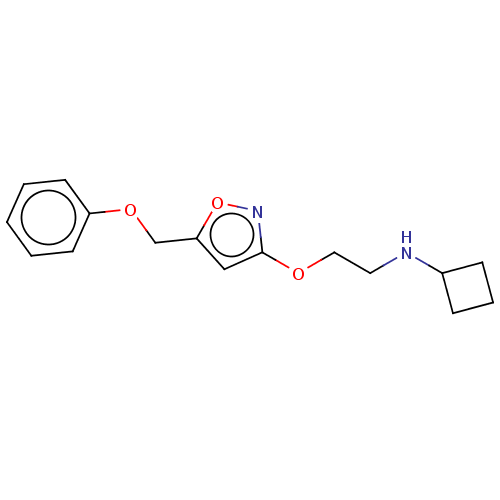
(CHEMBL4743313) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma1 receptor in guinea pig brain homogenate incubated for 150 mins by liquid scintillation counting meth... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01192
BindingDB Entry DOI: 10.7270/Q2XD15CH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM84994
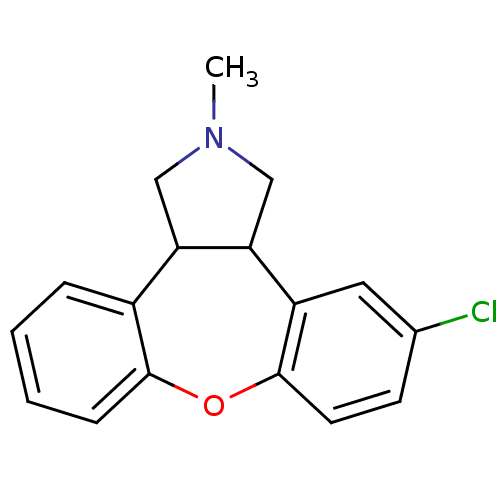
(CAS_163091 | NSC_163091 | ORG-5222)Show InChI InChI=1S/C17H16ClNO/c1-19-9-14-12-4-2-3-5-16(12)20-17-7-6-11(18)8-13(17)15(14)10-19/h2-8,14-15H,9-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 14: 87-96 (1996)
Article DOI: 10.1016/0893-133X(94)00129-N
BindingDB Entry DOI: 10.7270/Q2445K1M |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM347460
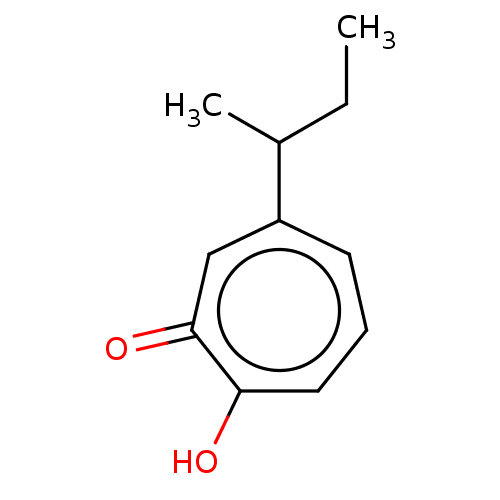
(US9790158, 11)Show InChI InChI=1S/C11H14O2/c1-3-8(2)9-5-4-6-10(12)11(13)7-9/h4-8H,3H2,1-2H3,(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50009338
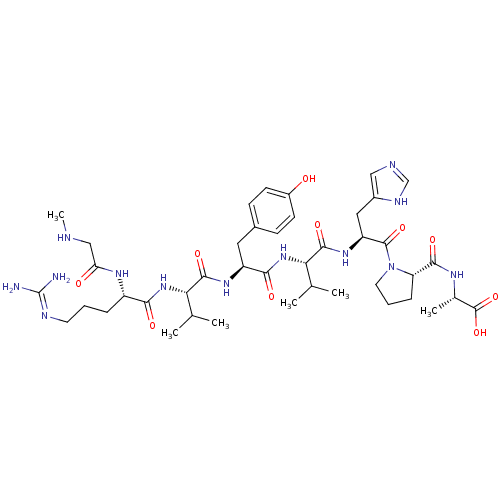
((S)-2-((S)-1-((S)-2-((S)-2-((S)-2-((S)-2-((S)-5-(d...)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |r,wU:60.63,wD:24.23,36.36,43.43,56.59,17.16,6.5,(6.84,-31.4,;5.3,-31.4,;4.53,-30.07,;2.99,-30.07,;2.22,-31.4,;2.22,-28.73,;2.99,-27.4,;4.53,-27.4,;5.3,-26.07,;4.53,-24.73,;5.3,-23.4,;6.84,-23.4,;7.61,-24.73,;7.61,-22.07,;2.22,-26.07,;2.99,-24.73,;.68,-26.07,;-.09,-24.73,;.68,-23.4,;2.22,-23.4,;-.09,-22.07,;-1.63,-24.73,;-2.4,-23.4,;-2.4,-26.07,;-3.94,-26.07,;-4.71,-24.73,;-6.25,-24.73,;-7.02,-23.4,;-8.56,-23.4,;-9.33,-24.73,;-10.87,-24.73,;-8.56,-26.07,;-7.02,-26.07,;-4.71,-27.4,;-6.25,-27.4,;-3.94,-28.73,;-4.71,-30.07,;-6.25,-30.07,;-7.02,-28.73,;-7.02,-31.4,;-3.94,-31.4,;-2.4,-31.4,;-4.71,-32.73,;-3.94,-34.07,;-4.71,-35.4,;-6.25,-35.4,;-7.15,-34.16,;-8.62,-34.63,;-8.62,-36.17,;-7.15,-36.65,;-2.4,-34.07,;-1.63,-32.73,;-1.63,-35.4,;-2.26,-36.81,;-1.12,-37.84,;.22,-37.07,;-.1,-35.56,;.93,-34.42,;.45,-32.95,;2.42,-34.82,;3.5,-33.73,;3.11,-32.24,;5,-34.13,;6.09,-33.04,;5.39,-35.61,)| Show InChI InChI=1S/C42H65N13O10/c1-22(2)33(53-35(58)28(50-32(57)20-45-6)9-7-15-47-42(43)44)38(61)51-29(17-25-11-13-27(56)14-12-25)36(59)54-34(23(3)4)39(62)52-30(18-26-19-46-21-48-26)40(63)55-16-8-10-31(55)37(60)49-24(5)41(64)65/h11-14,19,21-24,28-31,33-34,45,56H,7-10,15-18,20H2,1-6H3,(H,46,48)(H,49,60)(H,50,57)(H,51,61)(H,52,62)(H,53,58)(H,54,59)(H,64,65)(H4,43,44,47)/t24-,28-,29-,30-,31-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Binding affinity to AT1 receptor (unknown origin) |
ACS Med Chem Lett 5: 1129-32 (2014)
Article DOI: 10.1021/ml500278g
BindingDB Entry DOI: 10.7270/Q2BV7J7R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM347454
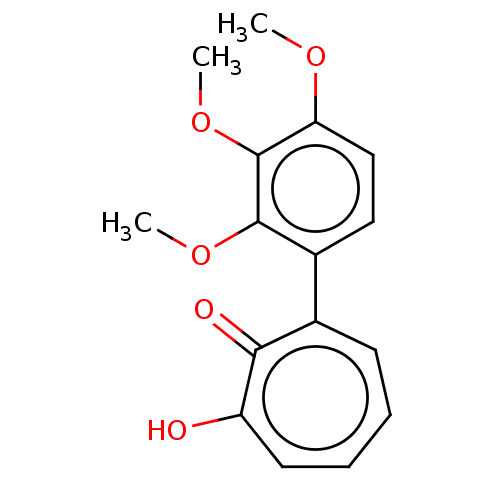
(MO-OH-TM | US9790158, 5)Show InChI InChI=1S/C16H16O5/c1-19-13-9-8-11(15(20-2)16(13)21-3)10-6-4-5-7-12(17)14(10)18/h4-9H,1-3H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM347451
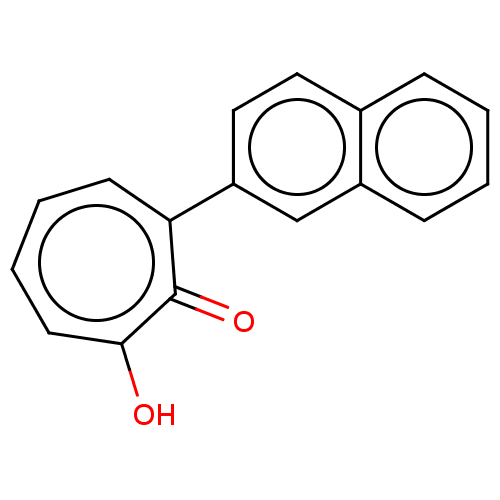
(MO-OH-NAP | US9790158, 2)Show InChI InChI=1S/C17H12O2/c18-16-8-4-3-7-15(17(16)19)14-10-9-12-5-1-2-6-13(12)11-14/h1-11H,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM347330
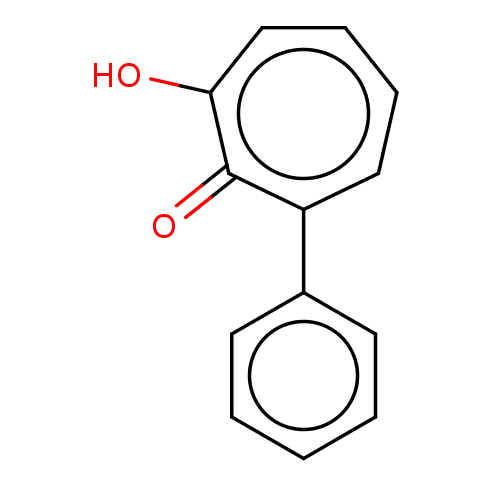
(MO-OH-PHE | US9790158, 1)Show InChI InChI=1S/C13H10O2/c14-12-9-5-4-8-11(13(12)15)10-6-2-1-3-7-10/h1-9H,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50559179
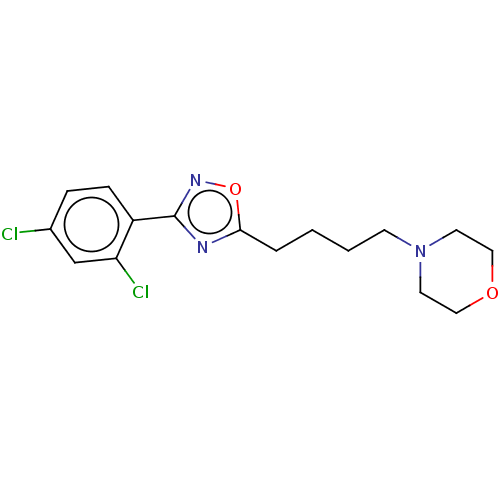
(CHEMBL4792161) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in Dunkin-Hartley guinea pig brain membranes incubated for 180 mins by liquid scintillatio... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01192
BindingDB Entry DOI: 10.7270/Q2XD15CH |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342635
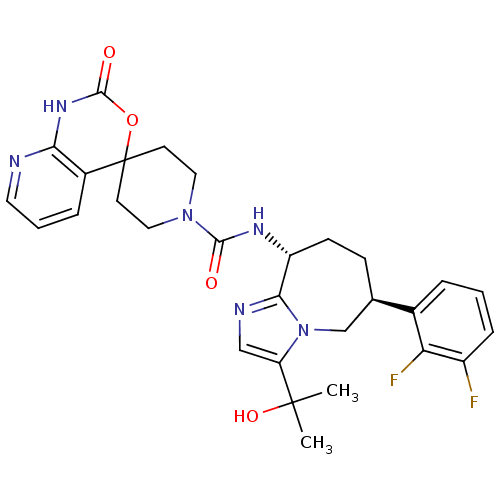
(CHEMBL1770725 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES CC(C)(O)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C29H32F2N6O4/c1-28(2,40)22-15-33-25-21(9-8-17(16-37(22)25)18-5-3-7-20(30)23(18)31)34-26(38)36-13-10-29(11-14-36)19-6-4-12-32-24(19)35-27(39)41-29/h3-7,12,15,17,21,40H,8-11,13-14,16H2,1-2H3,(H,34,38)(H,32,35,39)/t17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50224419
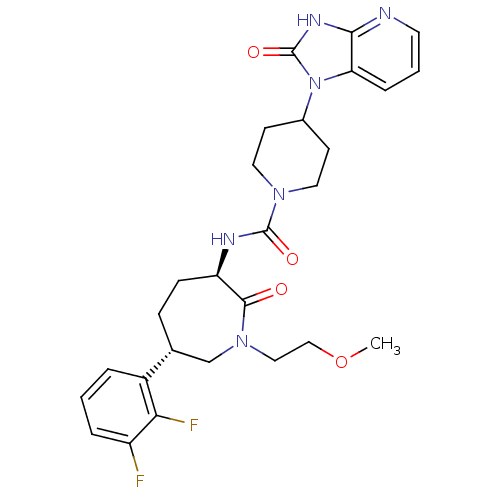
(CHEMBL237662 | N-[(3R,6S)-6-(2,3-difluorophenyl)-1...)Show SMILES COCCN1C[C@@H](CC[C@@H](NC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)C1=O)c1cccc(F)c1F Show InChI InChI=1S/C27H32F2N6O4/c1-39-15-14-34-16-17(19-4-2-5-20(28)23(19)29)7-8-21(25(34)36)31-26(37)33-12-9-18(10-13-33)35-22-6-3-11-30-24(22)32-27(35)38/h2-6,11,17-18,21H,7-10,12-16H2,1H3,(H,31,37)(H,30,32,38)/t17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGPR from human CL receptor expressed in HEK293 cells |
J Med Chem 50: 5564-7 (2007)
Article DOI: 10.1021/jm070668p
BindingDB Entry DOI: 10.7270/Q28C9W02 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370575
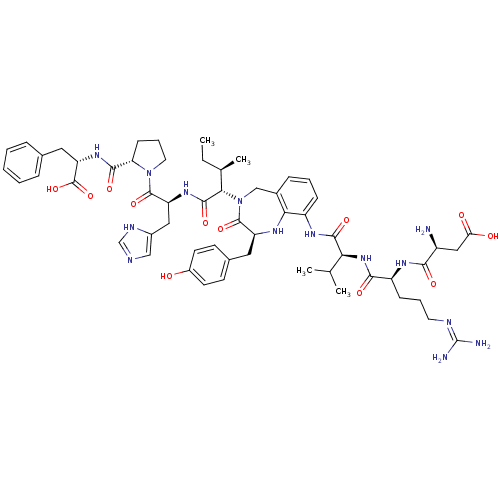
(CHEMBL1791308)Show SMILES CC[C@@H](C)[C@H](N1Cc2cccc(NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)c2N[C@@H](Cc2ccc(O)cc2)C1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:68.73,15.15,55.57,41.42,30.30,2.2,wD:4.4,19.26,72.76,(20.86,-11.16,;20.86,-9.62,;19.5,-8.85,;18.13,-9.62,;19.5,-7.31,;18.13,-6.53,;17.29,-7.83,;15.7,-8,;15.29,-9.47,;13.78,-9.86,;12.67,-8.77,;13.1,-7.3,;11.74,-6.61,;10.41,-7.27,;10.41,-8.62,;9.06,-6.59,;7.72,-7.27,;6.37,-6.59,;6.4,-5.24,;5.03,-7.24,;5.03,-8.61,;6.22,-9.28,;6.2,-10.63,;7.41,-11.31,;7.4,-12.65,;6.2,-13.32,;8.58,-13.35,;3.69,-6.58,;2.35,-7.24,;2.35,-8.58,;.99,-6.58,;-.35,-7.21,;1.02,-5.24,;-.33,-4.54,;-1.69,-5.21,;-.32,-3.2,;9.07,-5.22,;10.45,-4.54,;7.71,-4.51,;14.61,-6.9,;14.8,-5.36,;16.14,-4.57,;15.99,-3.04,;16.77,-1.69,;15.97,-.38,;16.74,.97,;18.32,.97,;19.09,2.32,;19.13,-.34,;18.35,-1.69,;17.63,-5.09,;18.76,-3.99,;20.86,-6.53,;20.86,-5,;22.25,-7.3,;23.67,-6.69,;23.73,-5.14,;24.52,-3.79,;26.07,-3.63,;26.4,-2.11,;25.03,-1.35,;23.84,-2.38,;25,-7.41,;24.92,-8.77,;26.39,-6.67,;25.88,-5.19,;27.18,-4.28,;28.45,-5.19,;27.97,-6.67,;29.35,-7.43,;29.34,-8.98,;30.71,-6.67,;32.02,-7.41,;31.94,-8.77,;33.27,-9.48,;34.67,-8.88,;35.98,-9.63,;35.9,-10.95,;34.48,-11.61,;33.19,-10.87,;33.44,-6.8,;34.77,-7.53,;33.52,-5.43,)| Show InChI InChI=1S/C57H76N14O12/c1-5-32(4)48(53(79)67-42(26-36-28-61-30-63-36)54(80)70-23-11-17-44(70)51(77)68-43(56(82)83)25-33-12-7-6-8-13-33)71-29-35-14-9-15-39(47(35)64-41(55(71)81)24-34-18-20-37(72)21-19-34)65-52(78)46(31(2)3)69-50(76)40(16-10-22-62-57(59)60)66-49(75)38(58)27-45(73)74/h6-9,12-15,18-21,28,30-32,38,40-44,46,48,64,72H,5,10-11,16-17,22-27,29,58H2,1-4H3,(H,61,63)(H,65,78)(H,66,75)(H,67,79)(H,68,77)(H,69,76)(H,73,74)(H,82,83)(H4,59,60,62)/t32-,38+,40+,41+,42+,43+,44+,46+,48+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370575

(CHEMBL1791308)Show SMILES CC[C@@H](C)[C@H](N1Cc2cccc(NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)c2N[C@@H](Cc2ccc(O)cc2)C1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:68.73,15.15,55.57,41.42,30.30,2.2,wD:4.4,19.26,72.76,(20.86,-11.16,;20.86,-9.62,;19.5,-8.85,;18.13,-9.62,;19.5,-7.31,;18.13,-6.53,;17.29,-7.83,;15.7,-8,;15.29,-9.47,;13.78,-9.86,;12.67,-8.77,;13.1,-7.3,;11.74,-6.61,;10.41,-7.27,;10.41,-8.62,;9.06,-6.59,;7.72,-7.27,;6.37,-6.59,;6.4,-5.24,;5.03,-7.24,;5.03,-8.61,;6.22,-9.28,;6.2,-10.63,;7.41,-11.31,;7.4,-12.65,;6.2,-13.32,;8.58,-13.35,;3.69,-6.58,;2.35,-7.24,;2.35,-8.58,;.99,-6.58,;-.35,-7.21,;1.02,-5.24,;-.33,-4.54,;-1.69,-5.21,;-.32,-3.2,;9.07,-5.22,;10.45,-4.54,;7.71,-4.51,;14.61,-6.9,;14.8,-5.36,;16.14,-4.57,;15.99,-3.04,;16.77,-1.69,;15.97,-.38,;16.74,.97,;18.32,.97,;19.09,2.32,;19.13,-.34,;18.35,-1.69,;17.63,-5.09,;18.76,-3.99,;20.86,-6.53,;20.86,-5,;22.25,-7.3,;23.67,-6.69,;23.73,-5.14,;24.52,-3.79,;26.07,-3.63,;26.4,-2.11,;25.03,-1.35,;23.84,-2.38,;25,-7.41,;24.92,-8.77,;26.39,-6.67,;25.88,-5.19,;27.18,-4.28,;28.45,-5.19,;27.97,-6.67,;29.35,-7.43,;29.34,-8.98,;30.71,-6.67,;32.02,-7.41,;31.94,-8.77,;33.27,-9.48,;34.67,-8.88,;35.98,-9.63,;35.9,-10.95,;34.48,-11.61,;33.19,-10.87,;33.44,-6.8,;34.77,-7.53,;33.52,-5.43,)| Show InChI InChI=1S/C57H76N14O12/c1-5-32(4)48(53(79)67-42(26-36-28-61-30-63-36)54(80)70-23-11-17-44(70)51(77)68-43(56(82)83)25-33-12-7-6-8-13-33)71-29-35-14-9-15-39(47(35)64-41(55(71)81)24-34-18-20-37(72)21-19-34)65-52(78)46(31(2)3)69-50(76)40(16-10-22-62-57(59)60)66-49(75)38(58)27-45(73)74/h6-9,12-15,18-21,28,30-32,38,40-44,46,48,64,72H,5,10-11,16-17,22-27,29,58H2,1-4H3,(H,61,63)(H,65,78)(H,66,75)(H,67,79)(H,68,77)(H,69,76)(H,73,74)(H,82,83)(H4,59,60,62)/t32-,38+,40+,41+,42+,43+,44+,46+,48+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM85603
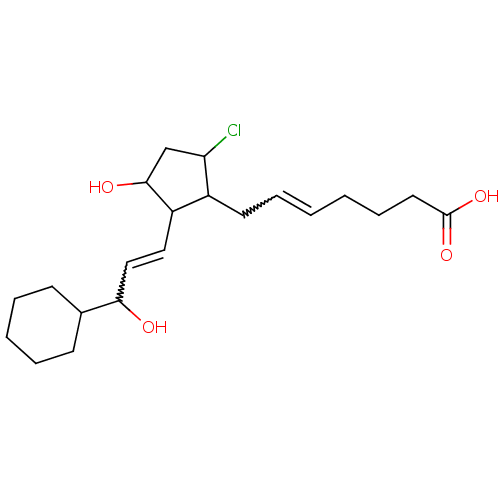
(CAS_5311503 | NSC_5311503 | ZK110841)Show SMILES OC(C=CC1C(O)CC(Cl)C1CC=CCCCC(O)=O)C1CCCCC1 |w:2.1,12.12| Show InChI InChI=1S/C21H33ClO4/c22-18-14-20(24)17(12-13-19(23)15-8-4-3-5-9-15)16(18)10-6-1-2-7-11-21(25)26/h1,6,12-13,15-20,23-24H,2-5,7-11,14H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50277668
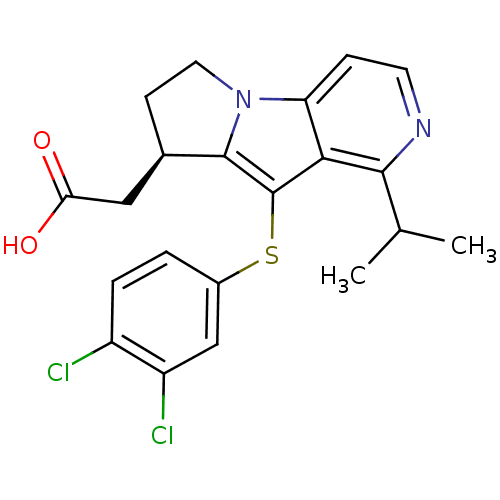
((R)-2-(9-(3,4-dichlorophenylthio)-1-isopropyl-7,8-...)Show SMILES CC(C)c1nccc2n3CC[C@H](CC(O)=O)c3c(Sc3ccc(Cl)c(Cl)c3)c12 |r| Show InChI InChI=1S/C21H20Cl2N2O2S/c1-11(2)19-18-16(5-7-24-19)25-8-6-12(9-17(26)27)20(25)21(18)28-13-3-4-14(22)15(23)10-13/h3-5,7,10-12H,6,8-9H2,1-2H3,(H,26,27)/t12-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human recombinant DP1 receptor expressed in HEK293 cells by scintillation proximity assay |
Bioorg Med Chem Lett 19: 2125-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.010
BindingDB Entry DOI: 10.7270/Q2W9593R |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1
(Homo sapiens (Human)) | BDBM50601067
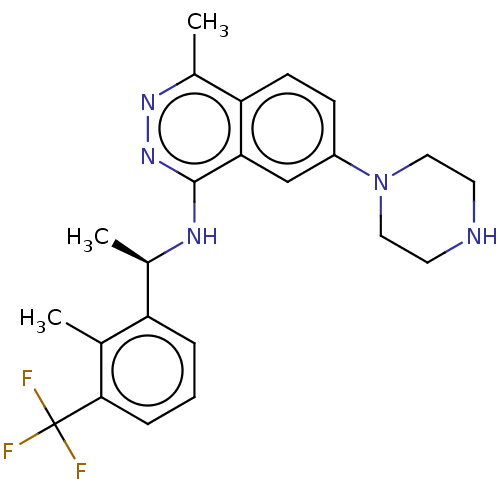
(CHEMBL5208088 | US11702418, Example 6-4)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCNCC1)c1cccc(c1C)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00741
BindingDB Entry DOI: 10.7270/Q2XD15Q0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85183
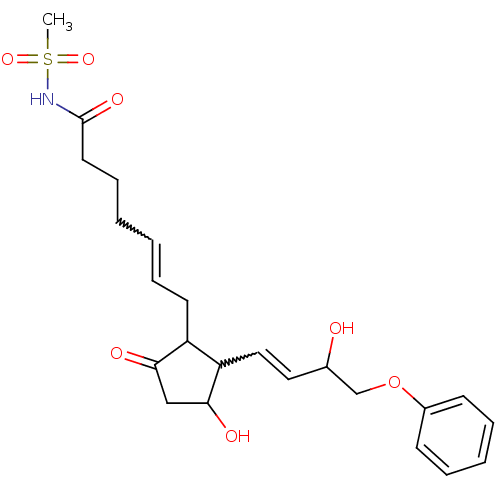
(CAS_60325-46-4 | NSC_43251 | SULPROSTONE)Show SMILES CS(=O)(=O)NC(=O)CCCC=CCC1C(C=CC(O)COc2ccccc2)C(O)CC1=O |w:10.9,15.14| Show InChI InChI=1S/C23H31NO7S/c1-32(29,30)24-23(28)12-8-3-2-7-11-19-20(22(27)15-21(19)26)14-13-17(25)16-31-18-9-5-4-6-10-18/h2,4-7,9-10,13-14,17,19-20,22,25,27H,3,8,11-12,15-16H2,1H3,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1
(Homo sapiens (Human)) | BDBM50601075
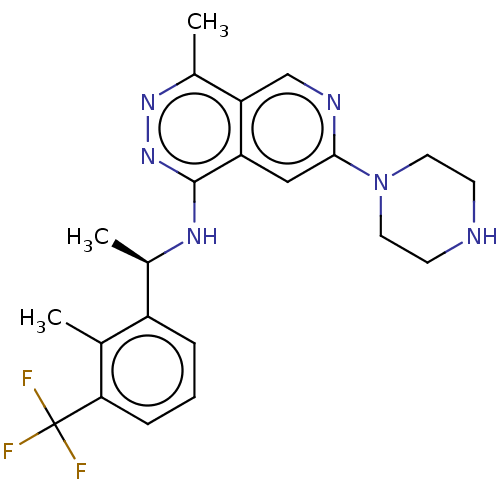
(CHEMBL5202472 | US11702418, Example 6-9)Show SMILES C[C@@H](Nc1nnc(C)c2cnc(cc12)N1CCNCC1)c1cccc(c1C)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00741
BindingDB Entry DOI: 10.7270/Q2XD15Q0 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342624
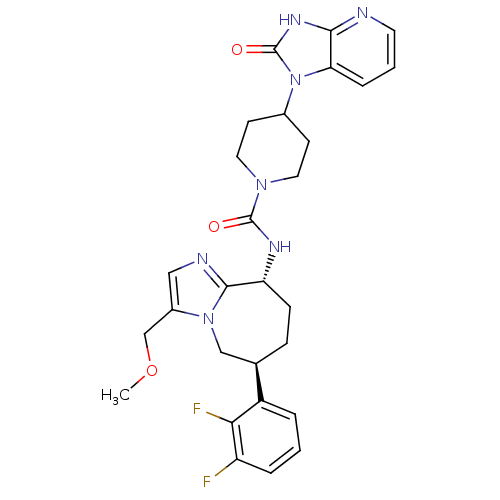
(CHEMBL1770714 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES COCc1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC(CC1)n1c2cccnc2[nH]c1=O |r| Show InChI InChI=1S/C28H31F2N7O3/c1-40-16-19-14-32-26-22(8-7-17(15-36(19)26)20-4-2-5-21(29)24(20)30)33-27(38)35-12-9-18(10-13-35)37-23-6-3-11-31-25(23)34-28(37)39/h2-6,11,14,17-18,22H,7-10,12-13,15-16H2,1H3,(H,33,38)(H,31,34,39)/t17-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50009338

((S)-2-((S)-1-((S)-2-((S)-2-((S)-2-((S)-2-((S)-5-(d...)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |r,wU:60.63,wD:24.23,36.36,43.43,56.59,17.16,6.5,(6.84,-31.4,;5.3,-31.4,;4.53,-30.07,;2.99,-30.07,;2.22,-31.4,;2.22,-28.73,;2.99,-27.4,;4.53,-27.4,;5.3,-26.07,;4.53,-24.73,;5.3,-23.4,;6.84,-23.4,;7.61,-24.73,;7.61,-22.07,;2.22,-26.07,;2.99,-24.73,;.68,-26.07,;-.09,-24.73,;.68,-23.4,;2.22,-23.4,;-.09,-22.07,;-1.63,-24.73,;-2.4,-23.4,;-2.4,-26.07,;-3.94,-26.07,;-4.71,-24.73,;-6.25,-24.73,;-7.02,-23.4,;-8.56,-23.4,;-9.33,-24.73,;-10.87,-24.73,;-8.56,-26.07,;-7.02,-26.07,;-4.71,-27.4,;-6.25,-27.4,;-3.94,-28.73,;-4.71,-30.07,;-6.25,-30.07,;-7.02,-28.73,;-7.02,-31.4,;-3.94,-31.4,;-2.4,-31.4,;-4.71,-32.73,;-3.94,-34.07,;-4.71,-35.4,;-6.25,-35.4,;-7.15,-34.16,;-8.62,-34.63,;-8.62,-36.17,;-7.15,-36.65,;-2.4,-34.07,;-1.63,-32.73,;-1.63,-35.4,;-2.26,-36.81,;-1.12,-37.84,;.22,-37.07,;-.1,-35.56,;.93,-34.42,;.45,-32.95,;2.42,-34.82,;3.5,-33.73,;3.11,-32.24,;5,-34.13,;6.09,-33.04,;5.39,-35.61,)| Show InChI InChI=1S/C42H65N13O10/c1-22(2)33(53-35(58)28(50-32(57)20-45-6)9-7-15-47-42(43)44)38(61)51-29(17-25-11-13-27(56)14-12-25)36(59)54-34(23(3)4)39(62)52-30(18-26-19-46-21-48-26)40(63)55-16-8-10-31(55)37(60)49-24(5)41(64)65/h11-14,19,21-24,28-31,33-34,45,56H,7-10,15-18,20H2,1-6H3,(H,46,48)(H,49,60)(H,50,57)(H,51,61)(H,52,62)(H,53,58)(H,54,59)(H,64,65)(H4,43,44,47)/t24-,28-,29-,30-,31-,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Binding affinity to AT2 receptor (unknown origin) |
ACS Med Chem Lett 5: 1129-32 (2014)
Article DOI: 10.1021/ml500278g
BindingDB Entry DOI: 10.7270/Q2BV7J7R |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50277629

(2-(9-(2,4-dichlorophenylthio)-1-isopropyl-7,8-dihy...)Show SMILES CC(C)c1nccc2n3CCC(CC(O)=O)c3c(Sc3ccc(Cl)cc3Cl)c12 Show InChI InChI=1S/C21H20Cl2N2O2S/c1-11(2)19-18-15(5-7-24-19)25-8-6-12(9-17(26)27)20(25)21(18)28-16-4-3-13(22)10-14(16)23/h3-5,7,10-12H,6,8-9H2,1-2H3,(H,26,27) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human recombinant DP1 receptor expressed in HEK293 cells by scintillation proximity assay |
Bioorg Med Chem Lett 19: 2125-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.010
BindingDB Entry DOI: 10.7270/Q2W9593R |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50277631

(2-(1-isopropyl-9-(2,4,5-trichlorophenylthio)-7,8-d...)Show SMILES CC(C)c1nccc2n3CCC(CC(O)=O)c3c(Sc3cc(Cl)c(Cl)cc3Cl)c12 Show InChI InChI=1S/C21H19Cl3N2O2S/c1-10(2)19-18-15(3-5-25-19)26-6-4-11(7-17(27)28)20(26)21(18)29-16-9-13(23)12(22)8-14(16)24/h3,5,8-11H,4,6-7H2,1-2H3,(H,27,28) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human recombinant DP1 receptor expressed in HEK293 cells by scintillation proximity assay |
Bioorg Med Chem Lett 19: 2125-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.010
BindingDB Entry DOI: 10.7270/Q2W9593R |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50445820

(CHEMBL3104783)Show SMILES CN(Cc1ccc(Cl)cc1)C(=O)[C@@H](CCN1CCC2(CC1)OC(=O)N(C)c1ccc(F)cc21)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C31H31Cl3FN3O3/c1-36(19-20-3-6-22(32)7-4-20)29(39)24(21-5-9-26(33)27(34)17-21)11-14-38-15-12-31(13-16-38)25-18-23(35)8-10-28(25)37(2)30(40)41-31/h3-10,17-18,24H,11-16,19H2,1-2H3/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SR142801 from human NK3 receptor expressed in recombinant CHO cells |
ACS Med Chem Lett 5: 550-5 (2014)
Article DOI: 10.1021/ml400528y
BindingDB Entry DOI: 10.7270/Q2G162CB |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Sus scrofa) | BDBM50156173
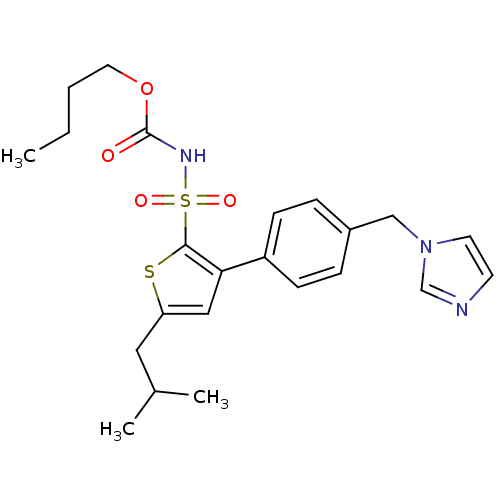
((1-((2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1sc(CC(C)C)cc1-c1ccc(Cn2ccnc2)cc1 Show InChI InChI=1S/C23H29N3O4S2/c1-4-5-12-30-23(27)25-32(28,29)22-21(14-20(31-22)13-17(2)3)19-8-6-18(7-9-19)15-26-11-10-24-16-26/h6-11,14,16-17H,4-5,12-13,15H2,1-3H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125]Ang2 from AT2 receptor in pig uterus membrane |
Bioorg Med Chem 16: 6841-9 (2008)
Article DOI: 10.1016/j.bmc.2008.05.066
BindingDB Entry DOI: 10.7270/Q22F7N7C |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50156173

((1-((2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1sc(CC(C)C)cc1-c1ccc(Cn2ccnc2)cc1 Show InChI InChI=1S/C23H29N3O4S2/c1-4-5-12-30-23(27)25-32(28,29)22-21(14-20(31-22)13-17(2)3)19-8-6-18(7-9-19)15-26-11-10-24-16-26/h6-11,14,16-17H,4-5,12-13,15H2,1-3H3,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Binding affinity to AT2 receptor (unknown origin) |
ACS Med Chem Lett 5: 1129-32 (2014)
Article DOI: 10.1021/ml500278g
BindingDB Entry DOI: 10.7270/Q2BV7J7R |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM85175
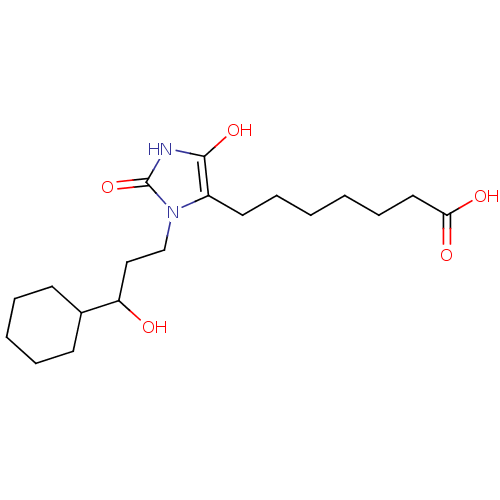
(BW245C | CAS_72814-32-5 | NSC_3080928)Show SMILES OC(CCn1c(CCCCCCC(O)=O)c(O)[nH]c1=O)C1CCCCC1 Show InChI InChI=1S/C19H32N2O5/c22-16(14-8-4-3-5-9-14)12-13-21-15(18(25)20-19(21)26)10-6-1-2-7-11-17(23)24/h14,16,22,25H,1-13H2,(H,20,26)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data