 Found 3176 hits with Last Name = 'meanwell' and Initial = 'na'
Found 3176 hits with Last Name = 'meanwell' and Initial = 'na' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50095105
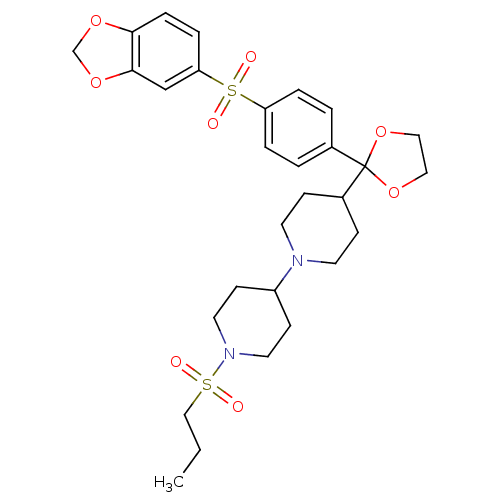
(4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...)Show SMILES CCCS(=O)(=O)N1CCC(CC1)N1CCC(CC1)C1(OCCO1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O8S2/c1-2-19-40(32,33)31-15-11-24(12-16-31)30-13-9-23(10-14-30)29(38-17-18-39-29)22-3-5-25(6-4-22)41(34,35)26-7-8-27-28(20-26)37-21-36-27/h3-8,20,23-24H,2,9-19,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50095523
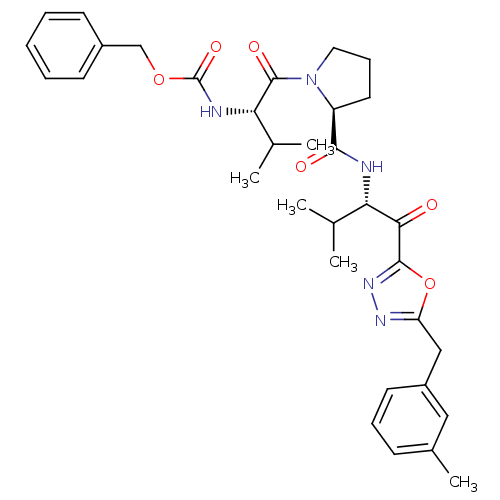
(CHEMBL285231 | [(S)-2-methyl-1-((S)-2-{(S)-2-methy...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)c1nnc(Cc2cccc(C)c2)o1 Show InChI InChI=1S/C33H41N5O6/c1-20(2)27(29(39)31-37-36-26(44-31)18-24-14-9-11-22(5)17-24)34-30(40)25-15-10-16-38(25)32(41)28(21(3)4)35-33(42)43-19-23-12-7-6-8-13-23/h6-9,11-14,17,20-21,25,27-28H,10,15-16,18-19H2,1-5H3,(H,34,40)(H,35,42)/t25-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123490
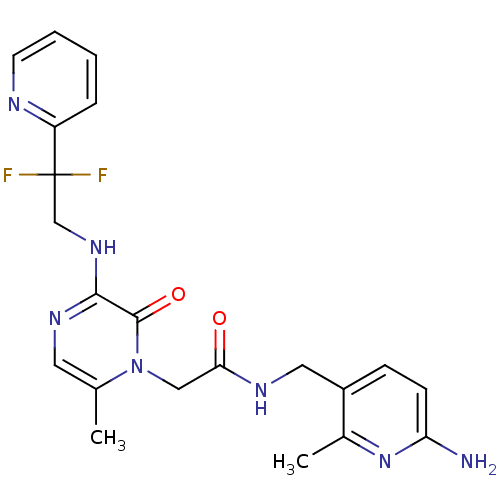
(CHEMBL143418 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1cnc(NCC(F)(F)c2ccccn2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C21H23F2N7O2/c1-13-9-27-19(28-12-21(22,23)16-5-3-4-8-25-16)20(32)30(13)11-18(31)26-10-15-6-7-17(24)29-14(15)2/h3-9H,10-12H2,1-2H3,(H2,24,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Thrombin (unknown origin) |
J Med Chem 58: 8315-59 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00258
BindingDB Entry DOI: 10.7270/Q22J6DPZ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123504
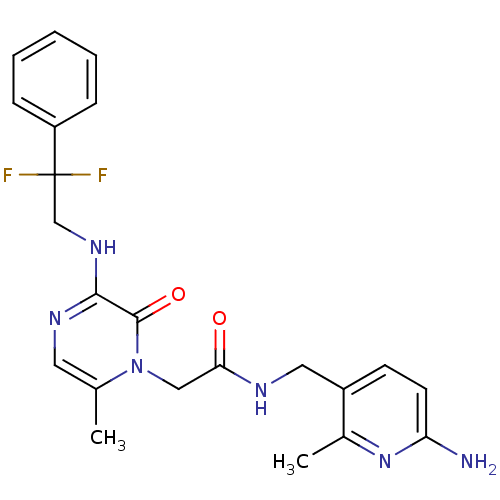
(CHEMBL142546 | N-((6-amino-2-methylpyridin-3-yl)me...)Show SMILES Cc1cnc(NCC(F)(F)c2ccccc2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C22H24F2N6O2/c1-14-10-27-20(28-13-22(23,24)17-6-4-3-5-7-17)21(32)30(14)12-19(31)26-11-16-8-9-18(25)29-15(16)2/h3-10H,11-13H2,1-2H3,(H2,25,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Thrombin (unknown origin) |
J Med Chem 58: 8315-59 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00258
BindingDB Entry DOI: 10.7270/Q22J6DPZ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123496

(CHEMBL143138 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)cnc(NCCc2ccccn2)c1=O Show InChI InChI=1S/C21H25N7O2/c1-14-11-26-20(24-10-8-17-5-3-4-9-23-17)21(30)28(14)13-19(29)25-12-16-6-7-18(22)27-15(16)2/h3-7,9,11H,8,10,12-13H2,1-2H3,(H2,22,27)(H,24,26)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Thrombin (unknown origin) |
J Med Chem 58: 8315-59 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00258
BindingDB Entry DOI: 10.7270/Q22J6DPZ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50517882
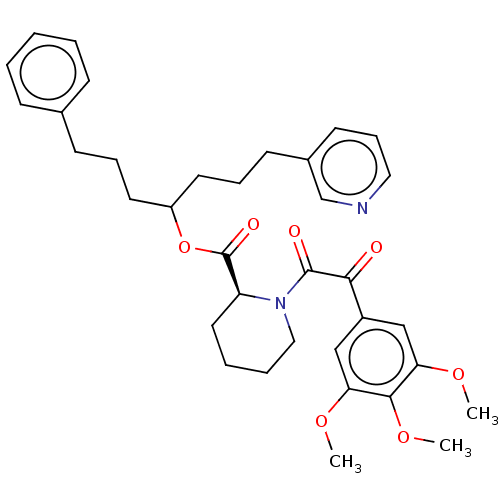
(CHEMBL4449096)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1CCCC[C@H]1C(=O)OC(CCCc1ccccc1)CCCc1cccnc1 |r| Show InChI InChI=1S/C35H42N2O7/c1-41-30-22-27(23-31(42-2)33(30)43-3)32(38)34(39)37-21-8-7-19-29(37)35(40)44-28(17-9-14-25-12-5-4-6-13-25)18-10-15-26-16-11-20-36-24-26/h4-6,11-13,16,20,22-24,28-29H,7-10,14-15,17-19,21H2,1-3H3/t28?,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of His6 tagged human FKBP12 expressed in Escherichia coli BL21(DE3) cells using succinylALPF-p-nitroanilide as substrate by fluorescence p... |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM580

((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...)Show SMILES Cc1ccccc1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(O)c1C |r| Show InChI InChI=1S/C32H37N3O5S/c1-20-11-8-9-14-23(20)18-33-30(39)28-32(3,4)41-19-35(28)31(40)27(37)25(17-22-12-6-5-7-13-22)34-29(38)24-15-10-16-26(36)21(24)2/h5-16,25,27-28,36-37H,17-19H2,1-4H3,(H,33,39)(H,34,38)/t25-,27-,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50204512
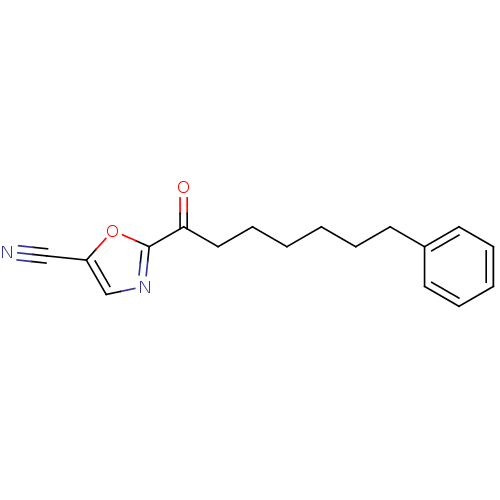
(2-(7-phenylheptanoyl)oxazole-5-carbonitrile | CHEM...)Show InChI InChI=1S/C17H18N2O2/c18-12-15-13-19-17(21-15)16(20)11-7-2-1-4-8-14-9-5-3-6-10-14/h3,5-6,9-10,13H,1-2,4,7-8,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50095526
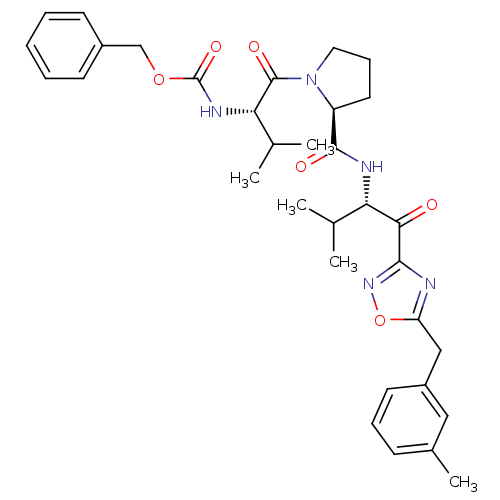
(CHEMBL24058 | [(S)-2-methyl-1-((S)-2-{(S)-2-methyl...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)c1noc(Cc2cccc(C)c2)n1 Show InChI InChI=1S/C33H41N5O6/c1-20(2)27(29(39)30-34-26(44-37-30)18-24-14-9-11-22(5)17-24)35-31(40)25-15-10-16-38(25)32(41)28(21(3)4)36-33(42)43-19-23-12-7-6-8-13-23/h6-9,11-14,17,20-21,25,27-28H,10,15-16,18-19H2,1-5H3,(H,35,40)(H,36,42)/t25-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50103773
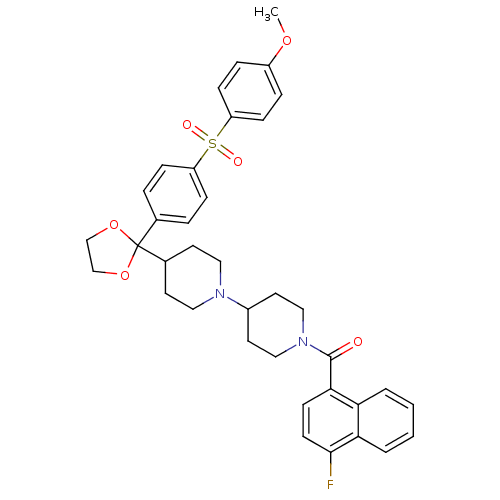
((4-Fluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C1(OCCO1)C1CCN(CC1)C1CCN(CC1)C(=O)c1ccc(F)c2ccccc12 Show InChI InChI=1S/C37H39FN2O6S/c1-44-29-8-12-31(13-9-29)47(42,43)30-10-6-26(7-11-30)37(45-24-25-46-37)27-16-20-39(21-17-27)28-18-22-40(23-19-28)36(41)34-14-15-35(38)33-5-3-2-4-32(33)34/h2-15,27-28H,16-25H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50400823

(CHEMBL2204343)Show SMILES O[Si]1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H23ClFNO2Si/c21-17-5-9-19(10-6-17)26(25)14-12-23(13-15-26)11-1-2-20(24)16-3-7-18(22)8-4-16/h3-10,25H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D2 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50400837
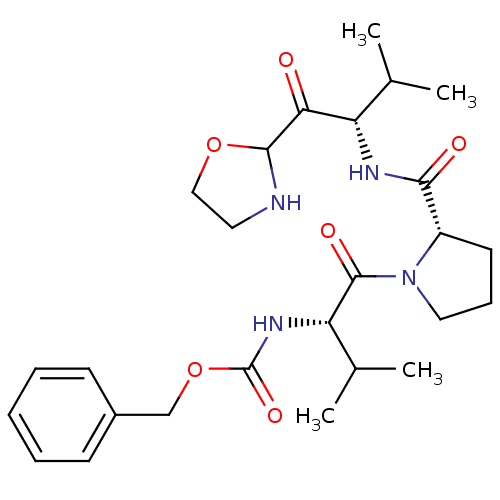
(CHEMBL2205678)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(=O)C1NCCO1 |r| Show InChI InChI=1S/C26H38N4O6/c1-16(2)20(22(31)24-27-12-14-35-24)28-23(32)19-11-8-13-30(19)25(33)21(17(3)4)29-26(34)36-15-18-9-6-5-7-10-18/h5-7,9-10,16-17,19-21,24,27H,8,11-15H2,1-4H3,(H,28,32)(H,29,34)/t19-,20-,21-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM579

((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...)Show SMILES CSC[C@H](NC(=O)COc1cccc2cnccc12)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C33H41N5O6S2/c1-33(2,3)37-31(42)26-19-46-20-38(26)32(43)29(40)24(15-21-9-6-5-7-10-21)36-30(41)25(18-45-4)35-28(39)17-44-27-12-8-11-22-16-34-14-13-23(22)27/h5-14,16,24-26,29,40H,15,17-20H2,1-4H3,(H,35,39)(H,36,41)(H,37,42)/t24-,25-,26-,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50067797
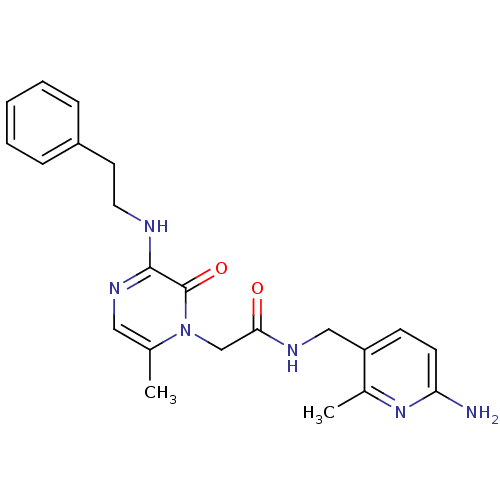
(CHEMBL19080 | L-37378 | N-(6-Amino-2-methyl-pyridi...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)cnc(NCCc2ccccc2)c1=O Show InChI InChI=1S/C22H26N6O2/c1-15-12-26-21(24-11-10-17-6-4-3-5-7-17)22(30)28(15)14-20(29)25-13-18-8-9-19(23)27-16(18)2/h3-9,12H,10-11,13-14H2,1-2H3,(H2,23,27)(H,24,26)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Thrombin (unknown origin) |
J Med Chem 58: 8315-59 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00258
BindingDB Entry DOI: 10.7270/Q22J6DPZ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50204483
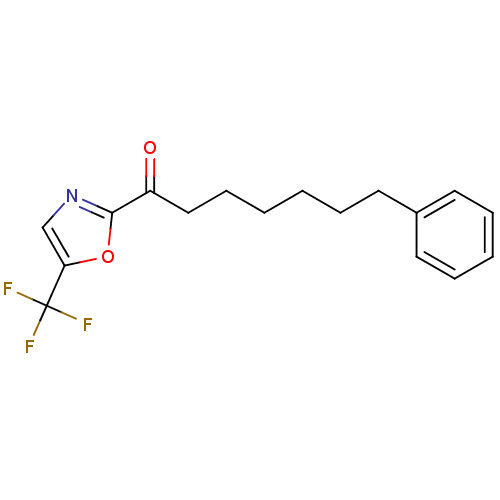
(7-phenyl-1-(5-(trifluoromethyl)oxazol-2-yl)heptan-...)Show InChI InChI=1S/C17H18F3NO2/c18-17(19,20)15-12-21-16(23-15)14(22)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50546436
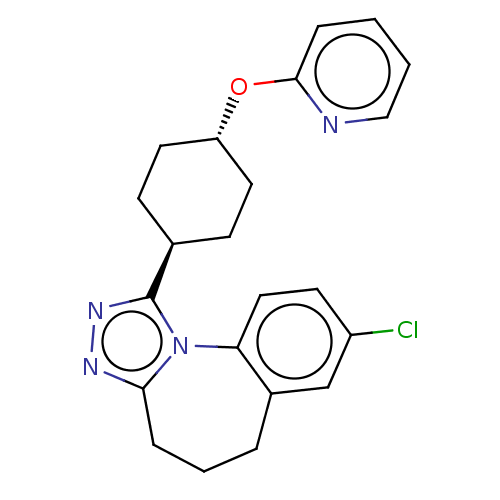
(CHEMBL4799793)Show SMILES Clc1ccc-2c(CCCc3nnc([C@H]4CC[C@@H](CC4)Oc4ccccn4)n-23)c1 |r,wU:13.12,wD:16.19,(20.56,-28.4,;21.91,-29.12,;23.23,-28.31,;24.58,-29.05,;24.62,-30.59,;23.31,-31.39,;23.13,-32.92,;24.2,-34.02,;25.73,-33.86,;26.56,-32.56,;28.09,-32.56,;28.56,-31.1,;27.32,-30.2,;27.31,-28.66,;25.98,-27.88,;25.99,-26.34,;27.34,-25.57,;28.67,-26.35,;28.66,-27.9,;27.34,-24.04,;26.01,-23.27,;26.02,-21.74,;24.69,-20.97,;23.36,-21.74,;23.36,-23.28,;24.69,-24.04,;26.07,-31.1,;21.96,-30.66,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50204490
(CHEMBL220784 | methyl 2-(7-phenylheptanoyl)oxazole...)Show InChI InChI=1S/C18H21NO4/c1-22-18(21)16-13-19-17(23-16)15(20)12-8-3-2-5-9-14-10-6-4-7-11-14/h4,6-7,10-11,13H,2-3,5,8-9,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50421796
(CHEMBL5286105)Show InChI InChI=1S/C11H10I2N4O/c12-7-2-5(3-8(13)9(7)18)1-6-4-16-11(15)17-10(6)14/h2-4,18H,1H2,(H4,14,15,16,17) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50421795

(CHEMBL5274111)Show InChI InChI=1S/C17H25N5/c1-4-10(3)14-8-11(6-12(5-2)15(14)18)7-13-9-21-17(20)22-16(13)19/h6,8-10H,4-5,7,18H2,1-3H3,(H4,19,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50008984

(4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...)Show InChI InChI=1S/C22H28N2O/c1-2-22(25)24(20-11-7-4-8-12-20)21-14-17-23(18-15-21)16-13-19-9-5-3-6-10-19/h3-12,21H,2,13-18H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor (unknown origin) expressed in HEK cells at pH 7.4 |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50123500

(CHEMBL143139 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)cnc(NCC(C)(C)c2ccccc2)c1=O Show InChI InChI=1S/C24H30N6O2/c1-16-12-27-22(28-15-24(3,4)19-8-6-5-7-9-19)23(32)30(16)14-21(31)26-13-18-10-11-20(25)29-17(18)2/h5-12H,13-15H2,1-4H3,(H2,25,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Thrombin (unknown origin) |
J Med Chem 58: 8315-59 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00258
BindingDB Entry DOI: 10.7270/Q22J6DPZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193921
(3-(2-(naphthalen-2-ylmethyl)phenyl)-N-(thiophen-2-...)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1Cc1ccc2ccccc2c1 Show InChI InChI=1S/C24H19NO3S2/c26-23(25-30(27,28)24-10-5-15-29-24)14-13-20-7-2-4-9-22(20)17-18-11-12-19-6-1-3-8-21(19)16-18/h1-16H,17H2,(H,25,26)/b14-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of EP3 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50421795

(CHEMBL5274111)Show InChI InChI=1S/C17H25N5/c1-4-10(3)14-8-11(6-12(5-2)15(14)18)7-13-9-21-17(20)22-16(13)19/h6,8-10H,4-5,7,18H2,1-3H3,(H4,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50213021
(CHEBI:63621 | Fortovase | Invirase | Ro-31-8959 | ...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50546428
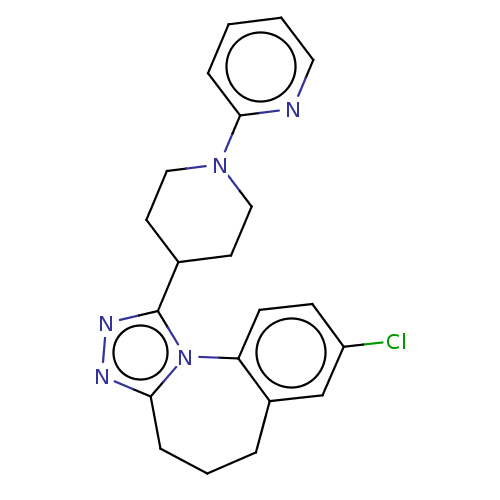
(CHEMBL4778839)Show SMILES Clc1ccc-2c(CCCc3nnc(C4CCN(CC4)c4ccccn4)n-23)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036058
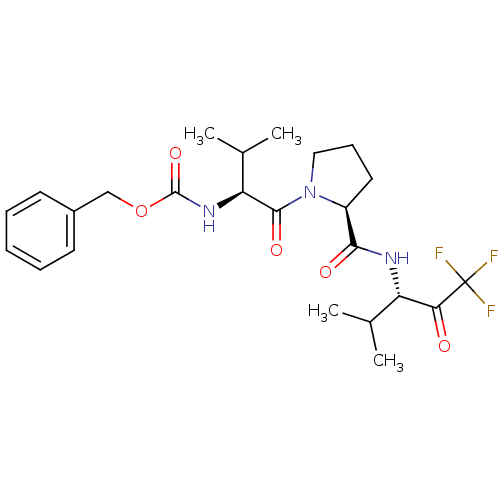
(CHEMBL354883 | benzyl (S)-1-((S)-2-(((S)-1,1,1-tri...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C24H32F3N3O5/c1-14(2)18(20(31)24(25,26)27)28-21(32)17-11-8-12-30(17)22(33)19(15(3)4)29-23(34)35-13-16-9-6-5-7-10-16/h5-7,9-10,14-15,17-19H,8,11-13H2,1-4H3,(H,28,32)(H,29,34)/t17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of sigma 1 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50400835
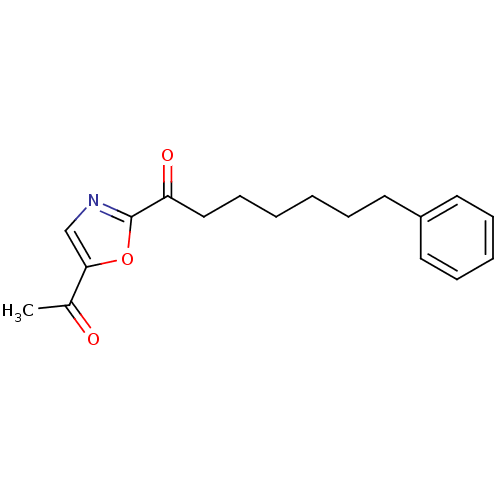
(CHEMBL219660)Show InChI InChI=1S/C18H21NO3/c1-14(20)17-13-19-18(22-17)16(21)12-8-3-2-5-9-15-10-6-4-7-11-15/h4,6-7,10-11,13H,2-3,5,8-9,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50400836

(CHEMBL219659)Show InChI InChI=1S/C19H24N2O3/c1-21(2)19(23)17-14-20-18(24-17)16(22)13-9-4-3-6-10-15-11-7-5-8-12-15/h5,7-8,11-12,14H,3-4,6,9-10,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50400818

(CHEMBL2204339)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C23H17NO5S3/c25-22(24-32(28,29)23-10-5-15-30-23)14-12-18-7-3-4-9-21(18)31(26,27)20-13-11-17-6-1-2-8-19(17)16-20/h1-16H,(H,24,25)/b14-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of EP3 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406931
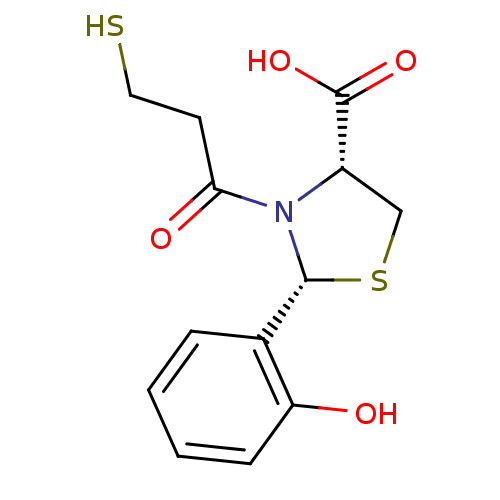
(RENTIAPRIL)Show InChI InChI=1S/C13H15NO4S2/c15-10-4-2-1-3-8(10)12-14(11(16)5-6-19)9(7-20-12)13(17)18/h1-4,9,12,15,19H,5-7H2,(H,17,18)/t9-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D2 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50400832
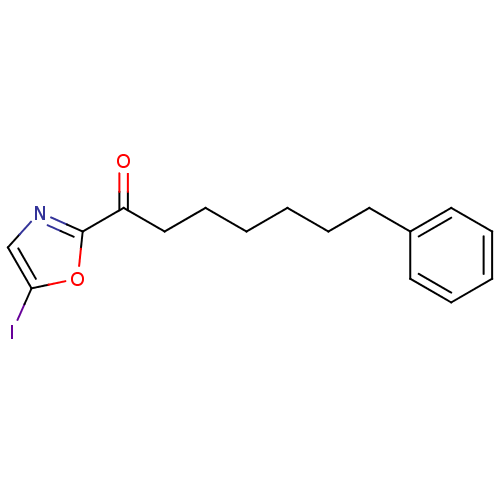
(CHEMBL220555)Show InChI InChI=1S/C16H18INO2/c17-15-12-18-16(20-15)14(19)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50204475
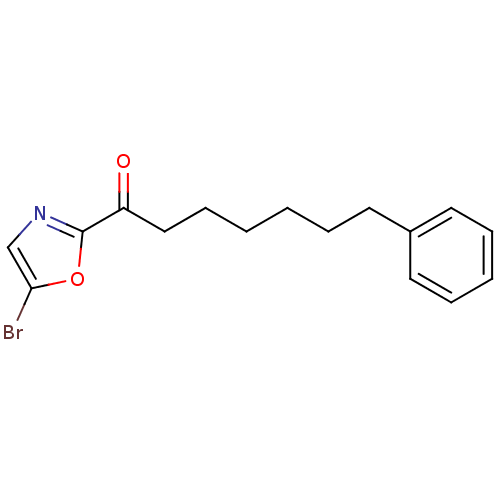
(1-(5-bromooxazol-2-yl)-7-phenylheptan-1-one | CHEM...)Show InChI InChI=1S/C16H18BrNO2/c17-15-12-18-16(20-15)14(19)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50110125

(2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(F)F)C(O)=O Show InChI InChI=1S/C44H56F2N6O14/c1-24(53)47-31(23-36(58)59)42(63)48-29(18-20-35(56)57)40(61)52-38(37(26-13-7-3-8-14-26)27-15-9-4-10-16-27)43(64)49-28(17-19-34(54)55)39(60)50-30(21-25-11-5-2-6-12-25)41(62)51-32(44(65)66)22-33(45)46/h3-4,7-10,13-16,25,28-33,37-38H,2,5-6,11-12,17-23H2,1H3,(H,47,53)(H,48,63)(H,49,64)(H,50,60)(H,51,62)(H,52,61)(H,54,55)(H,56,57)(H,58,59)(H,65,66)/t28-,29-,30-,31-,32-,38-/m0/s1 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50031199
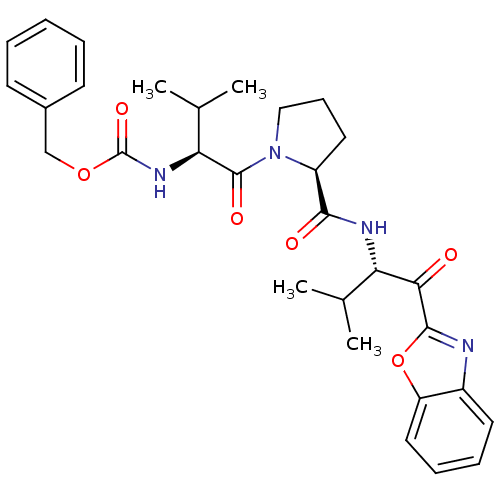
(((S)-1-{(S)-2-[(S)-1-(Benzooxazole-2-carbonyl)-2-m...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)c1nc2ccccc2o1 Show InChI InChI=1S/C30H36N4O6/c1-18(2)24(26(35)28-31-21-13-8-9-15-23(21)40-28)32-27(36)22-14-10-16-34(22)29(37)25(19(3)4)33-30(38)39-17-20-11-6-5-7-12-20/h5-9,11-13,15,18-19,22,24-25H,10,14,16-17H2,1-4H3,(H,32,36)(H,33,38)/t22-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50400823

(CHEMBL2204343)Show SMILES O[Si]1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H23ClFNO2Si/c21-17-5-9-19(10-6-17)26(25)14-12-23(13-15-26)11-1-2-20(24)16-3-7-18(22)8-4-16/h3-10,25H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of sigma 1 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50400833
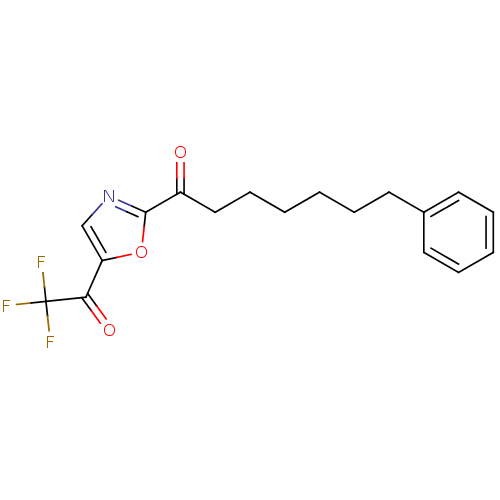
(CHEMBL220378)Show InChI InChI=1S/C18H18F3NO3/c19-18(20,21)16(24)15-12-22-17(25-15)14(23)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50612264
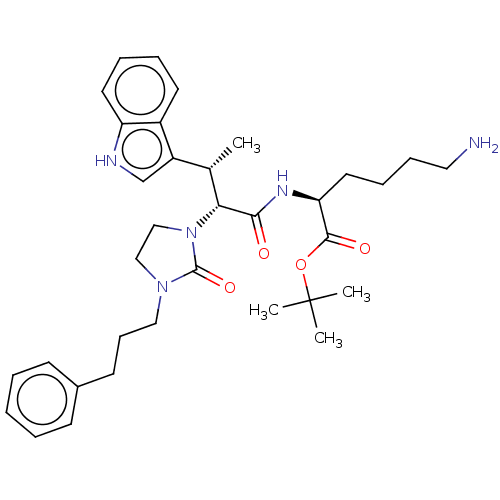
(CHEMBL5276641)Show SMILES C[C@H]([C@@H](N1CCN(CCCc2ccccc2)C1=O)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50502780
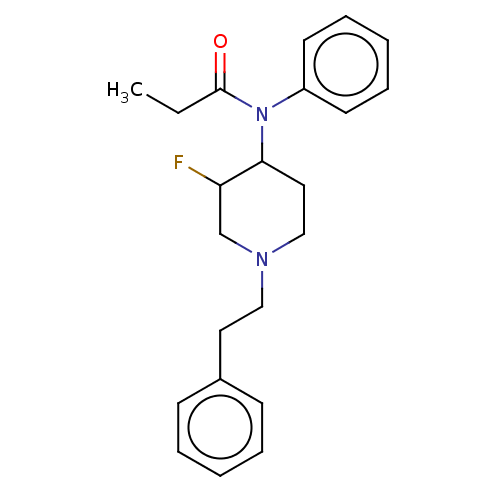
(CHEMBL4471584)Show InChI InChI=1S/C22H27FN2O/c1-2-22(26)25(19-11-7-4-8-12-19)21-14-16-24(17-20(21)23)15-13-18-9-5-3-6-10-18/h3-12,20-21H,2,13-17H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor (unknown origin) expressed in HEK cells at pH 6.5 |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50421797
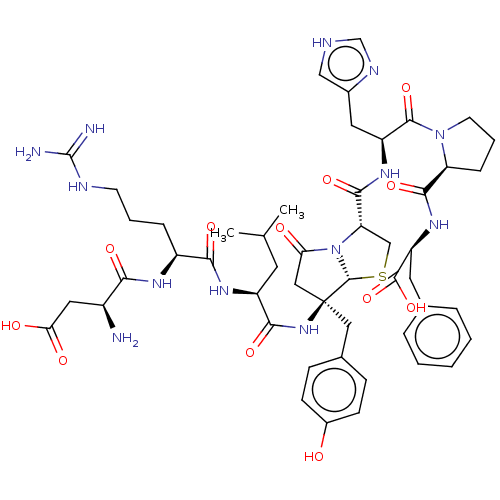
(CHEMBL5286767)Show SMILES Nc1ncc(Cc2cc(I)c(OCCCOc3ccccc3)c(I)c2)c(N)n1 Show InChI InChI=1S/C20H20I2N4O2/c21-16-10-13(9-14-12-25-20(24)26-19(14)23)11-17(22)18(16)28-8-4-7-27-15-5-2-1-3-6-15/h1-3,5-6,10-12H,4,7-9H2,(H4,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50400823

(CHEMBL2204343)Show SMILES O[Si]1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H23ClFNO2Si/c21-17-5-9-19(10-6-17)26(25)14-12-23(13-15-26)11-1-2-20(24)16-3-7-18(22)8-4-16/h3-10,25H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50204525
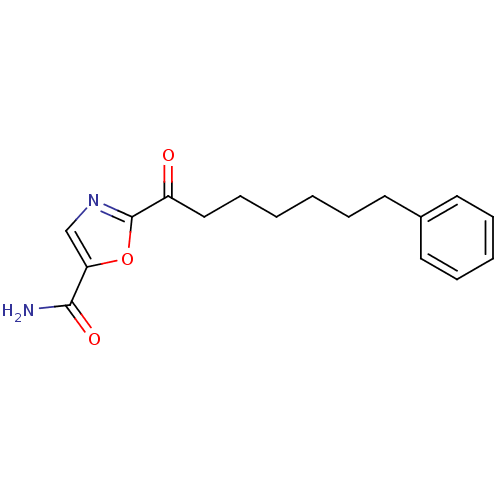
(2-(7-phenylheptanoyl)oxazole-5-carboxamide | CHEMB...)Show InChI InChI=1S/C17H20N2O3/c18-16(21)15-12-19-17(22-15)14(20)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2,(H2,18,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50130216

(CHEMBL3632874)Show SMILES [H][C@]12C3CCCN3[C@@H](c3ccc(cc3)C(N)=N)[C@@]1([H])C(=O)N(Cc1ccc(F)cc1)[C@@H]2C(C)C |r| Show InChI InChI=1S/C26H31FN4O/c1-15(2)23-21-20-4-3-13-30(20)24(17-7-9-18(10-8-17)25(28)29)22(21)26(32)31(23)14-16-5-11-19(27)12-6-16/h5-12,15,20-24H,3-4,13-14H2,1-2H3,(H3,28,29)/t20?,21-,22-,23+,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Thrombin using S-2238 as substrate assessed as release of p-nitroaniline preincubated for 240 secs followed by substrate addition... |
J Med Chem 58: 8315-59 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00258
BindingDB Entry DOI: 10.7270/Q22J6DPZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50095103
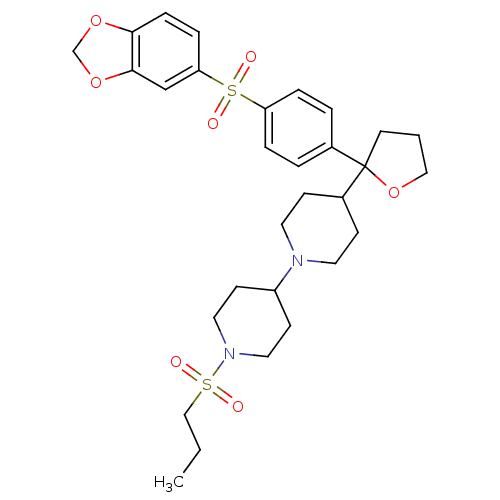
(4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-tet...)Show SMILES CCCS(=O)(=O)N1CCC(CC1)N1CCC(CC1)C1(CCCO1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C30H40N2O7S2/c1-2-20-40(33,34)32-17-12-25(13-18-32)31-15-10-24(11-16-31)30(14-3-19-39-30)23-4-6-26(7-5-23)41(35,36)27-8-9-28-29(21-27)38-22-37-28/h4-9,21,24-25H,2-3,10-20,22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50400831
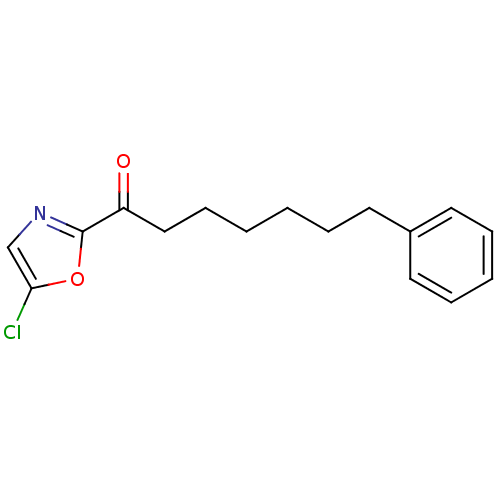
(CHEMBL220613)Show InChI InChI=1S/C16H18ClNO2/c17-15-12-18-16(20-15)14(19)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50154726
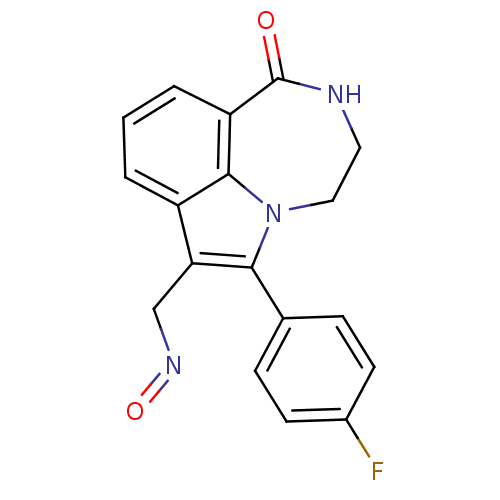
(6-(4-Fluoro-phenyl)-1-oxo-1,2,3,4-tetrahydro-[1,4]...)Show InChI InChI=1S/C18H14FN3O2/c19-12-6-4-11(5-7-12)16-15(10-21-24)13-2-1-3-14-17(13)22(16)9-8-20-18(14)23/h1-7H,8-10H2,(H,20,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PARP-1 |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50400834
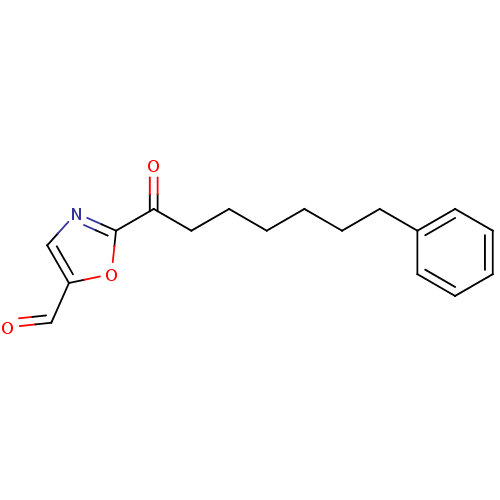
(CHEMBL220727)Show InChI InChI=1S/C17H19NO3/c19-13-15-12-18-17(21-15)16(20)11-7-2-1-4-8-14-9-5-3-6-10-14/h3,5-6,9-10,12-13H,1-2,4,7-8,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50120724
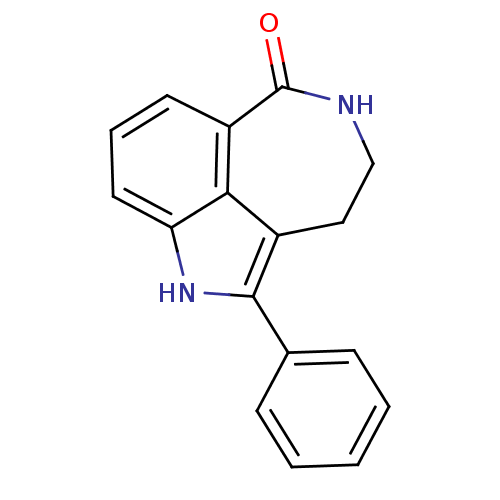
(2-Phenyl-1,3,4,5-tetrahydro-azepino[5,4,3-cd]indol...)Show InChI InChI=1S/C17H14N2O/c20-17-13-7-4-8-14-15(13)12(9-10-18-17)16(19-14)11-5-2-1-3-6-11/h1-8,19H,9-10H2,(H,18,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PARP-1 |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50400820
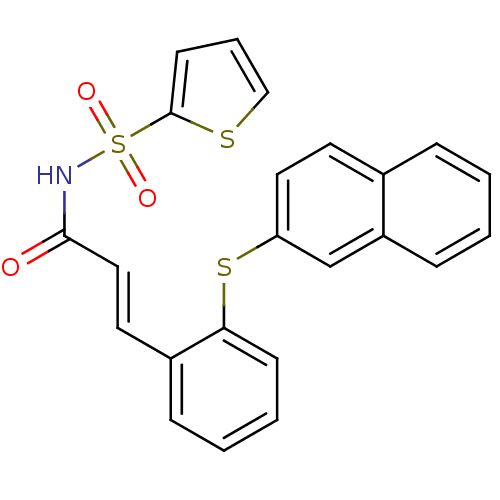
(CHEMBL2204337)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1Sc1ccc2ccccc2c1 Show InChI InChI=1S/C23H17NO3S3/c25-22(24-30(26,27)23-10-5-15-28-23)14-12-18-7-3-4-9-21(18)29-20-13-11-17-6-1-2-8-19(17)16-20/h1-16H,(H,24,25)/b14-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of EP3 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data