

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020654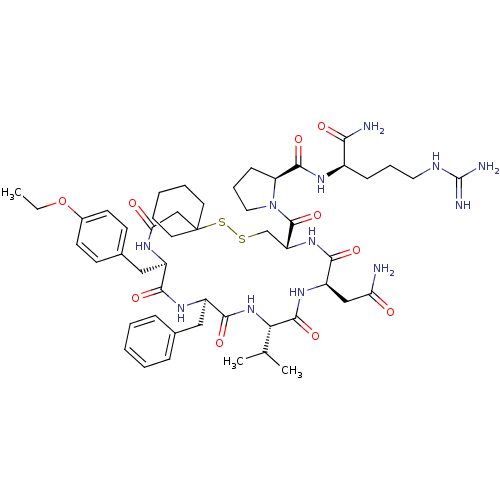 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations. | J Med Chem 29: 2425-6 (1987) BindingDB Entry DOI: 10.7270/Q23T9G6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020655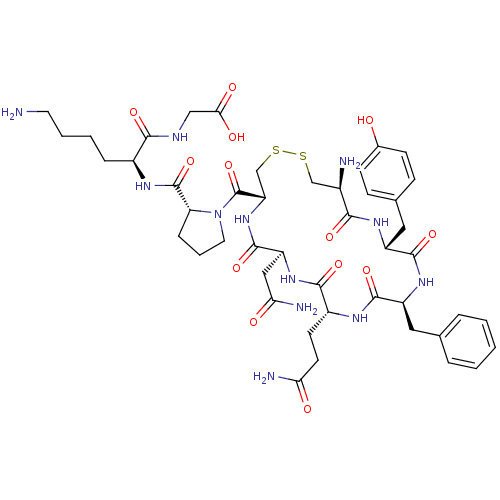 (CHEMBL408474 | [6-Amino-2-({1-[19-amino-13-benzyl-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of hog kidney renin | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020655 (CHEMBL408474 | [6-Amino-2-({1-[19-amino-13-benzyl-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of [3H]-LVP binding to vasopressin receptor in medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020675 (1-[13-Benzyl-7-carbamoylmethyl-16-(4-ethoxy-benzyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations. | J Med Chem 29: 2425-6 (1987) BindingDB Entry DOI: 10.7270/Q23T9G6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016760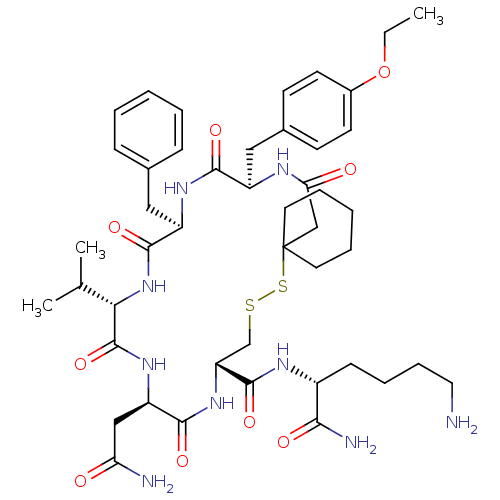 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020653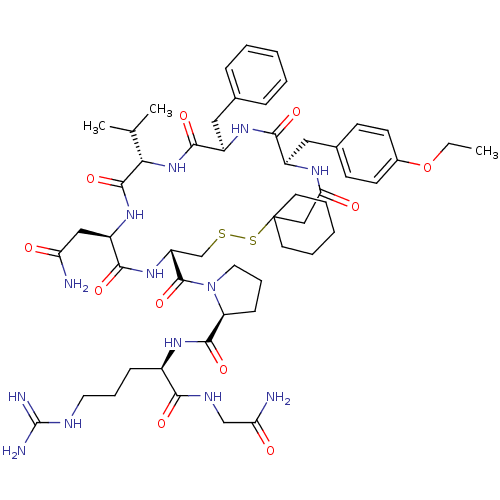 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020653 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations. | J Med Chem 29: 2425-6 (1987) BindingDB Entry DOI: 10.7270/Q23T9G6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020674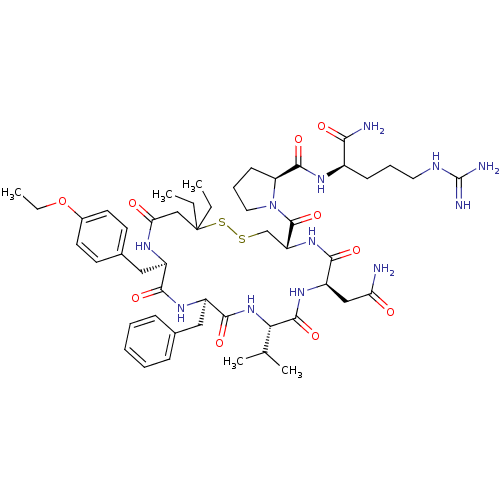 (1-[13-Benzyl-7-carbamoylmethyl-16-(4-ethoxy-benzyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations. | J Med Chem 29: 2425-6 (1987) BindingDB Entry DOI: 10.7270/Q23T9G6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020656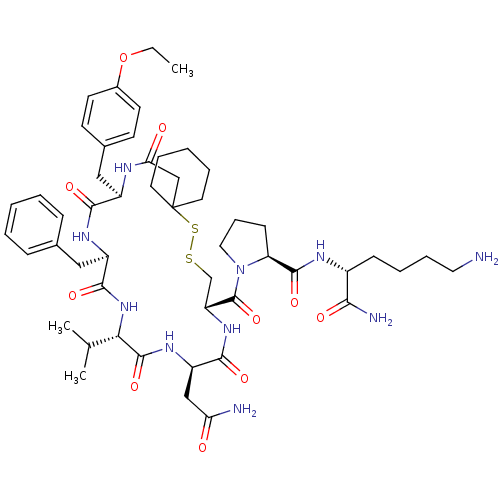 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020656 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020654 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of [3H]-LVP binding to vasopressin receptor in medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020653 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of [3H]-LVP binding to vasopressin receptor in medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020654 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020651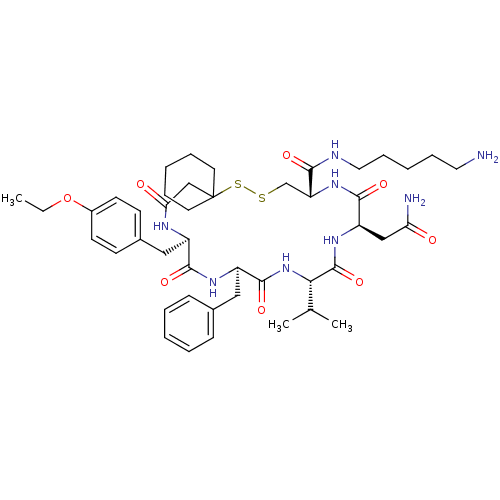 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020651 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of [3H]-LVP binding to vasopressin receptor in medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Rattus norvegicus) | BDBM50222264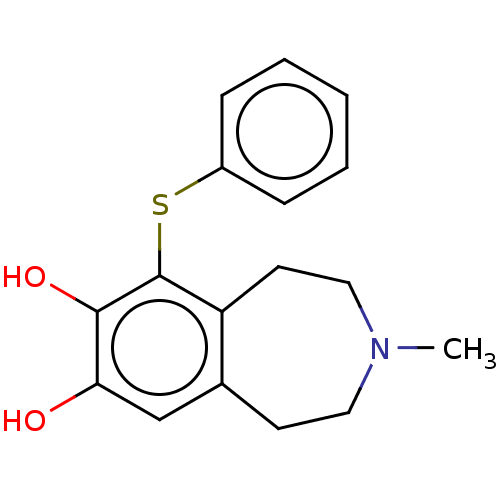 (CHEMBL552736) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine sensitive adenylate cyclase in rats | J Med Chem 23: 975-6 (1980) BindingDB Entry DOI: 10.7270/Q2RV0QWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016760 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020652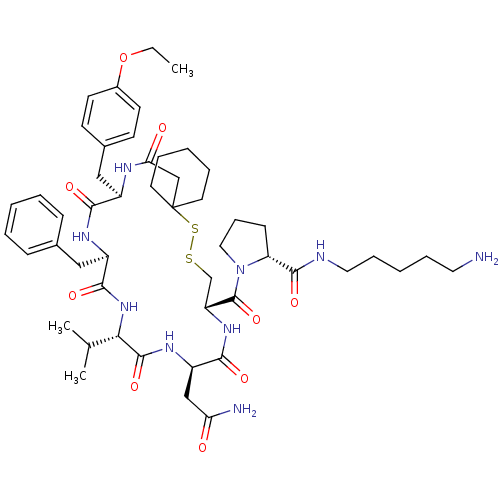 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of [3H]-LVP binding to vasopressin receptor in medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020652 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of [3H]-LVP binding to vasopressin receptor in medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Rattus norvegicus) | BDBM50222266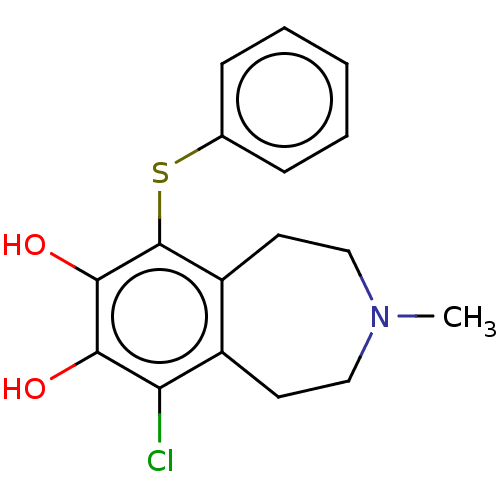 (CHEMBL369006) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine sensitive adenylate cyclase in rats | J Med Chem 23: 975-6 (1980) BindingDB Entry DOI: 10.7270/Q2RV0QWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Rattus norvegicus) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine sensitive adenylate cyclase in rats | J Med Chem 23: 975-6 (1980) BindingDB Entry DOI: 10.7270/Q2RV0QWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Rattus norvegicus) | BDBM50222265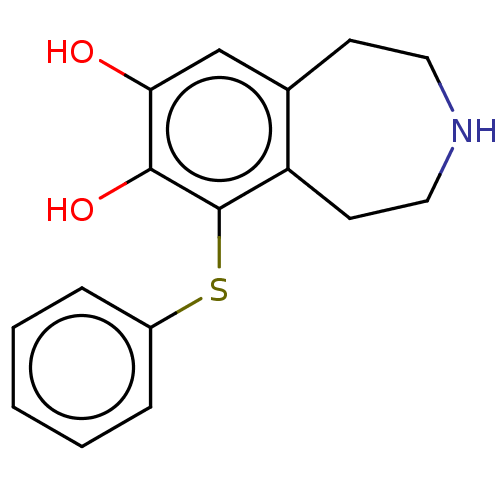 (CHEMBL541633) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine sensitive adenylate cyclase in rats | J Med Chem 23: 975-6 (1980) BindingDB Entry DOI: 10.7270/Q2RV0QWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Rattus norvegicus) | BDBM50222264 (CHEMBL552736) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against Dopamine sensitive adenylate cyclase in rats | J Med Chem 23: 975-6 (1980) BindingDB Entry DOI: 10.7270/Q2RV0QWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Rattus norvegicus) | BDBM50222266 (CHEMBL369006) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against Dopamine sensitive adenylate cyclase in rats | J Med Chem 23: 975-6 (1980) BindingDB Entry DOI: 10.7270/Q2RV0QWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1/Adenylate cyclase type 2/Adenylate cyclase type 3/Adenylate cyclase type 4/Adenylate cyclase type 5/Adenylate cyclase type 6/Adenylate cyclase type 8/Adenylyl cyclase 7 (Rattus norvegicus-RAT) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against Dopamine sensitive adenylate cyclase in rats | J Med Chem 23: 975-6 (1980) BindingDB Entry DOI: 10.7270/Q2RV0QWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Rattus norvegicus) | BDBM50222265 (CHEMBL541633) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against Dopamine sensitive adenylate cyclase in rats | J Med Chem 23: 975-6 (1980) BindingDB Entry DOI: 10.7270/Q2RV0QWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||