

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133948 ((S)-2-[(3aS,6S,6aR)-4-(6-Hydroxymethyl-benzothiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50323701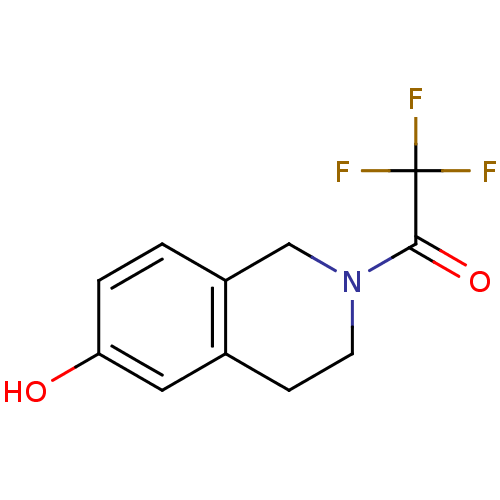 (2-(Trifluoroacetyl)-1,2,3,4-tetrahydro-6-isoquinol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Molekulare Physiologie Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human ERalpha ligand binding domain expressed in Escherichia coli BL21 (DE3) | Nat Chem Biol 5: 585-92 (2009) Article DOI: 10.1038/nchembio.188 BindingDB Entry DOI: 10.7270/Q2MP53F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133962 ((3S,3aR,6aS)-4-({(2S)-1-[(5-Chloro-3-methyl-1-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133960 ((S)-2-((3aS,6S,6aR)-4-Cyclopropanecarbonyl-6-methy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133951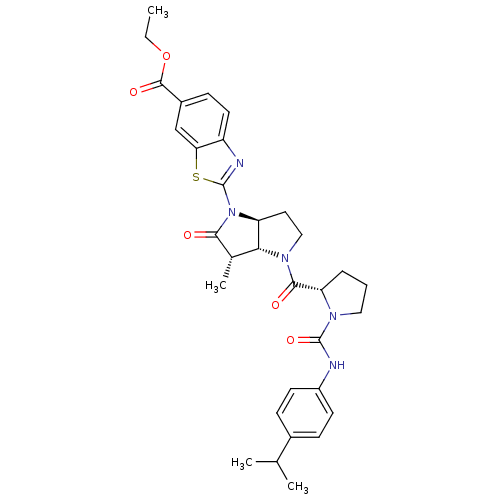 (2-{(3S,3aR,6aS)-4-[(S)-1-(4-Isopropyl-phenylcarbam...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133981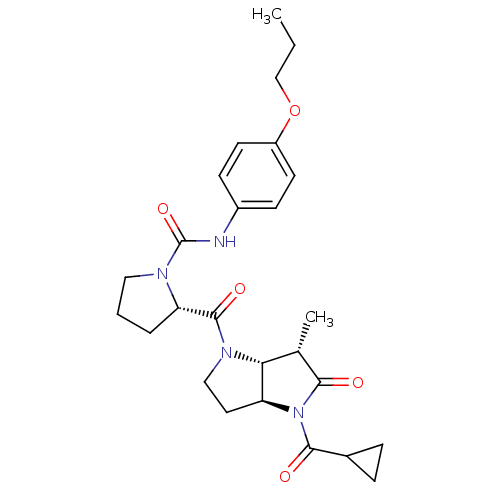 ((2S)-2-{[(3aS,6S,6aR)-4-(Cyclopropylcarbonyl)-6-me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133989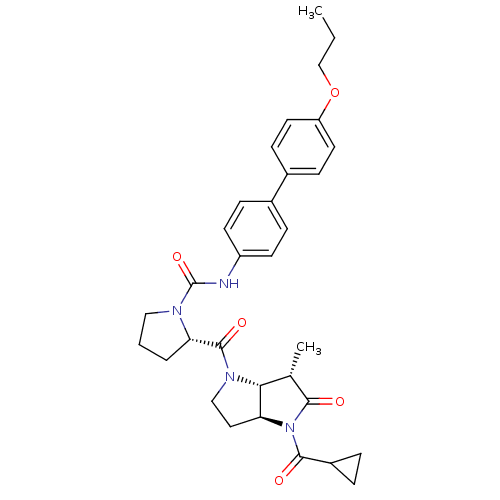 ((S)-2-((3aS,6S,6aR)-4-Cyclopropanecarbonyl-6-methy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50133961 ((S)-2-{(3aS,6S,6aR)-4-[5-(Difluoro-methanesulfonyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133952 ((S)-2-((3aS,6S,6aR)-4-Benzothiazol-2-yl-6-methyl-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133969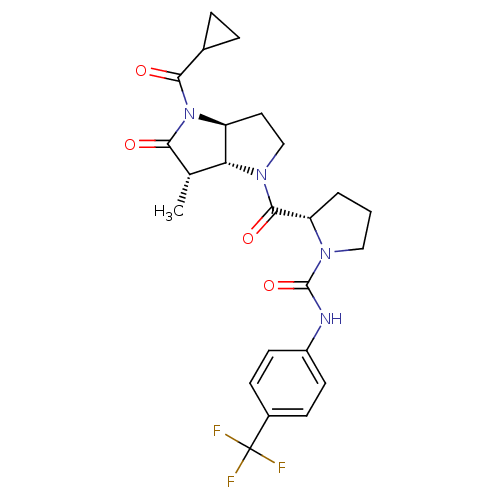 ((S)-2-((3aS,6S,6aR)-4-Cyclopropanecarbonyl-6-methy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133957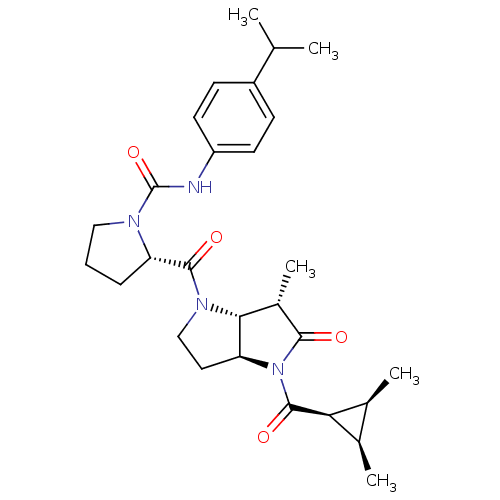 ((S)-2-[(3aS,6S,6aR)-6-Methyl-4-((2R,3S)-2-(S)-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50133973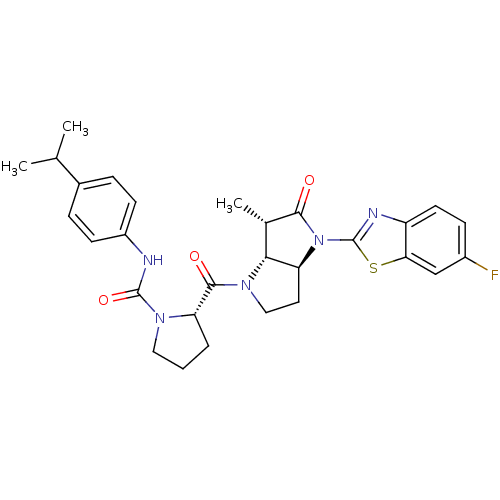 ((S)-2-[(3aS,6S,6aR)-4-(6-Fluoro-benzothiazol-2-yl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133980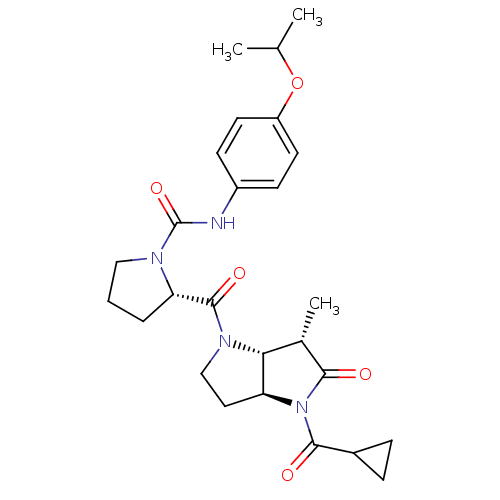 ((2S)-2-{[(3aS,6S,6aR)-4-(Cyclopropylcarbonyl)-6-me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133950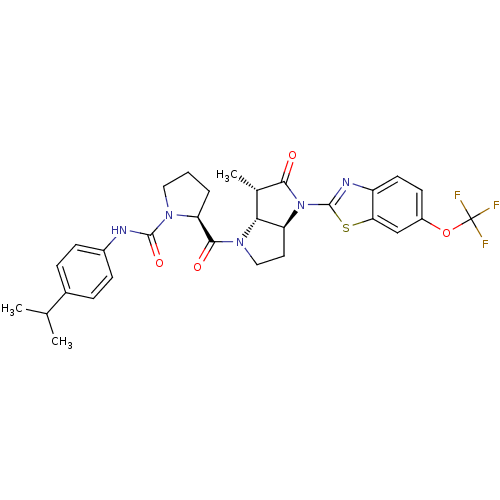 ((S)-2-[(3aS,6S,6aR)-6-Methyl-5-oxo-4-(6-trifluorom...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50107904 ((3S,3aR,6aS)-1-Cyclopropanecarbonyl-4-[(S)-1-(5-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50107904 ((3S,3aR,6aS)-1-Cyclopropanecarbonyl-4-[(S)-1-(5-di...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity towards Human cytomegalovirus (HCMV) protease | J Med Chem 45: 1-18 (2001) BindingDB Entry DOI: 10.7270/Q2Q52NZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133953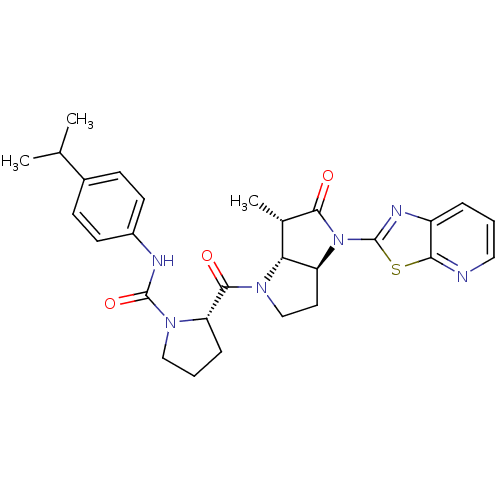 ((S)-2-((3aS,6S,6aR)-6-Methyl-5-oxo-4-thiazolo[5,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133992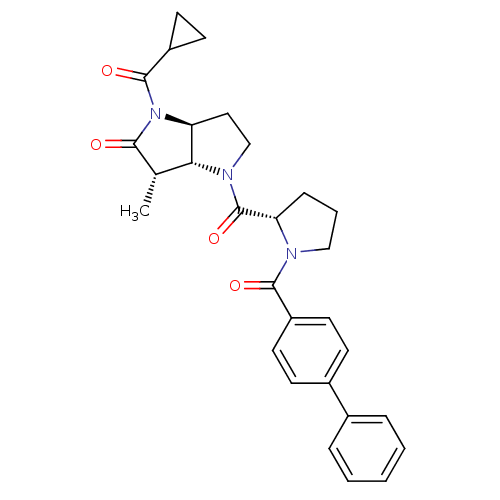 ((3S,3aR,6aS)-4-[(S)-1-(Biphenyl-4-carbonyl)-pyrrol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50133974 ((S)-2-[(3aS,6S,6aR)-4-(5-Methoxy-benzothiazol-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133975 ((S)-2-[(3aS,6S,6aR)-6-Methyl-4-(6-nitro-benzothiaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50133959 ((S)-2-[(3aS,6S,6aR)-4-(5-Chloro-benzothiazol-2-yl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133964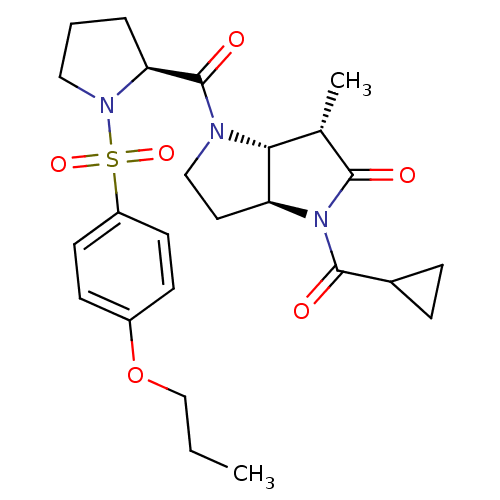 ((3S,3aR,6aS)-1-Cyclopropanecarbonyl-3-methyl-4-[(S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133978 ((3S,3aR,6aS)-1-Cyclopropanecarbonyl-3-methyl-4-[(S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50107914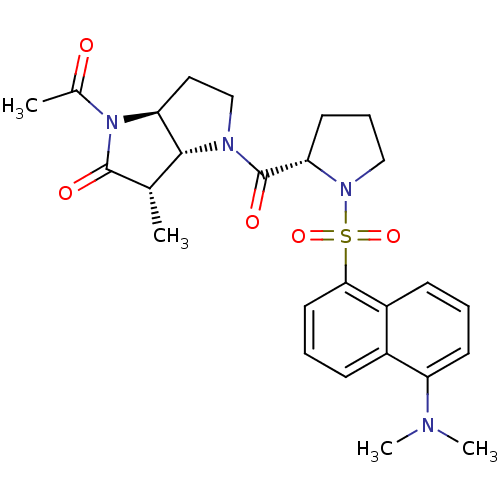 (4-Acetyl-1-[1-(5-dimethylamino-naphthalene-1-sulfo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity towards Human cytomegalovirus (HCMV) protease | J Med Chem 45: 1-18 (2001) BindingDB Entry DOI: 10.7270/Q2Q52NZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50133956 ((S)-2-[(3aS,6S,6aR)-4-(4-Methoxy-benzothiazol-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133977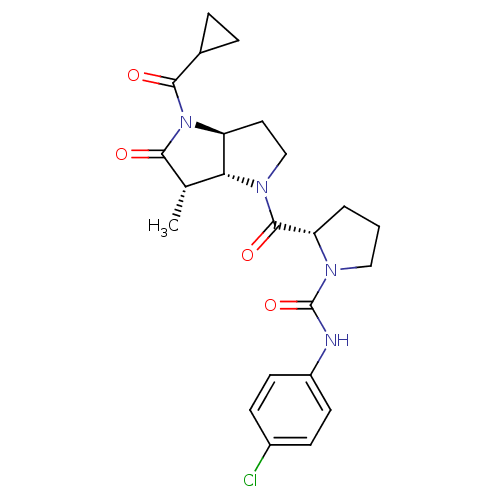 ((2S)-2-{[(3aS,6S,6aR)-4-(Cyclopropylcarbonyl)-6-me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133979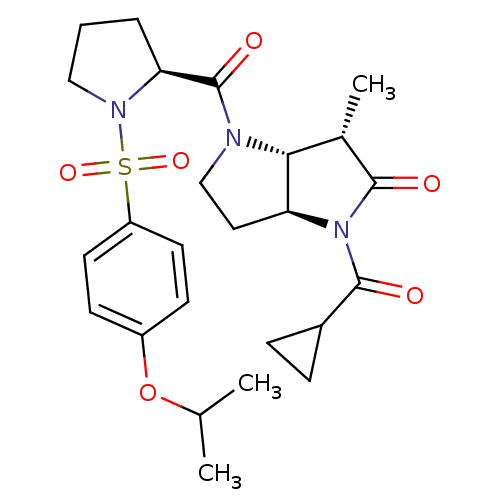 ((3S,3aR,6aS)-1-Cyclopropanecarbonyl-4-[(S)-1-(4-is...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133988 ((3S,3aR,6aS)-1-(Cyclopropylcarbonyl)-3-methyl-4-{[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50133982 ((S)-2-[(3aS,6S,6aR)-4-(4-Chloro-benzothiazol-2-yl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133985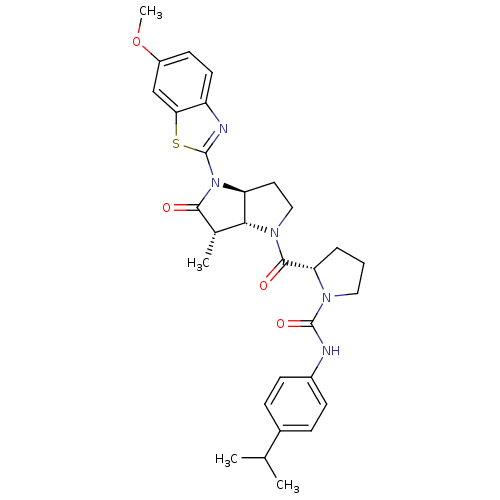 ((S)-2-[(3aS,6S,6aR)-4-(6-Methoxy-benzothiazol-2-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50392323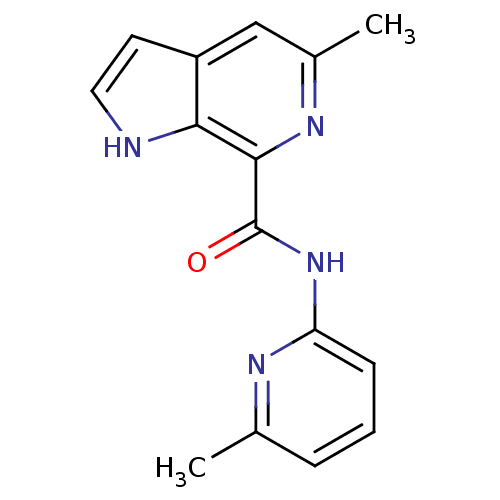 (CHEMBL2153782) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from human mGluR5a transmembrane region expressed in mouse L(tk-) cells | Bioorg Med Chem Lett 22: 6454-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.053 BindingDB Entry DOI: 10.7270/Q2765GDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133949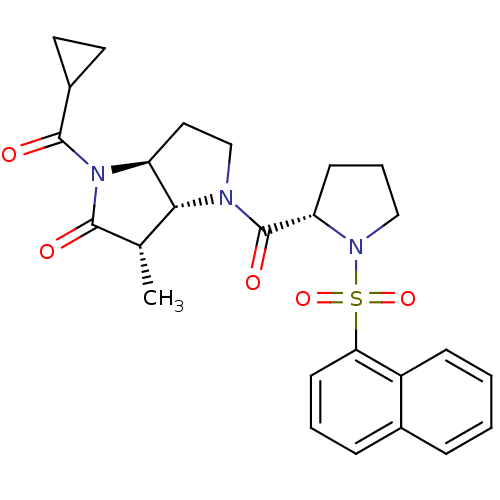 ((3S,3aR,6aS)-1-Cyclopropanecarbonyl-3-methyl-4-[(S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133991 ((3S,3aR,6aS)-1-Cyclopropanecarbonyl-3-methyl-4-[(S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133954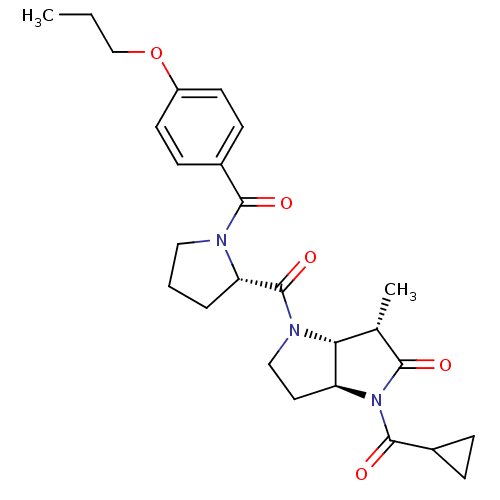 ((3S,3aR,6aS)-1-Cyclopropanecarbonyl-3-methyl-4-[(S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133987 ((3S,3aR,6aS)-1-Cyclopropanecarbonyl-4-[(S)-1-(4-is...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50133965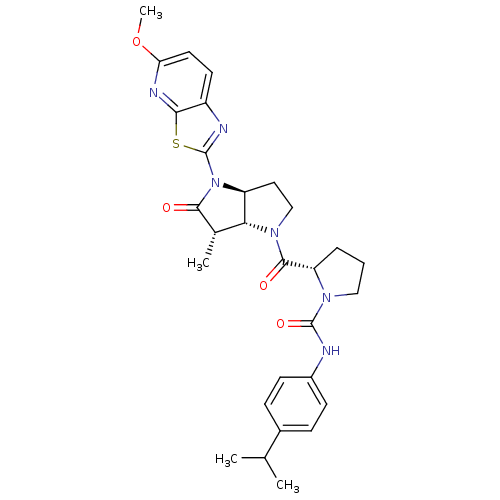 ((S)-2-[(3aS,6S,6aR)-4-(5-Methoxy-thiazolo[5,4-b]py...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133967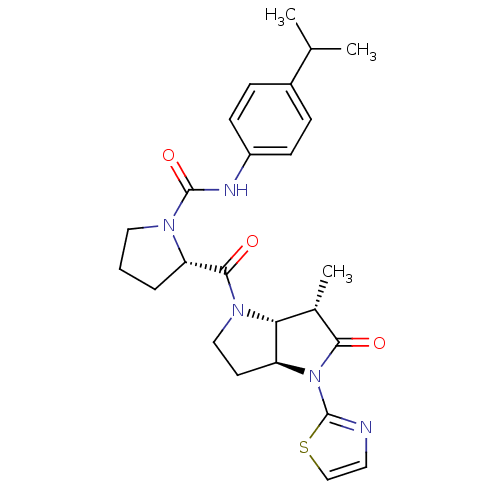 ((S)-2-((3aS,6S,6aR)-6-Methyl-5-oxo-4-thiazol-2-yl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 446 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency against human cytomegalovirus protease in HCMV pNA assay | J Med Chem 46: 4428-49 (2003) Article DOI: 10.1021/jm030810w BindingDB Entry DOI: 10.7270/Q2WH2PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136048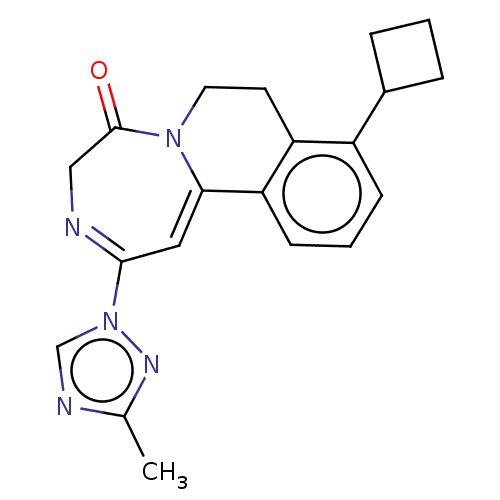 (US8853203, 92 | US9650377, Example 92) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136108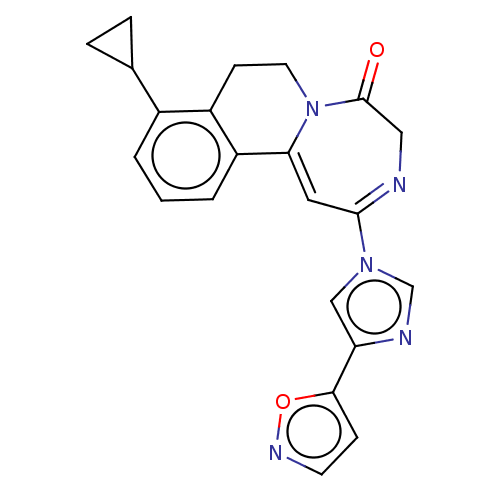 (US8853203, 123b | US9650377, Example 123b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136115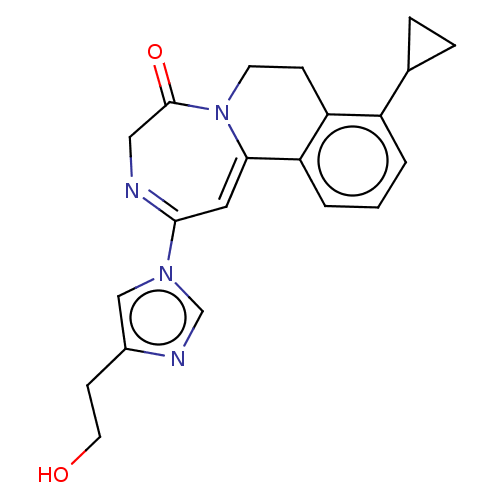 (US8853203, 128 | US9650377, Example 128) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136048 (US8853203, 92 | US9650377, Example 92) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136108 (US8853203, 123b | US9650377, Example 123b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136115 (US8853203, 128 | US9650377, Example 128) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136132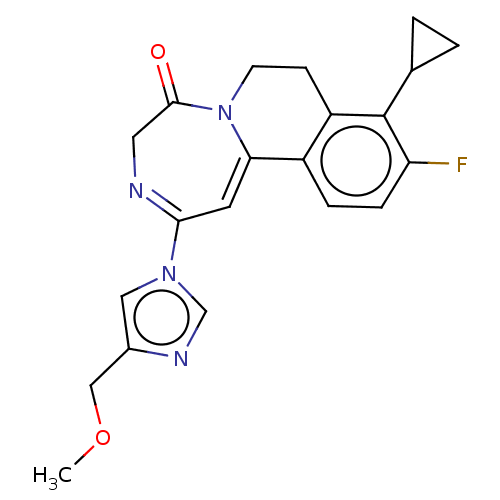 (US8853203, 96-2 | US9650377, Example 96-2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136067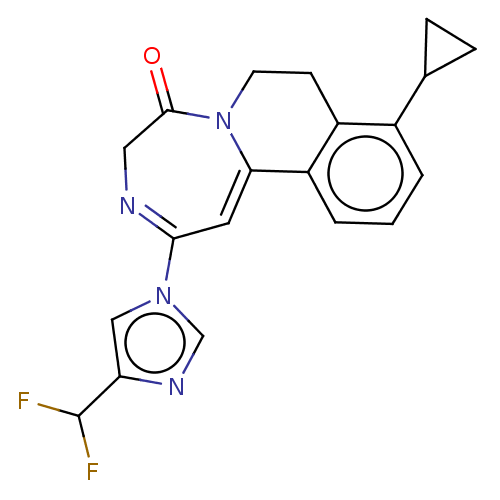 (US8853203, 99l) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136068 (US8853203, 99m) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136071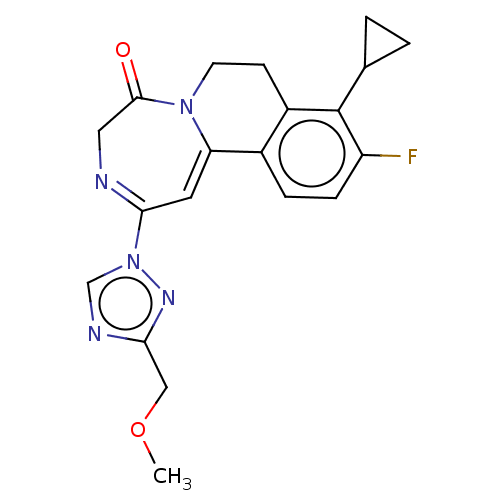 (US8853203, 99p) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136140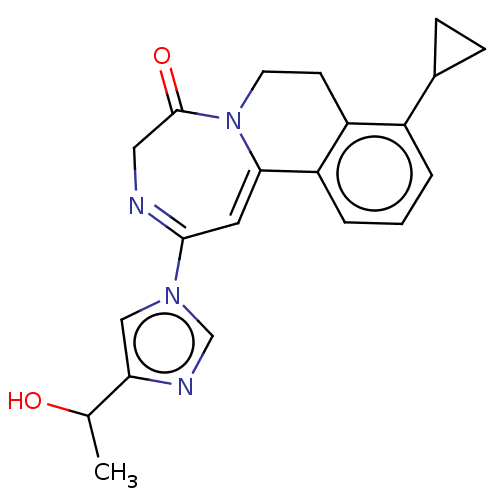 (US8853203, 129 | US9650377, Example 129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136140 (US8853203, 129 | US9650377, Example 129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136055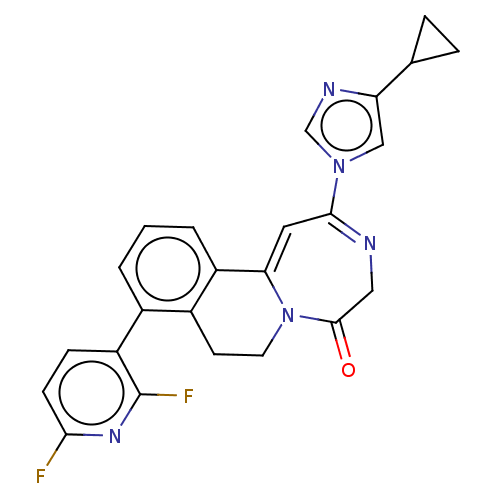 (US8853203, 99 | US9650377, Example 99s) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 953 total ) | Next | Last >> |