

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50281388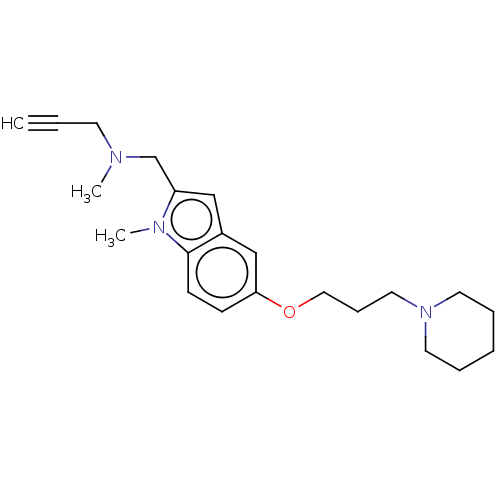 (CHEMBL4173258) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human H3R expressed in HEK293 cell membranes incubated for 90 mins by liquid scintillation counting... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50458225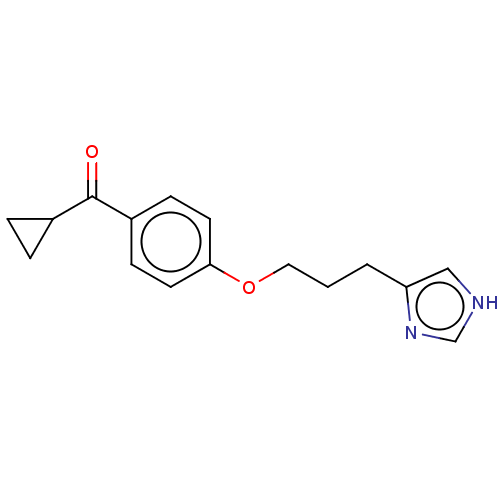 (Ciproxifan) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human H3R expressed in HEK293 cell membranes incubated for 90 mins by liquid scintillation counting... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50359391 (CHEMBL1929421) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human H3R expressed in HEK293 cell membranes incubated for 90 mins by liquid scintillation counting... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50182133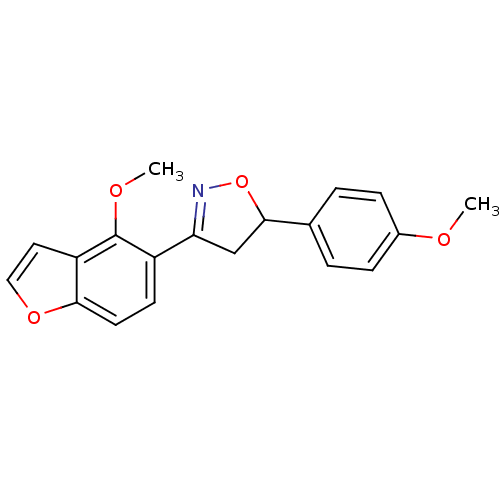 (3-(4-methoxybenzofuran-5-yl)-5-(4-methoxyphenyl)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of PTP1B | Bioorg Med Chem Lett 16: 2139-43 (2006) Article DOI: 10.1016/j.bmcl.2006.01.062 BindingDB Entry DOI: 10.7270/Q29K49ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50182134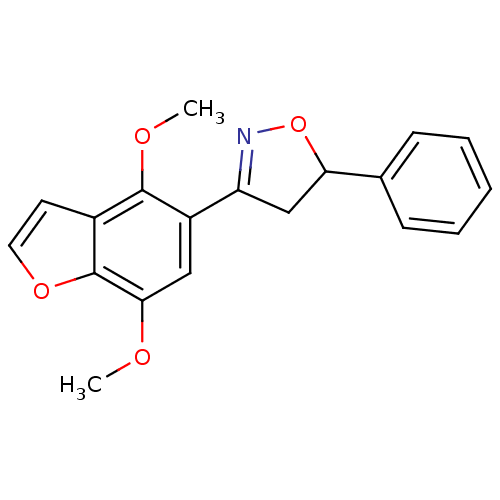 (3-(4,7-dimethoxybenzofuran-5-yl)-5-phenyl-4,5-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of PTP1B | Bioorg Med Chem Lett 16: 2139-43 (2006) Article DOI: 10.1016/j.bmcl.2006.01.062 BindingDB Entry DOI: 10.7270/Q29K49ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50182131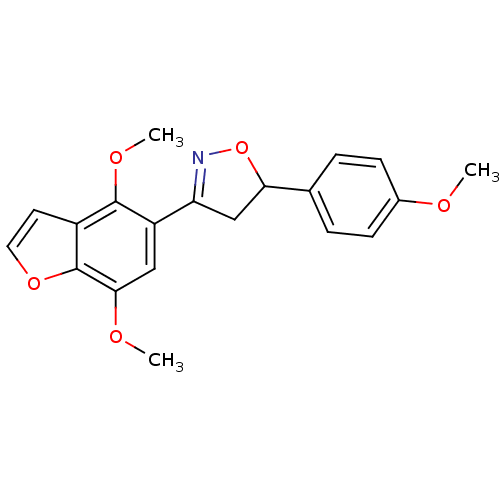 (3-(4,7-dimethoxybenzofuran-5-yl)-5-(4-methoxypheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of PTP1B | Bioorg Med Chem Lett 16: 2139-43 (2006) Article DOI: 10.1016/j.bmcl.2006.01.062 BindingDB Entry DOI: 10.7270/Q29K49ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50182135 (3-(4-methoxybenzofuran-5-yl)-5-phenyl-4,5-dihydroi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of PTP1B | Bioorg Med Chem Lett 16: 2139-43 (2006) Article DOI: 10.1016/j.bmcl.2006.01.062 BindingDB Entry DOI: 10.7270/Q29K49ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50182132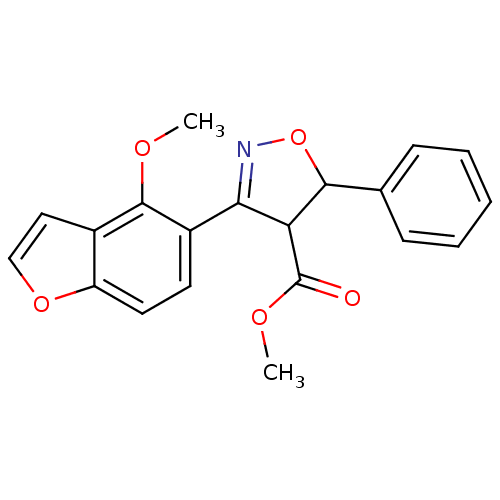 (CHEMBL205730 | methyl 3-(4-methoxybenzofuran-5-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of PTP1B | Bioorg Med Chem Lett 16: 2139-43 (2006) Article DOI: 10.1016/j.bmcl.2006.01.062 BindingDB Entry DOI: 10.7270/Q29K49ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9007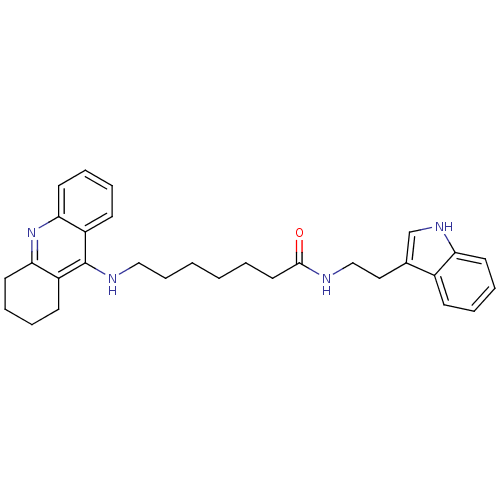 (7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50510845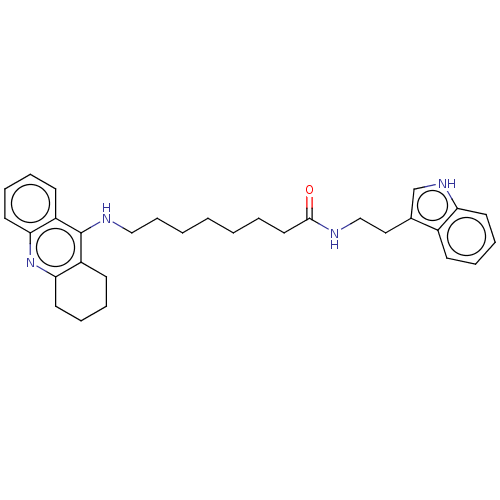 (CHEMBL4556734) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9008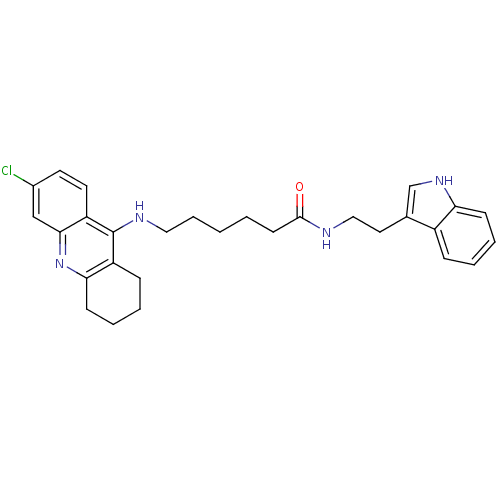 (6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50510846 (CHEMBL4590945) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50510847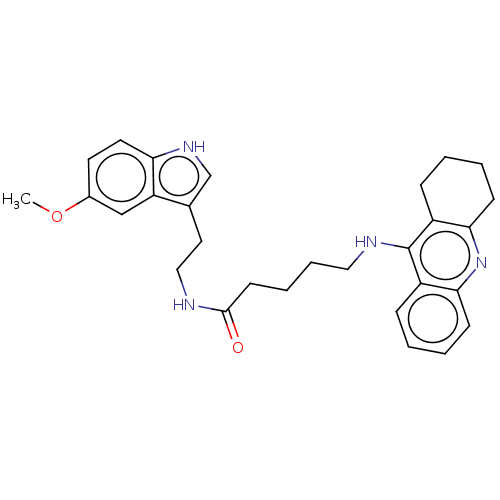 (CHEMBL4455677) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9013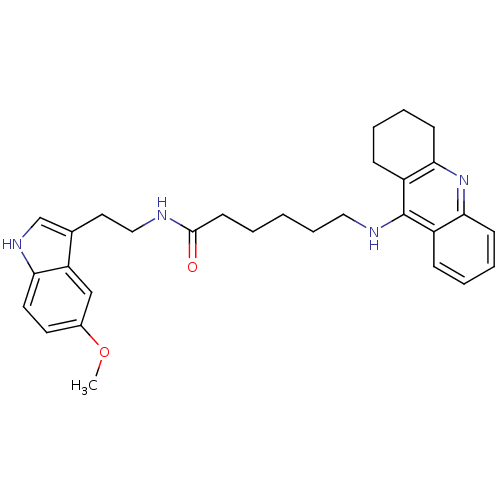 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9014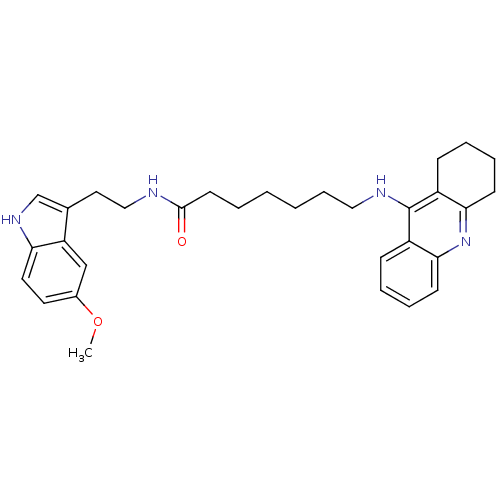 (7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9015 (6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50510848 (CHEMBL4468781) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9017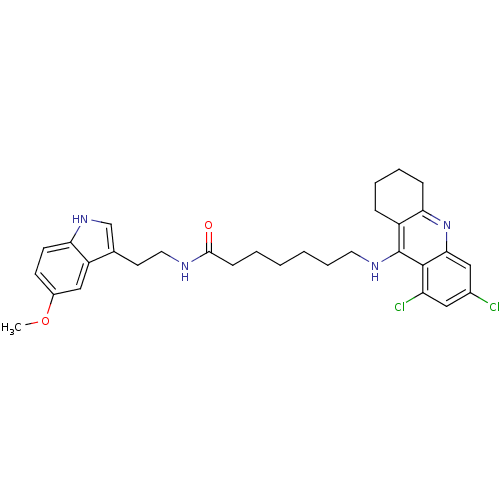 (7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50510849 (CHEMBL4579667) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9006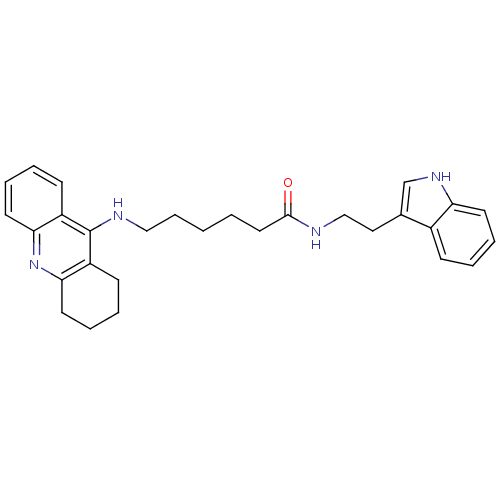 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9011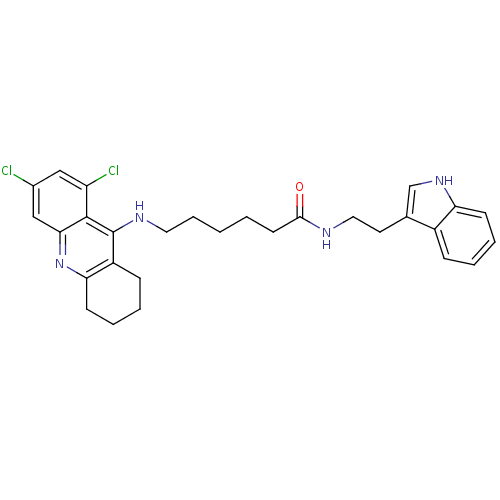 (6-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50510865 (CHEMBL4532770) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9018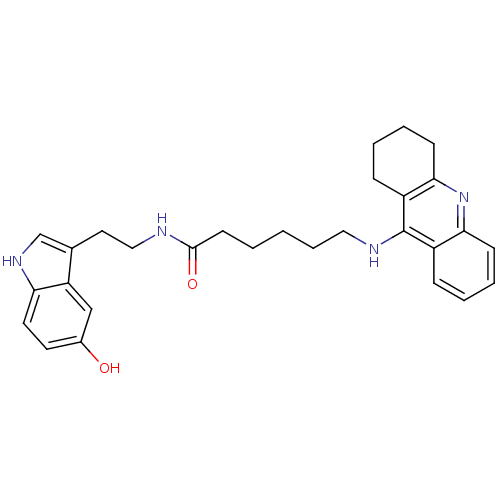 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50510867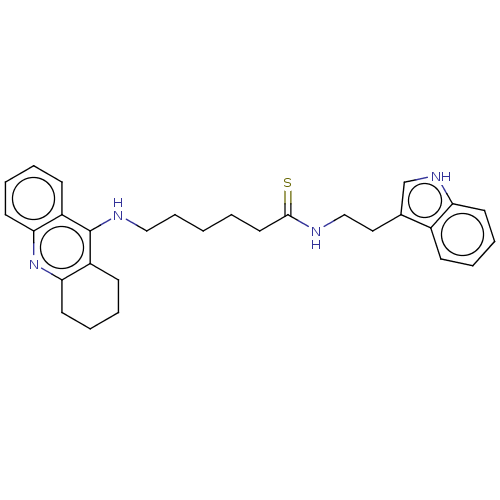 (CHEMBL4469239) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50510868 (CHEMBL4448350) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50510869 (CHEMBL4443426) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9009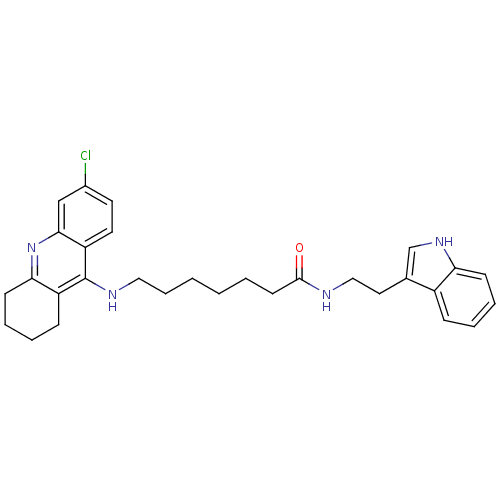 (7-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50510870 (CHEMBL4543251) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9016 (6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50510837 (CHEMBL4467284) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9012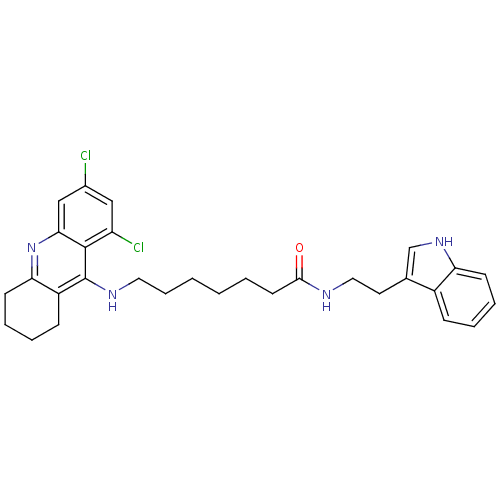 (7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50359391 (CHEMBL1929421) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOA expressed in baculovirus infected BTI insect cells using p-tyramine as substrate preincubated for 30 mins follow... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of rat brain Acetylcholinesterase using acetylthiocholine as substrate in presence of BChE inhibitor ethopropazine by spectrophotometric m... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8964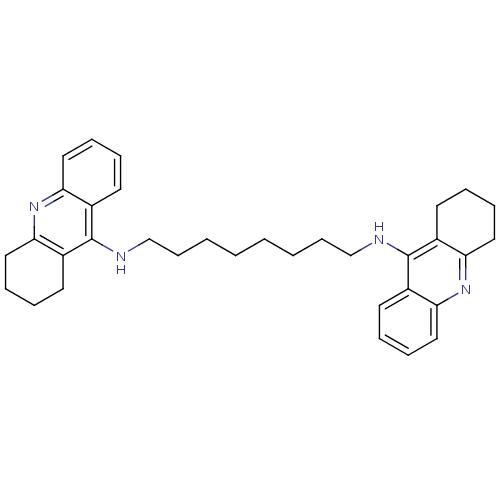 (CHEMBL75274 | Homodimeric Tacrine Analog 3c | N-[8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of rat brain Acetylcholinesterase using acetylthiocholine as substrate in presence of BChE inhibitor ethopropazine by spectrophotometric m... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10469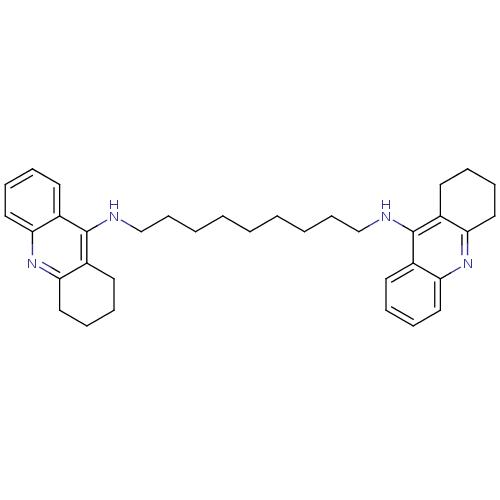 (Bis-THA inhibitor 1c | Bis-THA inhibitor 8 | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of rat brain Acetylcholinesterase using acetylthiocholine as substrate in presence of BChE inhibitor ethopropazine by spectrophotometric m... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human Acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human Acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50510840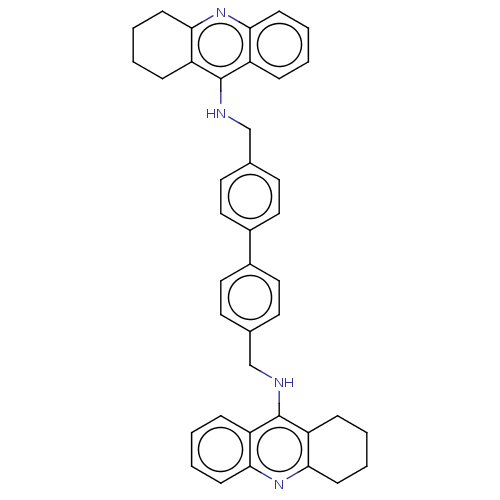 (CHEMBL4456928) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition ... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50510844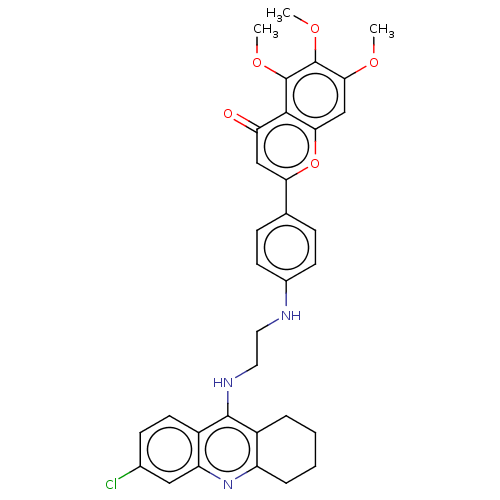 (CHEMBL4451532) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition by E... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50510843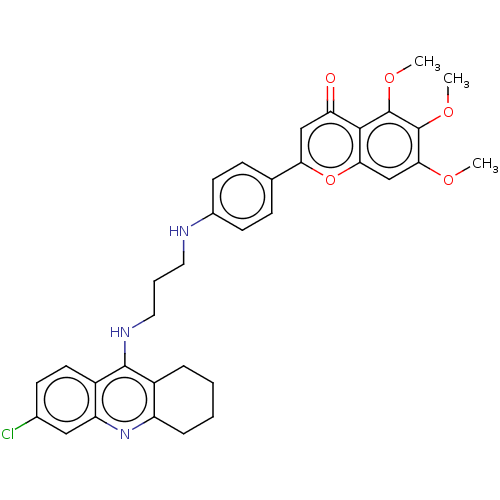 (CHEMBL4466652) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition by E... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50510840 (CHEMBL4456928) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human Acetylcholinesterase using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50433323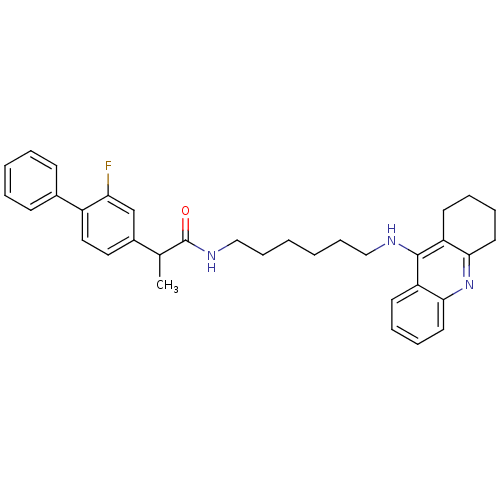 (CHEMBL2376472) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated 5 mins followed by substrate addition and measured after 2 m... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.23 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132072 (US8841453, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human plasma butyrylcholinesterase using butyrylthiocholine as substrate preincubated for 30 mins followed by substrate addition and me... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50006627 (CHEMBL3235503) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellma... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50510834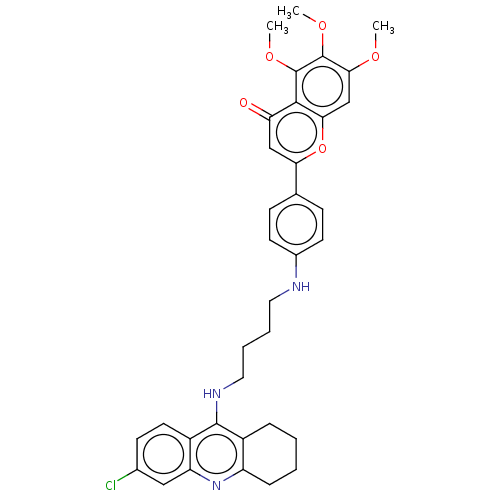 (CHEMBL4569511) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition by E... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50006627 (CHEMBL3235503) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition ... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50510853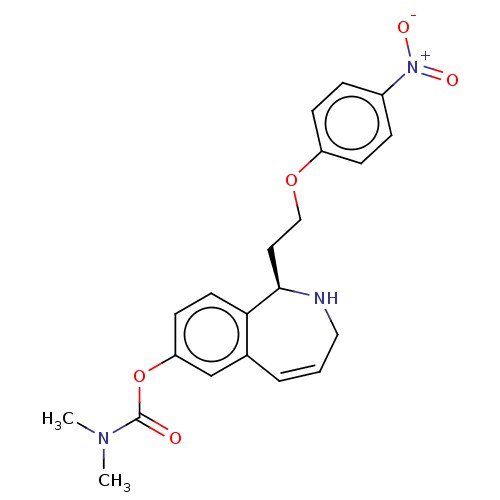 (CHEMBL4563881) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of rat synaptosome SERT assessed as reduction in [3H]5-HT uptake | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 297 total ) | Next | Last >> |