

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527524 (CHEMBL4566742) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50146972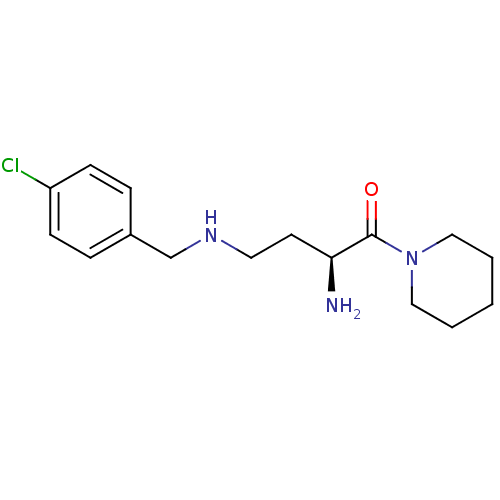 ((S)-4-(4-chlorobenzylamino)-2-amino-1-(piperidin-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of DPP2 (unknown origin) | Bioorg Med Chem Lett 18: 4154-8 (2008) Article DOI: 10.1016/j.bmcl.2008.05.080 BindingDB Entry DOI: 10.7270/Q2G160N3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50146972 ((S)-4-(4-chlorobenzylamino)-2-amino-1-(piperidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry University of Antwerp Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 16: 4777-9 (2006) Article DOI: 10.1016/j.bmcl.2006.06.082 BindingDB Entry DOI: 10.7270/Q2GM86XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527552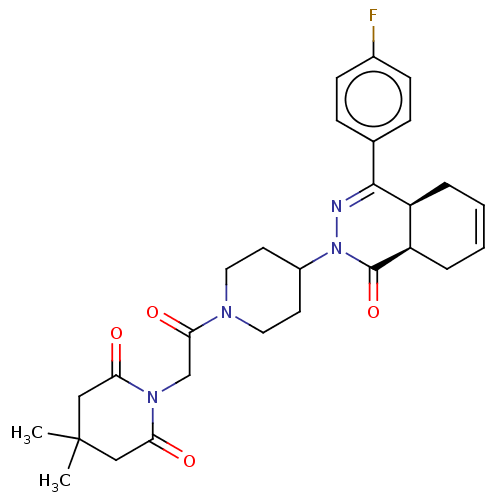 (CHEMBL4435111) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527532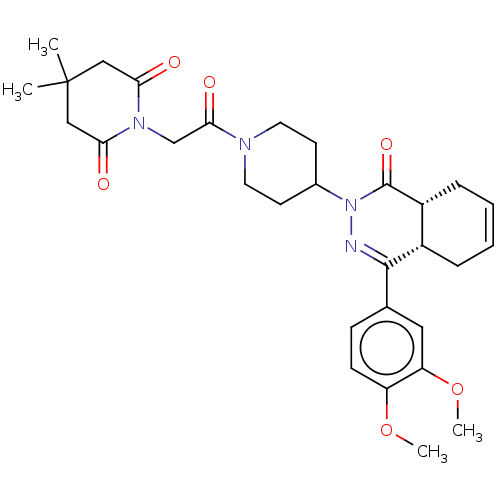 (CHEMBL4441748) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527531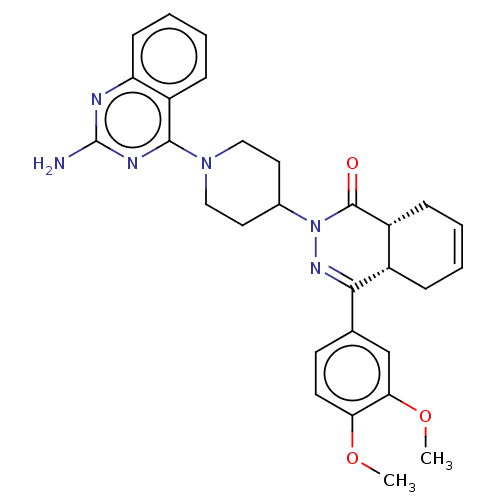 (CHEMBL4524402) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527537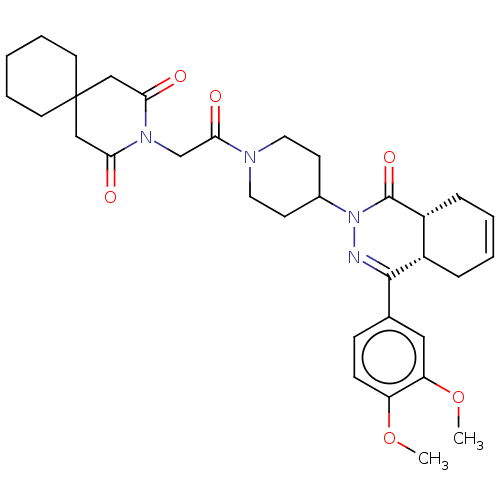 (CHEMBL4453005) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527528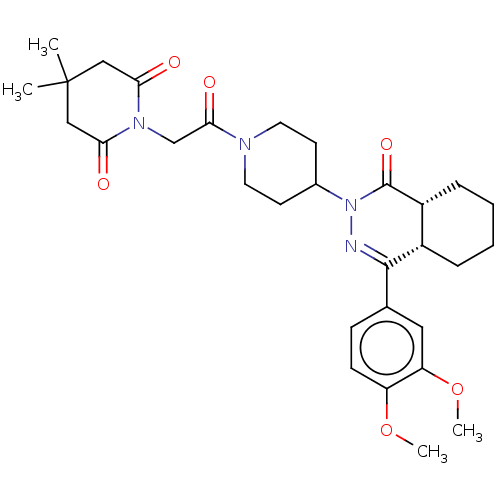 (CHEMBL4441871) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527550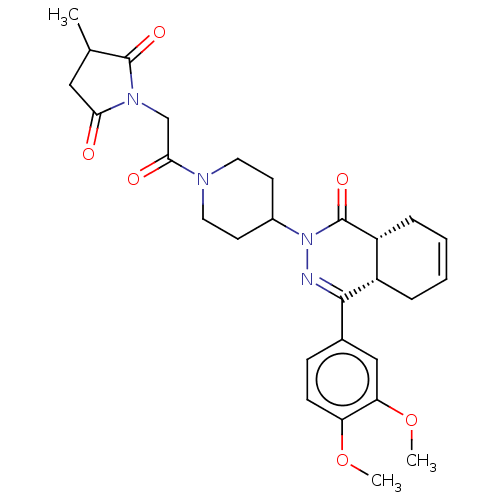 (CHEMBL4461456) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527535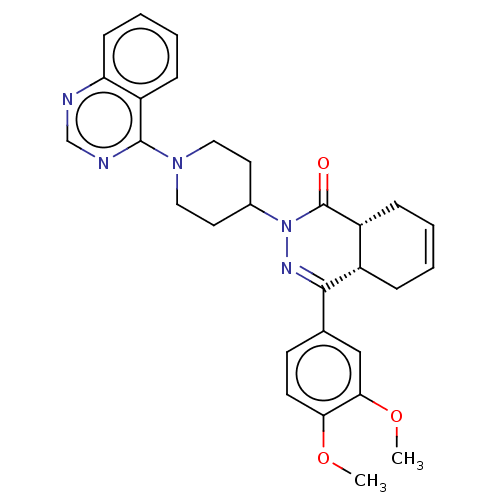 (CHEMBL4589927) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527533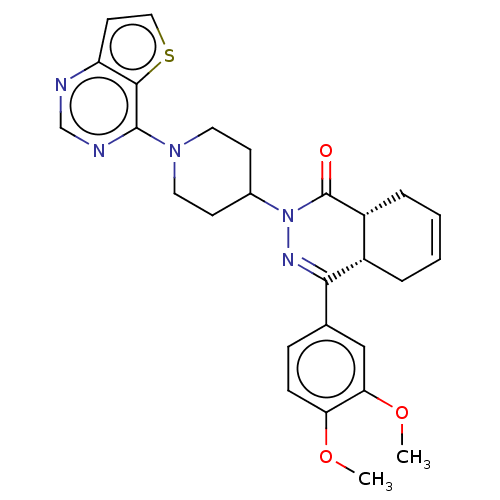 (CHEMBL4592553) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135635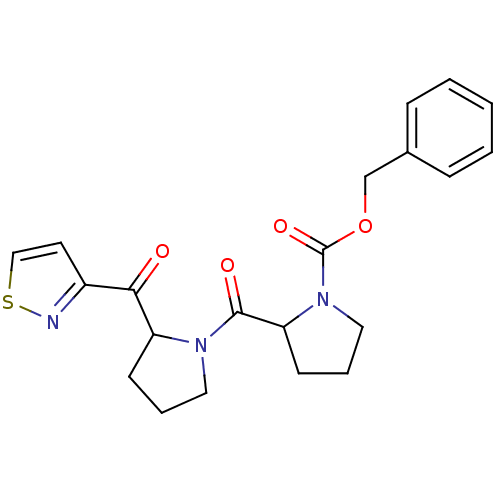 (2-[2-(Isothiazole-3-carbonyl)-pyrrolidine-1-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527555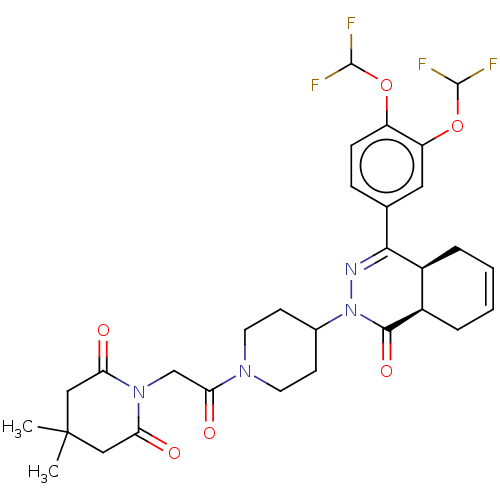 (CHEMBL4530777) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527560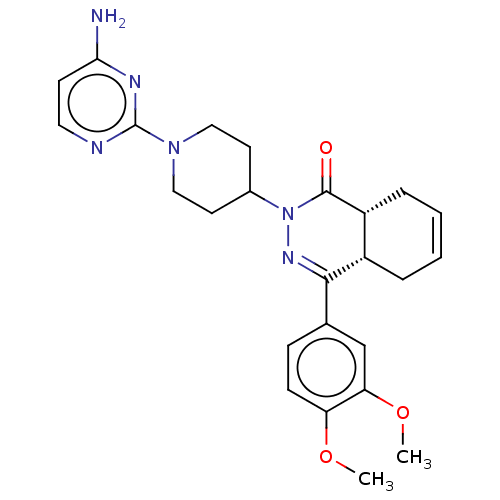 (CHEMBL4444578) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527567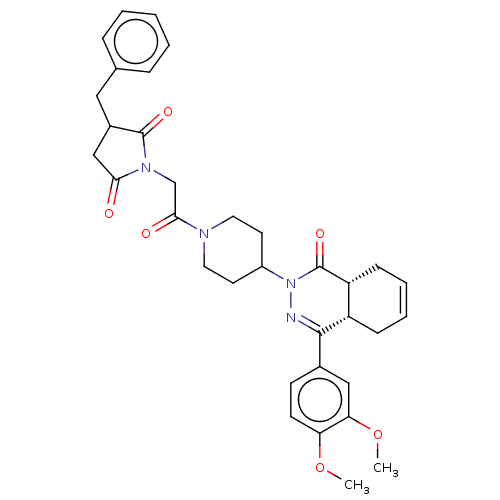 (CHEMBL4473426) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135637 (1-{(S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527554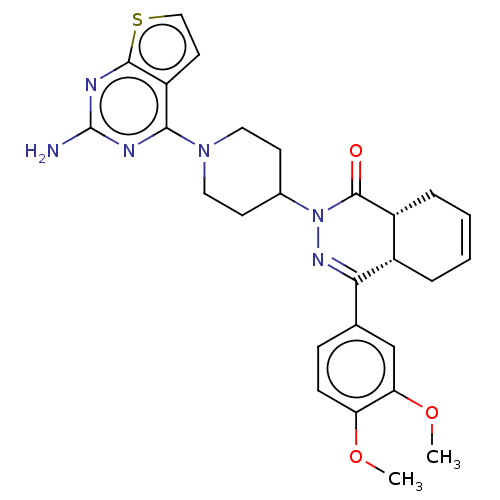 (CHEMBL4460623) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527559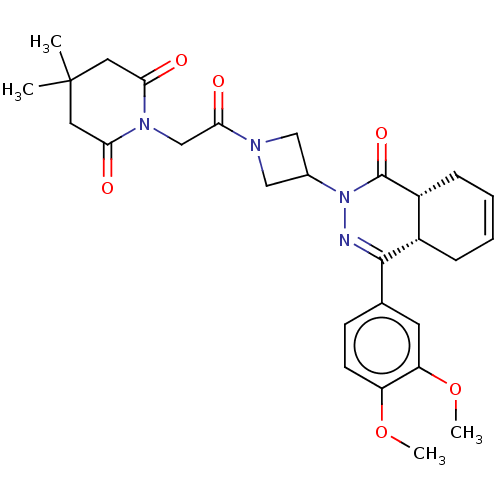 (CHEMBL4591859) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135634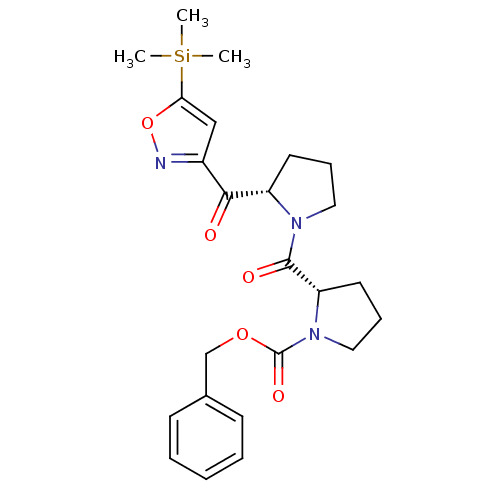 ((S)-2-[(S)-2-(5-Trimethylsilanyl-isoxazole-3-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527525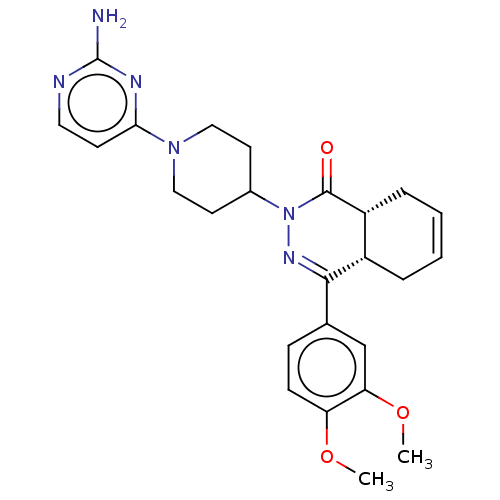 (CHEMBL4522249) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527529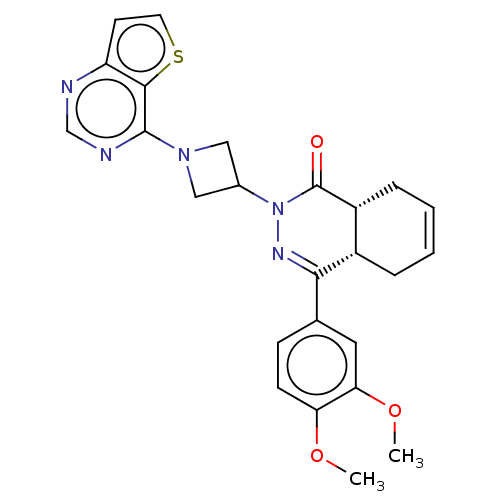 (CHEMBL4454600) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527527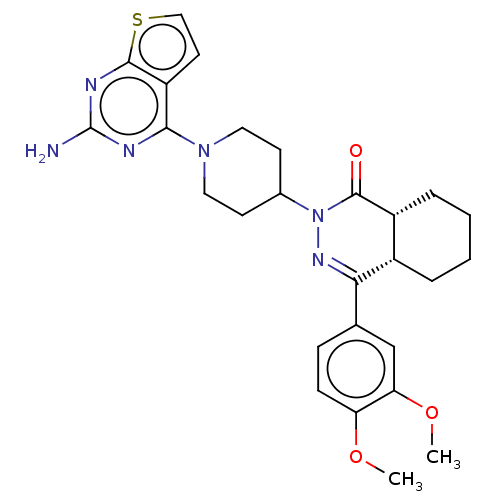 (CHEMBL4466777) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527530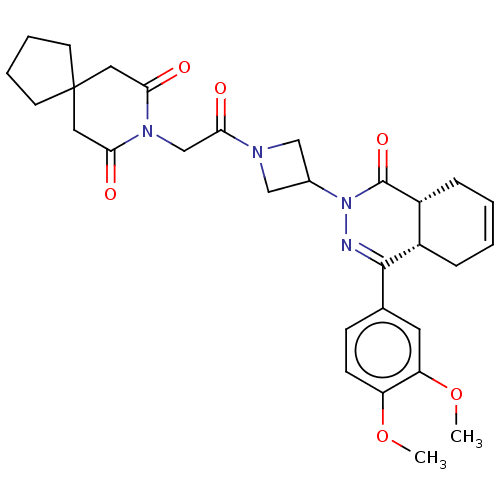 (CHEMBL4461705) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527534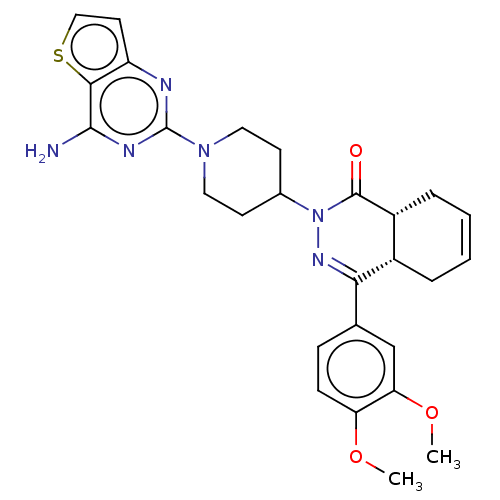 (CHEMBL4472307) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527548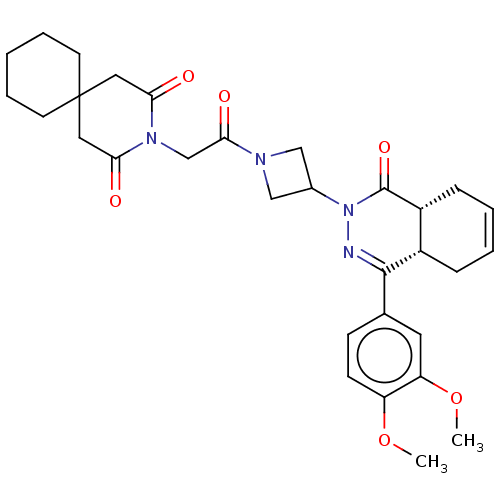 (CHEMBL4465097) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527549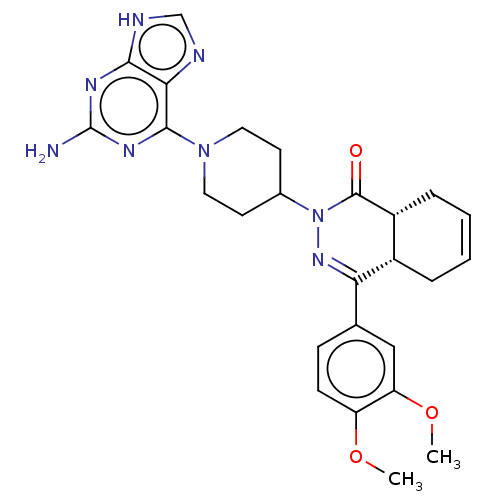 (CHEMBL4452707) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527523 (CHEMBL4461658) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527557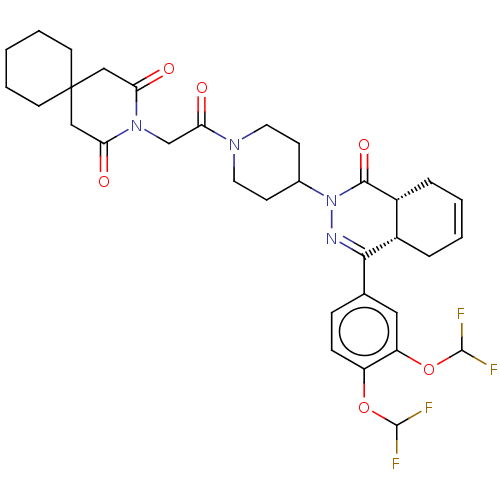 (CHEMBL4533993) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibition of Dipeptidyl Peptidase IV | Bioorg Med Chem Lett 12: 2825-8 (2002) BindingDB Entry DOI: 10.7270/Q279457M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527566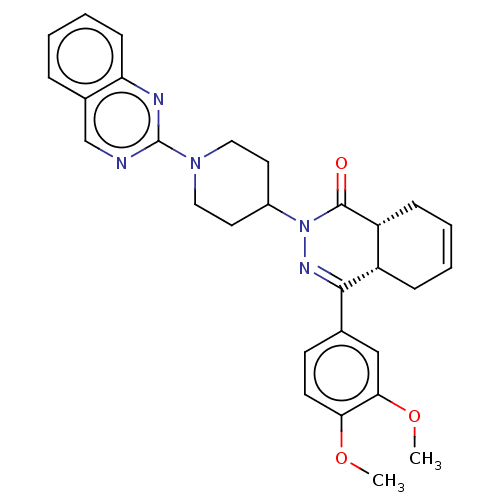 (CHEMBL4472329) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527551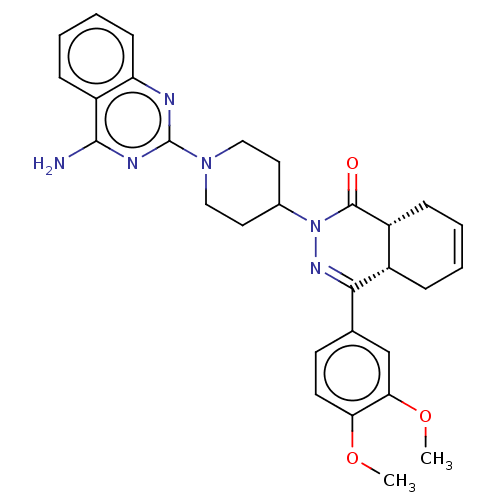 (CHEMBL4575003) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527536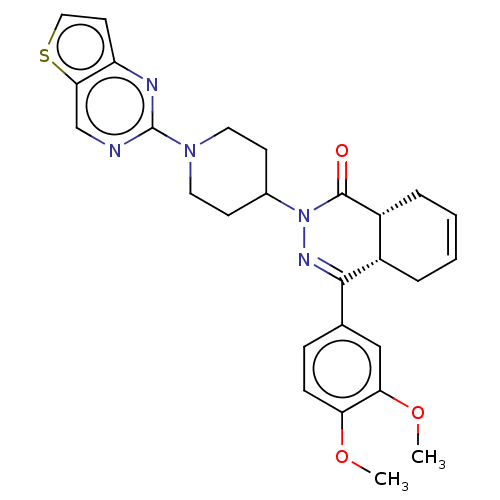 (CHEMBL4466905) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50434188 (CHEMBL2385281 | US9346814, Cmpd No 2 Example 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of mouse recombinant FAP expressed in HEK293 cells using Ala-Pro-p-nitroanilide as substrate incubated for 15 mins prior to substrate addi... | ACS Med Chem Lett 4: 491-6 (2013) Article DOI: 10.1021/ml300410d BindingDB Entry DOI: 10.7270/Q2KP83J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of PKD2 ( assessed as residual activity at 1 uM ) by TR-FRET assay | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50434169 (CHEMBL2385300 | US9346814, Cmpd No 22 Example 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of mouse recombinant FAP expressed in HEK293 cells using Ala-Pro-p-nitroanilide as substrate incubated for 15 mins prior to substrate addi... | ACS Med Chem Lett 4: 491-6 (2013) Article DOI: 10.1021/ml300410d BindingDB Entry DOI: 10.7270/Q2KP83J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50350167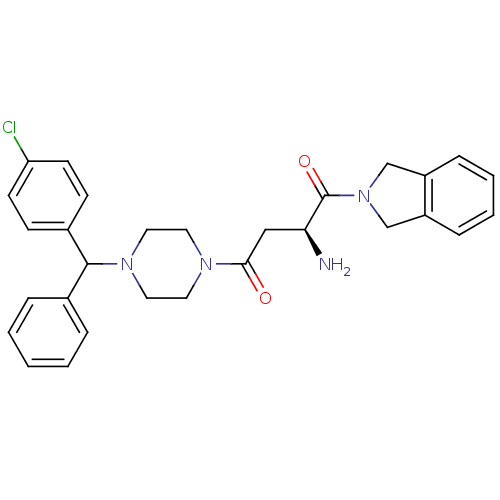 (CHEMBL1814741) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human DPP8 assessed as pNA release from Ala-Pro- p-nitroanilide substrate pre-incubated with enzyme for 15 min ... | J Med Chem 54: 5737-46 (2011) Article DOI: 10.1021/jm200383j BindingDB Entry DOI: 10.7270/Q2G73F37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135636 ((S)-2-[(S)-2-(5-Cyano-isoxazole-3-carbonyl)-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50110025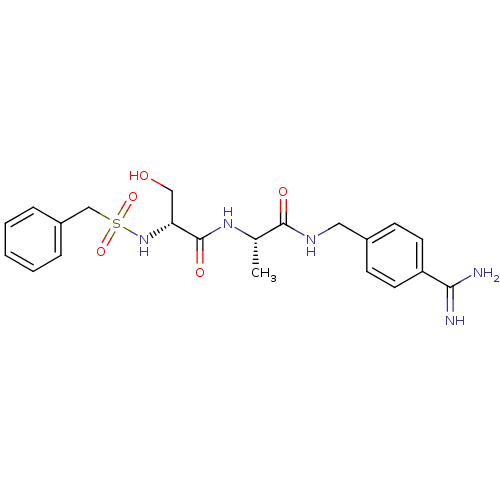 (CHEMBL158405 | N-(BENZYLSULFONYL)-D-SERYL-N-{4-[AM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human uPA using pyroGlu-Gly-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins by spectrop... | Bioorg Med Chem 20: 1557-68 (2012) Article DOI: 10.1016/j.bmc.2011.12.040 BindingDB Entry DOI: 10.7270/Q2QR4XK1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16152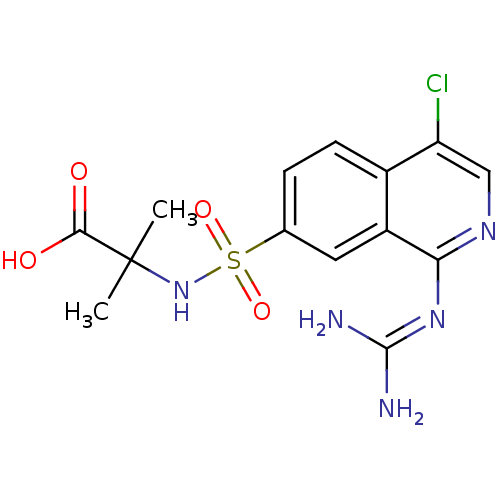 (2-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using S-2444 as substrate | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human; Moderately active | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135635 (2-[2-(Isothiazole-3-carbonyl)-pyrrolidine-1-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50228427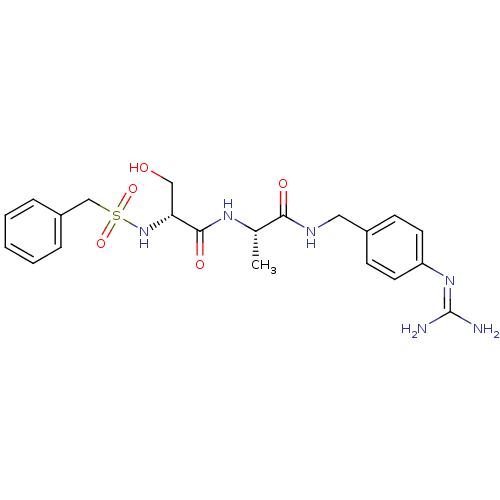 ((R)-N-[(S)-1-(4-guanidino-benzylcarbamoyl)-ethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human uPA | J Med Chem 50: 6638-46 (2007) Article DOI: 10.1021/jm700962j BindingDB Entry DOI: 10.7270/Q2MW2J0J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase IV (Porphyromonas gingivalis) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrate preincubate... | Eur J Med Chem 139: 482-491 (2017) Article DOI: 10.1016/j.ejmech.2017.08.024 BindingDB Entry DOI: 10.7270/Q25B050P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2723 total ) | Next | Last >> |