 Found 4011 hits with Last Name = 'thomas' and Initial = 'r'
Found 4011 hits with Last Name = 'thomas' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50527134

(CHEMBL4471306 | US20230295213, Compound a)Show SMILES C[C@H](Nc1cc(Cl)nc2n(ncc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O)c1ccccc1F |r| Show InChI InChI=1S/C20H24ClFN4O9P2/c1-10(11-4-2-3-5-13(11)22)24-14-6-16(21)25-19-12(14)7-23-26(19)20-18(28)17(27)15(35-20)8-34-37(32,33)9-36(29,30)31/h2-7,10,15,17-18,20,27-28H,8-9H2,1H3,(H,24,25)(H,32,33)(H2,29,30,31)/t10-,15+,17+,18+,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arcus Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in HEK293 cells using AMP as substrate preincubated for 1 h... |
J Med Chem 63: 3935-3955 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01713
BindingDB Entry DOI: 10.7270/Q2G1648T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
WD repeat-containing protein 5
(Homo sapiens (Human)) | BDBM50520121
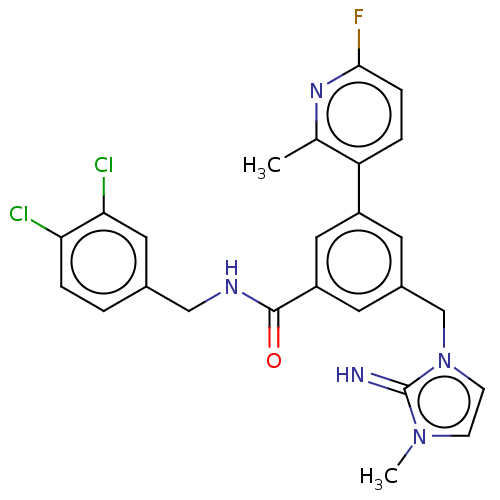
(CHEMBL4441671 | US10844044, Example 1)Show SMILES Cc1nc(F)ccc1-c1cc(Cn2ccn(C)c2=N)cc(c1)C(=O)NCc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H22Cl2FN5O/c1-15-20(4-6-23(28)31-15)18-9-17(14-33-8-7-32(2)25(33)29)10-19(12-18)24(34)30-13-16-3-5-21(26)22(27)11-16/h3-12,29H,13-14H2,1-2H3,(H,30,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research
Curated by ChEMBL
| Assay Description
Competitive inhibition of human N-terminal His6-SUMO tagged WDR5 (22 to 334 residues) expressed in Escherichia coli BL21-Gold(DE3) cells using 10-mer... |
J Med Chem 63: 656-675 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01608
BindingDB Entry DOI: 10.7270/Q2HD801W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061031
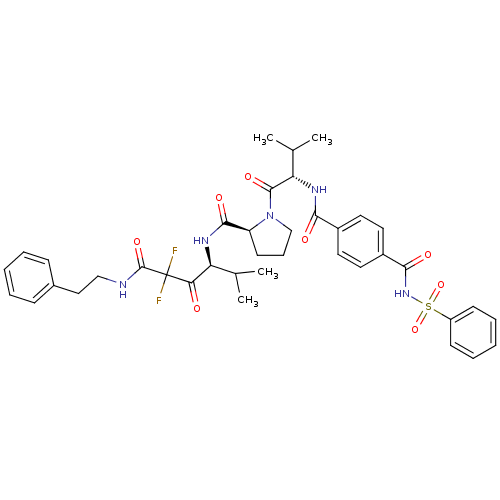
((S)-1-[(S)-2-(4-Benzenesulfonylaminocarbonyl-benzo...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C39H45F2N5O8S/c1-24(2)31(33(47)39(40,41)38(52)42-22-21-26-12-7-5-8-13-26)43-36(50)30-16-11-23-46(30)37(51)32(25(3)4)44-34(48)27-17-19-28(20-18-27)35(49)45-55(53,54)29-14-9-6-10-15-29/h5-10,12-15,17-20,24-25,30-32H,11,16,21-23H2,1-4H3,(H,42,52)(H,43,50)(H,44,48)(H,45,49)/t30-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474415

(CHEMBL2112882)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)C(=O)N[C@]1(C)O[C@@]2(O)[C@H](Cc5ccccc5)NC(=O)[C@H](CC(C)C)N2C1=O)c34 |c:12| Show InChI InChI=1S/C34H39N5O5/c1-19(2)13-27-31(41)36-28(14-20-9-6-5-7-10-20)34(43)39(27)32(42)33(3,44-34)37-30(40)22-15-24-23-11-8-12-25-29(23)21(17-35-25)16-26(24)38(4)18-22/h5-12,15,17,19,22,26-28,35,43H,13-14,16,18H2,1-4H3,(H,36,41)(H,37,40)/t22-,26-,27+,28+,33-,34+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 1A human cloned receptors in HEK293 cells using [3H]8-OH-DPAT as radioligand |
J Med Chem 46: 5117-20 (2003)
Article DOI: 10.1021/jm0341204
BindingDB Entry DOI: 10.7270/Q2Q242ZQ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50179929

(2'-Fluoro-5'-[8-fluoro-7-(1-hydroxy-1-methyl-ethyl...)Show SMILES CC(C)(O)c1ccn2c(cnc2c1F)-c1ccc(F)c(c1)-c1ccccc1C#N Show InChI InChI=1S/C23H17F2N3O/c1-23(2,29)18-9-10-28-20(13-27-22(28)21(18)25)14-7-8-19(24)17(11-14)16-6-4-3-5-15(16)12-26/h3-11,13,29H,1-2H3 | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 15-1788 from human GABA-Aalpha1 receptor plus beta3gamma2 expressed in mouse L(tk-) cells |
Bioorg Med Chem Lett 16: 1518-22 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.037
BindingDB Entry DOI: 10.7270/Q2HM582K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412114
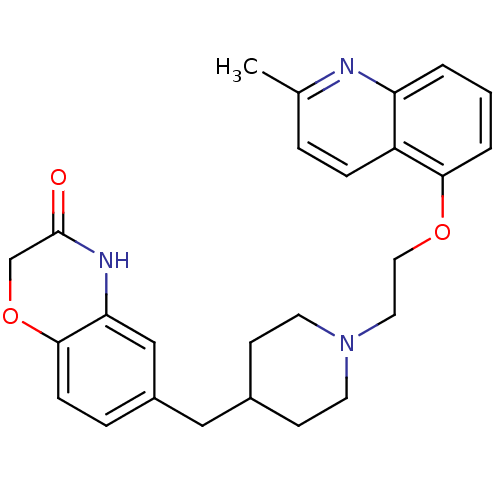
(CHEMBL183460 | SB-649915)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4ccc5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H29N3O3/c1-18-5-7-21-22(27-18)3-2-4-24(21)31-14-13-29-11-9-19(10-12-29)15-20-6-8-25-23(16-20)28-26(30)17-32-25/h2-8,16,19H,9-15,17H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50179929

(2'-Fluoro-5'-[8-fluoro-7-(1-hydroxy-1-methyl-ethyl...)Show SMILES CC(C)(O)c1ccn2c(cnc2c1F)-c1ccc(F)c(c1)-c1ccccc1C#N Show InChI InChI=1S/C23H17F2N3O/c1-23(2,29)18-9-10-28-20(13-27-22(28)21(18)25)14-7-8-19(24)17(11-14)16-6-4-3-5-15(16)12-26/h3-11,13,29H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 15-1788 from human GABA-Aalpha3 receptor plus beta3gamma2 expressed in mouse L(tk-) cells |
Bioorg Med Chem Lett 16: 1518-22 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.037
BindingDB Entry DOI: 10.7270/Q2HM582K |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061043
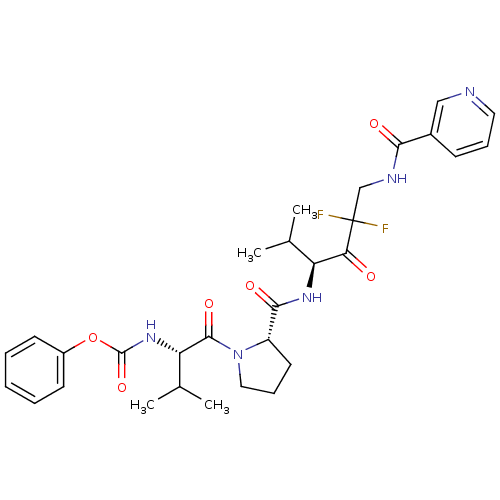
(CHEMBL106592 | [(S)-1-((S)-2-{(S)-3,3-Difluoro-1-i...)Show SMILES CC(C)[C@H](NC(=O)Oc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)CNC(=O)c1cccnc1 Show InChI InChI=1S/C30H37F2N5O6/c1-18(2)23(25(38)30(31,32)17-34-26(39)20-10-8-14-33-16-20)35-27(40)22-13-9-15-37(22)28(41)24(19(3)4)36-29(42)43-21-11-6-5-7-12-21/h5-8,10-12,14,16,18-19,22-24H,9,13,15,17H2,1-4H3,(H,34,39)(H,35,40)(H,36,42)/t22-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061047
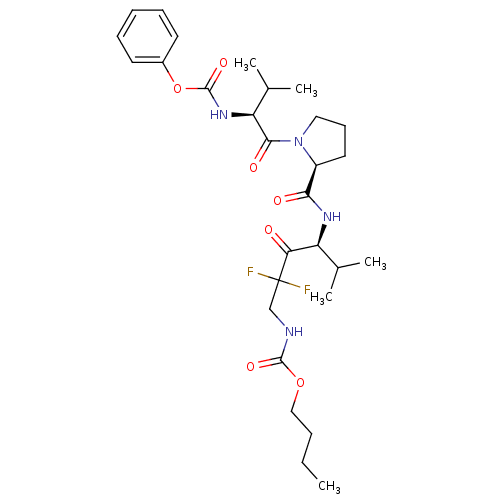
(((S)-2,2-Difluoro-5-methyl-4-{[(S)-1-((S)-3-methyl...)Show SMILES CCCCOC(=O)NCC(F)(F)C(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)Oc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C29H42F2N4O7/c1-6-7-16-41-27(39)32-17-29(30,31)24(36)22(18(2)3)33-25(37)21-14-11-15-35(21)26(38)23(19(4)5)34-28(40)42-20-12-9-8-10-13-20/h8-10,12-13,18-19,21-23H,6-7,11,14-17H2,1-5H3,(H,32,39)(H,33,37)(H,34,40)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061028
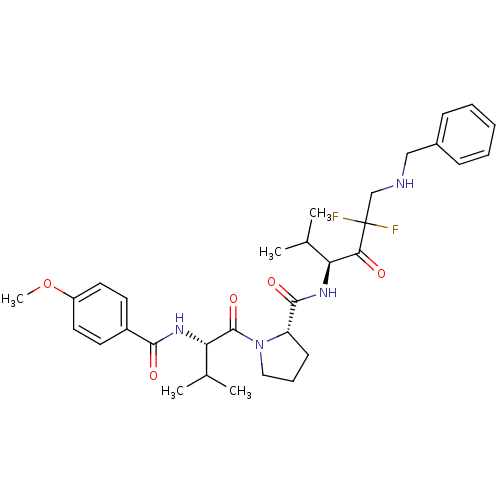
((S)-1-[(S)-2-(4-Methoxy-benzoylamino)-3-methyl-but...)Show SMILES COc1ccc(cc1)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)CNCc1ccccc1 Show InChI InChI=1S/C32H42F2N4O5/c1-20(2)26(28(39)32(33,34)19-35-18-22-10-7-6-8-11-22)36-30(41)25-12-9-17-38(25)31(42)27(21(3)4)37-29(40)23-13-15-24(43-5)16-14-23/h6-8,10-11,13-16,20-21,25-27,35H,9,12,17-19H2,1-5H3,(H,36,41)(H,37,40)/t25-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061037
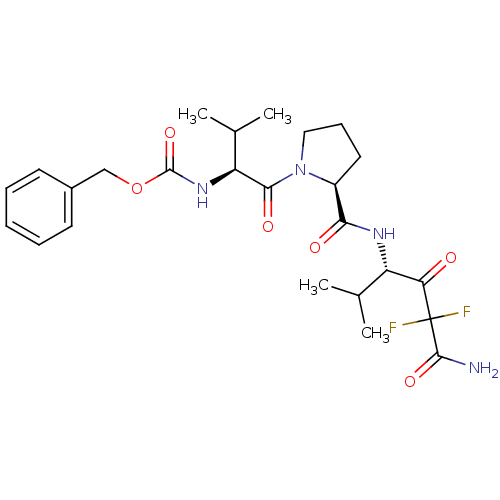
(CHEMBL302961 | {(S)-1-[(S)-2-((S)-3-Carbamoyl-3,3-...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)C(N)=O Show InChI InChI=1S/C25H34F2N4O6/c1-14(2)18(20(32)25(26,27)23(28)35)29-21(33)17-11-8-12-31(17)22(34)19(15(3)4)30-24(36)37-13-16-9-6-5-7-10-16/h5-7,9-10,14-15,17-19H,8,11-13H2,1-4H3,(H2,28,35)(H,29,33)(H,30,36)/t17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM21392

(3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...)Show InChI InChI=1S/C11H13N3O/c12-4-3-8-6-14-10-2-1-7(11(13)15)5-9(8)10/h1-2,5-6,14H,3-4,12H2,(H2,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 7 human receptors |
J Med Chem 46: 5117-20 (2003)
Article DOI: 10.1021/jm0341204
BindingDB Entry DOI: 10.7270/Q2Q242ZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222900
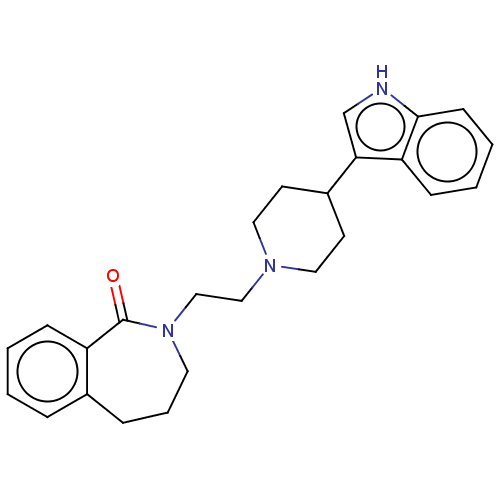
(CHEMBL267062)Show SMILES O=C1N(CCN2CCC(CC2)c2c[nH]c3ccccc23)CCCc2ccccc12 Show InChI InChI=1S/C25H29N3O/c29-25-21-8-2-1-6-19(21)7-5-13-28(25)17-16-27-14-11-20(12-15-27)23-18-26-24-10-4-3-9-22(23)24/h1-4,6,8-10,18,20,26H,5,7,11-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50179931
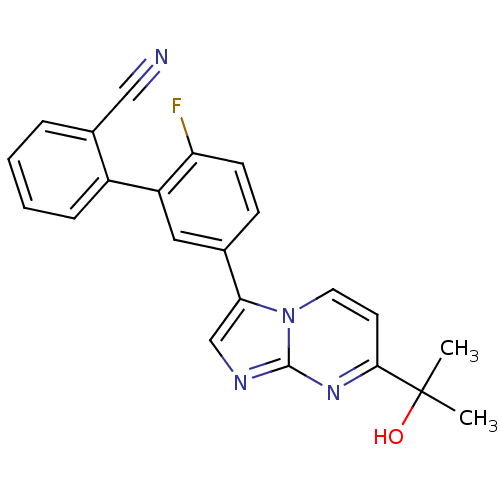
(2'-Fluoro-5'-[7-(1-hydroxy-1-methyl-ethyl)-imidazo...)Show SMILES CC(C)(O)c1ccn2c(cnc2n1)-c1ccc(F)c(c1)-c1ccccc1C#N Show InChI InChI=1S/C22H17FN4O/c1-22(2,28)20-9-10-27-19(13-25-21(27)26-20)14-7-8-18(23)17(11-14)16-6-4-3-5-15(16)12-24/h3-11,13,28H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 15-1788 from human GABA-Aalpha3 receptor plus beta3gamma2 expressed in mouse L(tk-) cells |
Bioorg Med Chem Lett 16: 1518-22 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.037
BindingDB Entry DOI: 10.7270/Q2HM582K |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM86210
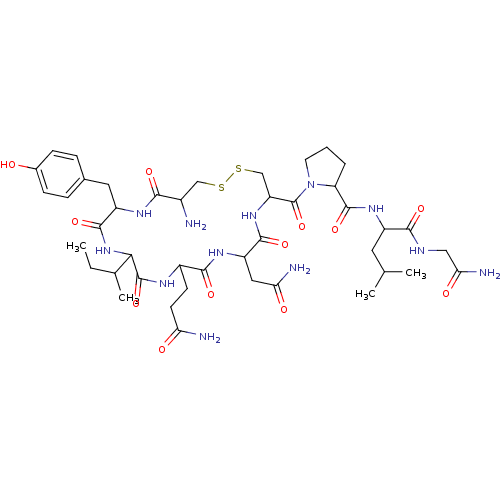
(CAS_50-56-6 | NSC_439302 | Oxytocin)Show SMILES CCC(C)C1NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(N)CSSCC(NC(=O)C(CC(N)=O)NC(=O)C(CCC(N)=O)NC1=O)C(=O)N1CCCC1C(=O)NC(CC(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LCG Bioscience
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 253-61 (2003)
Article DOI: 10.1124/jpet.103.049395
BindingDB Entry DOI: 10.7270/Q2MC8XKT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50219087

(CHEMBL97637)Show SMILES O=S(=O)(Nc1ccc2nccc(N3CCNCC3)c2c1)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C25H24N4O2S/c30-32(31,22-9-6-20(7-10-22)19-4-2-1-3-5-19)28-21-8-11-24-23(18-21)25(12-13-27-24)29-16-14-26-15-17-29/h1-13,18,26,28H,14-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned 5-HT6 receptor in HeLa cells using [3H]- LSD as radioligand |
Bioorg Med Chem Lett 11: 2843-6 (2001)
BindingDB Entry DOI: 10.7270/Q2TB192H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217828

(CHEMBL413707)Show SMILES Fc1ccc(OC2CCN(CC[C@H]3CCCN3S(=O)(=O)c3ccc4cc[nH]c4c3)CC2)cc1 Show InChI InChI=1S/C25H30FN3O3S/c26-20-4-6-22(7-5-20)32-23-11-16-28(17-12-23)15-10-21-2-1-14-29(21)33(30,31)24-8-3-19-9-13-27-25(19)18-24/h3-9,13,18,21,23,27H,1-2,10-12,14-17H2/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50219087

(CHEMBL97637)Show SMILES O=S(=O)(Nc1ccc2nccc(N3CCNCC3)c2c1)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C25H24N4O2S/c30-32(31,22-9-6-20(7-10-22)19-4-2-1-3-5-19)28-21-8-11-24-23(18-21)25(12-13-27-24)29-16-14-26-15-17-29/h1-13,18,26,28H,14-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against human 5-hydroxytryptamine 6 receptor |
Bioorg Med Chem Lett 11: 2843-6 (2001)
BindingDB Entry DOI: 10.7270/Q2TB192H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222781
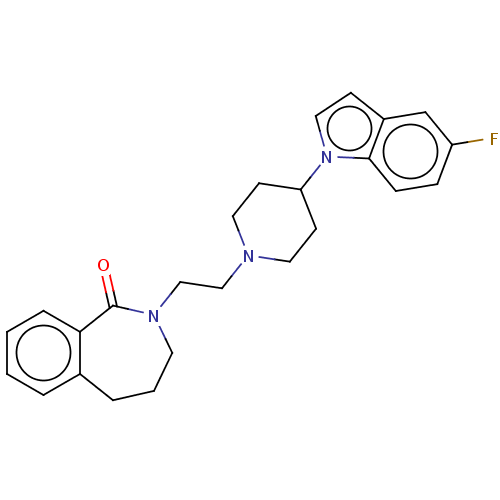
(CHEMBL9951)Show SMILES Fc1ccc2n(ccc2c1)C1CCN(CCN2CCCc3ccccc3C2=O)CC1 Show InChI InChI=1S/C25H28FN3O/c26-21-7-8-24-20(18-21)9-15-29(24)22-10-13-27(14-11-22)16-17-28-12-3-5-19-4-1-2-6-23(19)25(28)30/h1-2,4,6-9,15,18,22H,3,5,10-14,16-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50031210
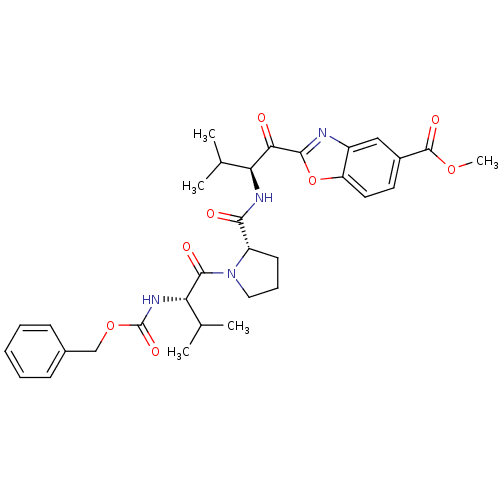
(2-((S)-2-{[(S)-1-((S)-2-Benzyloxycarbonylamino-3-m...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C32H38N4O8/c1-18(2)25(27(37)29-33-22-16-21(31(40)42-5)13-14-24(22)44-29)34-28(38)23-12-9-15-36(23)30(39)26(19(3)4)35-32(41)43-17-20-10-7-6-8-11-20/h6-8,10-11,13-14,16,18-19,23,25-26H,9,12,15,17H2,1-5H3,(H,34,38)(H,35,41)/t23-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50219081
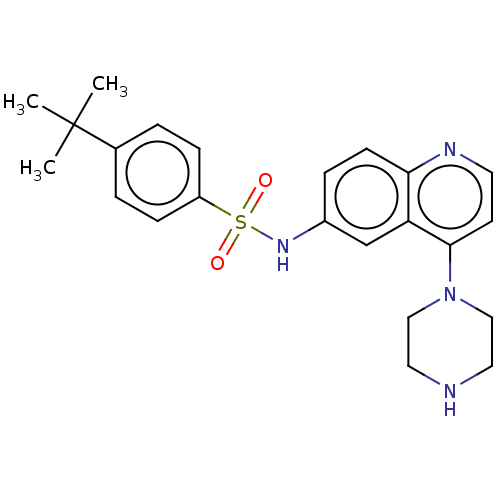
(CHEMBL261917)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Nc1ccc2nccc(N3CCNCC3)c2c1 Show InChI InChI=1S/C23H28N4O2S/c1-23(2,3)17-4-7-19(8-5-17)30(28,29)26-18-6-9-21-20(16-18)22(10-11-25-21)27-14-12-24-13-15-27/h4-11,16,24,26H,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned 5-hydroxytryptamine 6 receptor in HeLa cells using [3H]- LSD as radioligand |
Bioorg Med Chem Lett 11: 2843-6 (2001)
BindingDB Entry DOI: 10.7270/Q2TB192H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50130286
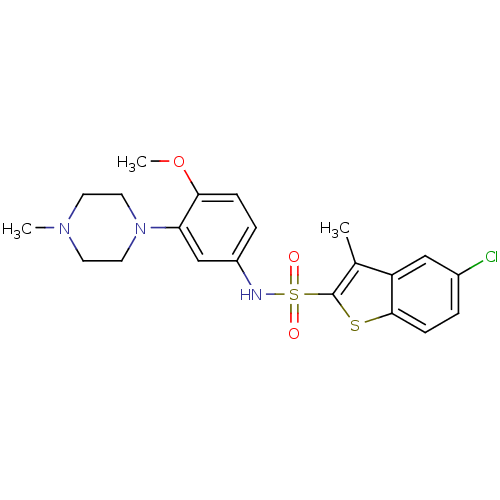
(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonic aci...)Show SMILES COc1ccc(NS(=O)(=O)c2sc3ccc(Cl)cc3c2C)cc1N1CCN(C)CC1 Show InChI InChI=1S/C21H24ClN3O3S2/c1-14-17-12-15(22)4-7-20(17)29-21(14)30(26,27)23-16-5-6-19(28-3)18(13-16)25-10-8-24(2)9-11-25/h4-7,12-13,23H,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
The compound was tested for the binding affinity towards human cloned 5-hydroxytryptamine 6 receptor in HeLa cells, using [3H]5-LSD as radioligand; n... |
Bioorg Med Chem Lett 12: 1357-60 (2002)
BindingDB Entry DOI: 10.7270/Q2NK3H7V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM21392

(3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...)Show InChI InChI=1S/C11H13N3O/c12-4-3-8-6-14-10-2-1-7(11(13)15)5-9(8)10/h1-2,5-6,14H,3-4,12H2,(H2,13,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 1A human cloned receptors in HEK293 cells using [3H]8-OH-DPAT as radioligand |
J Med Chem 46: 5117-20 (2003)
Article DOI: 10.1021/jm0341204
BindingDB Entry DOI: 10.7270/Q2Q242ZQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50219081

(CHEMBL261917)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Nc1ccc2nccc(N3CCNCC3)c2c1 Show InChI InChI=1S/C23H28N4O2S/c1-23(2,3)17-4-7-19(8-5-17)30(28,29)26-18-6-9-21-20(16-18)22(10-11-25-21)27-14-12-24-13-15-27/h4-11,16,24,26H,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against human 5-hydroxytryptamine 6 receptor |
Bioorg Med Chem Lett 11: 2843-6 (2001)
BindingDB Entry DOI: 10.7270/Q2TB192H |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061035
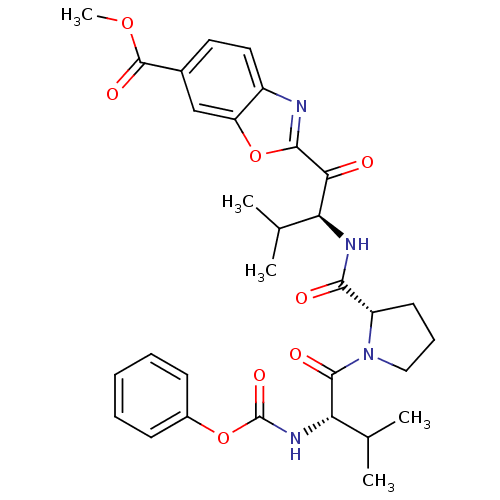
(2-((S)-3-Methyl-2-{[(S)-1-((S)-3-methyl-2-phenoxyc...)Show SMILES COC(=O)c1ccc2nc(oc2c1)C(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)Oc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C31H36N4O8/c1-17(2)24(26(36)28-32-21-14-13-19(30(39)41-5)16-23(21)43-28)33-27(37)22-12-9-15-35(22)29(38)25(18(3)4)34-31(40)42-20-10-7-6-8-11-20/h6-8,10-11,13-14,16-18,22,24-25H,9,12,15H2,1-5H3,(H,33,37)(H,34,40)/t22-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061044
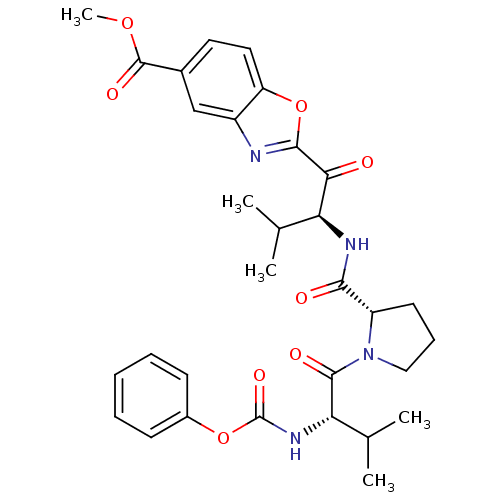
(2-((S)-3-Methyl-2-{[(S)-1-((S)-3-methyl-2-phenoxyc...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)Oc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C31H36N4O8/c1-17(2)24(26(36)28-32-21-16-19(30(39)41-5)13-14-23(21)43-28)33-27(37)22-12-9-15-35(22)29(38)25(18(3)4)34-31(40)42-20-10-7-6-8-11-20/h6-8,10-11,13-14,16-18,22,24-25H,9,12,15H2,1-5H3,(H,33,37)(H,34,40)/t22-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061049
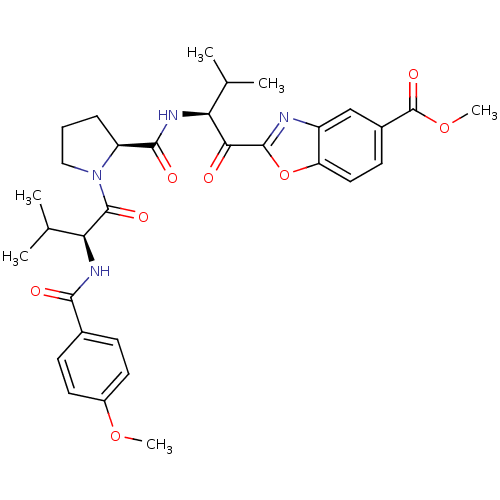
(2-[(S)-2-({(S)-1-[(S)-2-(4-Methoxy-benzoylamino)-3...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)c1ccc(OC)cc1)C(C)C)C(C)C Show InChI InChI=1S/C32H38N4O8/c1-17(2)25(27(37)30-33-22-16-20(32(41)43-6)11-14-24(22)44-30)34-29(39)23-8-7-15-36(23)31(40)26(18(3)4)35-28(38)19-9-12-21(42-5)13-10-19/h9-14,16-18,23,25-26H,7-8,15H2,1-6H3,(H,34,39)(H,35,38)/t23-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50326722
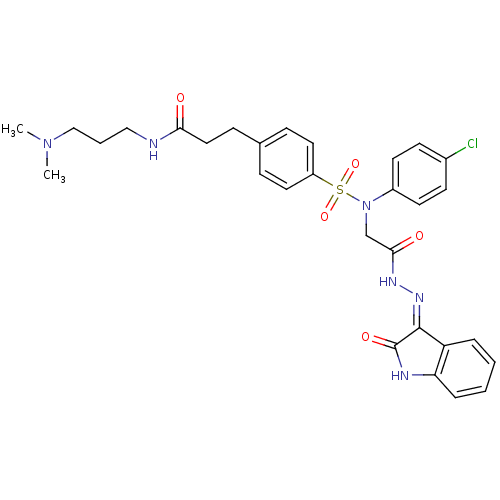
((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...)Show SMILES CN(C)CCCNC(=O)CCc1ccc(cc1)S(=O)(=O)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C30H33ClN6O5S/c1-36(2)19-5-18-32-27(38)17-10-21-8-15-24(16-9-21)43(41,42)37(23-13-11-22(31)12-14-23)20-28(39)34-35-29-25-6-3-4-7-26(25)33-30(29)40/h3-4,6-9,11-16H,5,10,17-20H2,1-2H3,(H,32,38)(H,34,39)(H,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells |
J Med Chem 48: 7882-905 (2005)
Article DOI: 10.1021/jm050645f
BindingDB Entry DOI: 10.7270/Q2QF8TN3 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50326722

((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...)Show SMILES CN(C)CCCNC(=O)CCc1ccc(cc1)S(=O)(=O)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C30H33ClN6O5S/c1-36(2)19-5-18-32-27(38)17-10-21-8-15-24(16-9-21)43(41,42)37(23-13-11-22(31)12-14-23)20-28(39)34-35-29-25-6-3-4-7-26(25)33-30(29)40/h3-4,6-9,11-16H,5,10,17-20H2,1-2H3,(H,32,38)(H,34,39)(H,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells |
J Med Chem 48: 7882-905 (2005)
Article DOI: 10.1021/jm050645f
BindingDB Entry DOI: 10.7270/Q2QF8TN3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217831

(CHEMBL430706)Show SMILES O=S(=O)(N1CCC[C@@H]1CCN1CCC(CC1)c1c[nH]c2ccccc12)c1ccc2cc[nH]c2c1 |r| Show InChI InChI=1S/C27H32N4O2S/c32-34(33,23-8-7-21-9-13-28-27(21)18-23)31-14-3-4-22(31)12-17-30-15-10-20(11-16-30)25-19-29-26-6-2-1-5-24(25)26/h1-2,5-9,13,18-20,22,28-29H,3-4,10-12,14-17H2/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217829
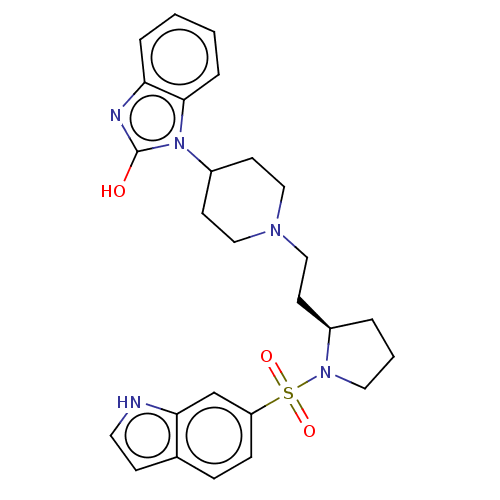
(CHEMBL115262)Show SMILES Oc1nc2ccccc2n1C1CCN(CC[C@H]2CCCN2S(=O)(=O)c2ccc3cc[nH]c3c2)CC1 Show InChI InChI=1S/C26H31N5O3S/c32-26-28-23-5-1-2-6-25(23)31(26)21-11-16-29(17-12-21)15-10-20-4-3-14-30(20)35(33,34)22-8-7-19-9-13-27-24(19)18-22/h1-2,5-9,13,18,20-21,27H,3-4,10-12,14-17H2,(H,28,32)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM86210

(CAS_50-56-6 | NSC_439302 | Oxytocin)Show SMILES CCC(C)C1NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(N)CSSCC(NC(=O)C(CC(N)=O)NC(=O)C(CCC(N)=O)NC1=O)C(=O)N1CCCC1C(=O)NC(CC(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LCG Bioscience
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 253-61 (2003)
Article DOI: 10.1124/jpet.103.049395
BindingDB Entry DOI: 10.7270/Q2MC8XKT |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50179931

(2'-Fluoro-5'-[7-(1-hydroxy-1-methyl-ethyl)-imidazo...)Show SMILES CC(C)(O)c1ccn2c(cnc2n1)-c1ccc(F)c(c1)-c1ccccc1C#N Show InChI InChI=1S/C22H17FN4O/c1-22(2,28)20-9-10-27-19(13-25-21(27)26-20)14-7-8-18(23)17(11-14)16-6-4-3-5-15(16)12-24/h3-11,13,28H,1-2H3 | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 15-1788 from human GABA-Aalpha1 receptor plus beta3gamma2 expressed in mouse L(tk-) cells |
Bioorg Med Chem Lett 16: 1518-22 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.037
BindingDB Entry DOI: 10.7270/Q2HM582K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50130270
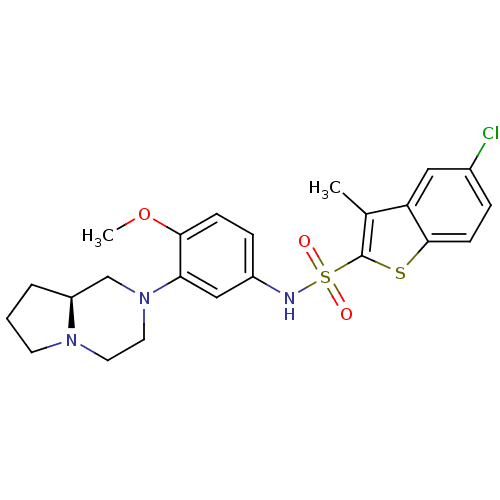
(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonic aci...)Show SMILES COc1ccc(NS(=O)(=O)c2sc3ccc(Cl)cc3c2C)cc1N1CCN2CCC[C@H]2C1 Show InChI InChI=1S/C23H26ClN3O3S2/c1-15-19-12-16(24)5-8-22(19)31-23(15)32(28,29)25-17-6-7-21(30-2)20(13-17)27-11-10-26-9-3-4-18(26)14-27/h5-8,12-13,18,25H,3-4,9-11,14H2,1-2H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
The compound was tested for the binding affinity towards human cloned 5-hydroxytryptamine 6 receptor in HeLa cells, using [3H]5-LSD as radioligand; n... |
Bioorg Med Chem Lett 12: 1357-60 (2002)
BindingDB Entry DOI: 10.7270/Q2NK3H7V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217832
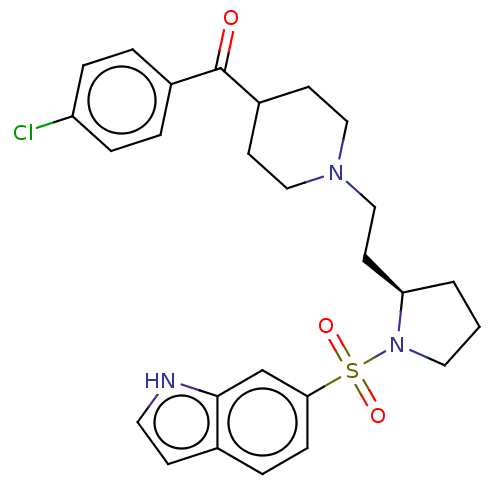
(CHEMBL116292)Show SMILES Clc1ccc(cc1)C(=O)C1CCN(CC[C@H]2CCCN2S(=O)(=O)c2ccc3cc[nH]c3c2)CC1 Show InChI InChI=1S/C26H30ClN3O3S/c27-22-6-3-20(4-7-22)26(31)21-10-15-29(16-11-21)17-12-23-2-1-14-30(23)34(32,33)24-8-5-19-9-13-28-25(19)18-24/h3-9,13,18,21,23,28H,1-2,10-12,14-17H2/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50001824
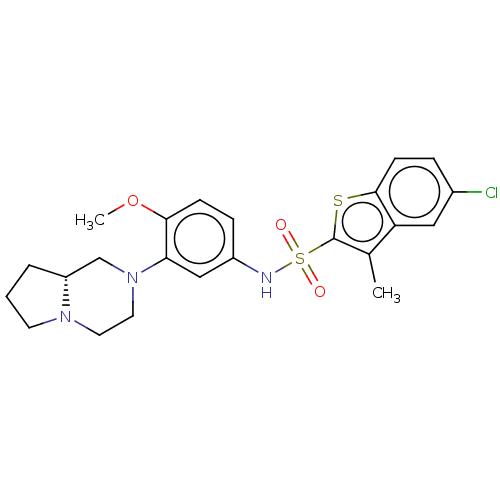
(CHEMBL282971)Show SMILES [H][C@]12CCCN1CCN(C2)c1cc(NS(=O)(=O)c2sc3ccc(Cl)cc3c2C)ccc1OC Show InChI InChI=1S/C23H26ClN3O3S2/c1-15-19-12-16(24)5-8-22(19)31-23(15)32(28,29)25-17-6-7-21(30-2)20(13-17)27-11-10-26-9-3-4-18(26)14-27/h5-8,12-13,18,25H,3-4,9-11,14H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
The compound was tested for the binding affinity towards human cloned 5-hydroxytryptamine 6 receptor in HeLa cells, using [3H]5-LSD as radioligand; n... |
Bioorg Med Chem Lett 12: 1357-60 (2002)
BindingDB Entry DOI: 10.7270/Q2NK3H7V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061054
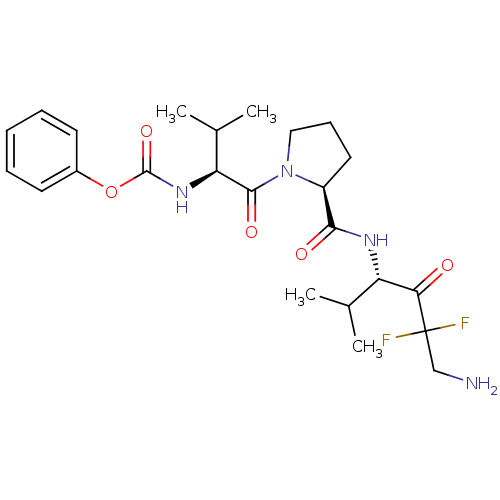
(CHEMBL110125 | CHEMBL66128 | {(S)-1-[(S)-2-((S)-4-...)Show SMILES CC(C)[C@H](NC(=O)Oc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)CN Show InChI InChI=1S/C24H34F2N4O5/c1-14(2)18(20(31)24(25,26)13-27)28-21(32)17-11-8-12-30(17)22(33)19(15(3)4)29-23(34)35-16-9-6-5-7-10-16/h5-7,9-10,14-15,17-19H,8,11-13,27H2,1-4H3,(H,28,32)(H,29,34)/t17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217835
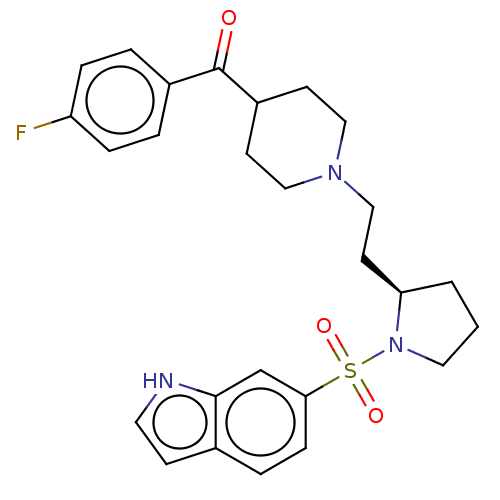
(CHEMBL114345)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CC[C@H]2CCCN2S(=O)(=O)c2ccc3cc[nH]c3c2)CC1 Show InChI InChI=1S/C26H30FN3O3S/c27-22-6-3-20(4-7-22)26(31)21-10-15-29(16-11-21)17-12-23-2-1-14-30(23)34(32,33)24-8-5-19-9-13-28-25(19)18-24/h3-9,13,18,21,23,28H,1-2,10-12,14-17H2/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061036
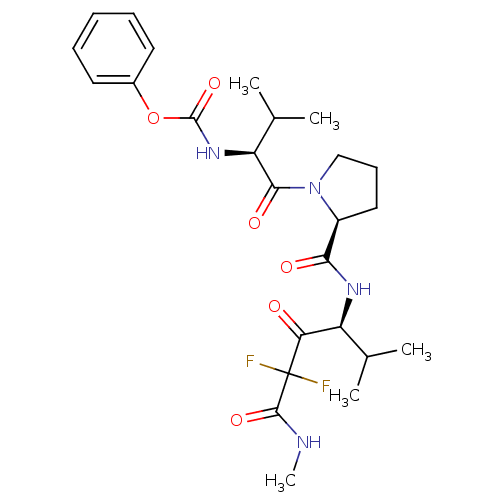
(CHEMBL304114 | {(S)-1-[(S)-2-((S)-3,3-Difluoro-1-i...)Show SMILES CNC(=O)C(F)(F)C(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)Oc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C25H34F2N4O6/c1-14(2)18(20(32)25(26,27)23(35)28-5)29-21(33)17-12-9-13-31(17)22(34)19(15(3)4)30-24(36)37-16-10-7-6-8-11-16/h6-8,10-11,14-15,17-19H,9,12-13H2,1-5H3,(H,28,35)(H,29,33)(H,30,36)/t17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50410618
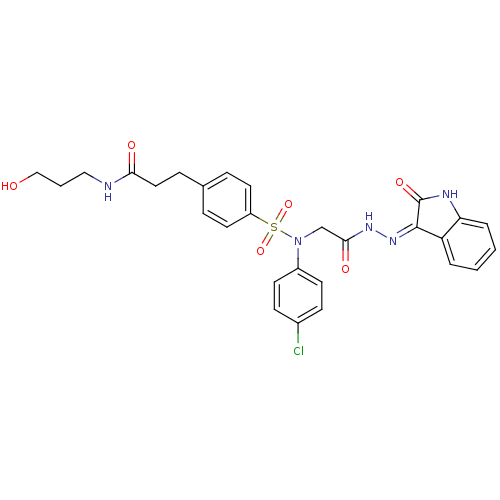
(CHEMBL2113181)Show SMILES OCCCNC(=O)CCc1ccc(cc1)S(=O)(=O)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H28ClN5O6S/c29-20-9-11-21(12-10-20)34(18-26(37)32-33-27-23-4-1-2-5-24(23)31-28(27)38)41(39,40)22-13-6-19(7-14-22)8-15-25(36)30-16-3-17-35/h1-2,4-7,9-14,35H,3,8,15-18H2,(H,30,36)(H,32,37)(H,31,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells |
J Med Chem 48: 7882-905 (2005)
Article DOI: 10.1021/jm050645f
BindingDB Entry DOI: 10.7270/Q2QF8TN3 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50410616
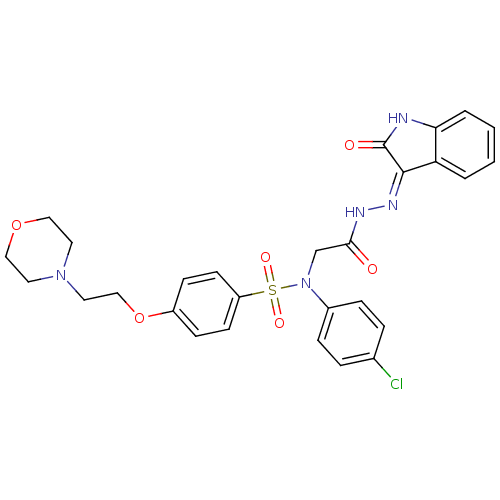
(CHEMBL2113185)Show SMILES Clc1ccc(cc1)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)S(=O)(=O)c1ccc(OCCN2CCOCC2)cc1 Show InChI InChI=1S/C28H28ClN5O6S/c29-20-5-7-21(8-6-20)34(19-26(35)31-32-27-24-3-1-2-4-25(24)30-28(27)36)41(37,38)23-11-9-22(10-12-23)40-18-15-33-13-16-39-17-14-33/h1-12H,13-19H2,(H,31,35)(H,30,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells |
J Med Chem 48: 7882-905 (2005)
Article DOI: 10.1021/jm050645f
BindingDB Entry DOI: 10.7270/Q2QF8TN3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50219083
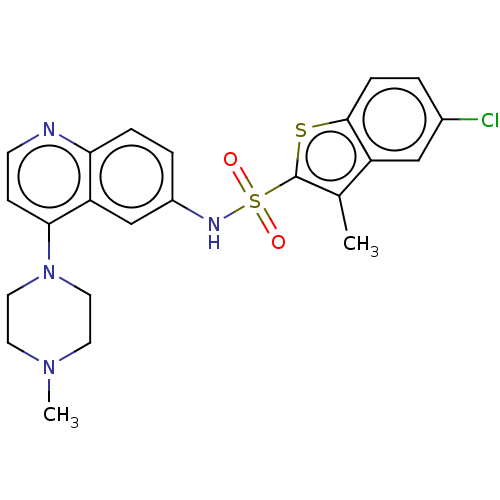
(CHEMBL316081)Show SMILES CN1CCN(CC1)c1ccnc2ccc(NS(=O)(=O)c3sc4ccc(Cl)cc4c3C)cc12 Show InChI InChI=1S/C23H23ClN4O2S2/c1-15-18-13-16(24)3-6-22(18)31-23(15)32(29,30)26-17-4-5-20-19(14-17)21(7-8-25-20)28-11-9-27(2)10-12-28/h3-8,13-14,26H,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against human 5-hydroxytryptamine 6 receptor |
Bioorg Med Chem Lett 11: 2843-6 (2001)
BindingDB Entry DOI: 10.7270/Q2TB192H |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412939
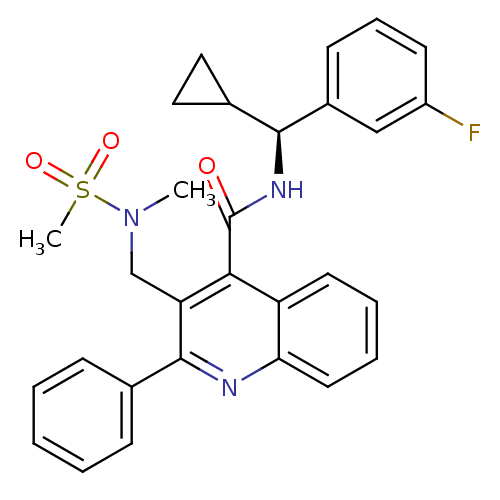
(CHEMBL479463 | GSK-256471)Show SMILES CN(Cc1c(nc2ccccc2c1C(=O)N[C@@H](C1CC1)c1cccc(F)c1)-c1ccccc1)S(C)(=O)=O |r| Show InChI InChI=1S/C29H28FN3O3S/c1-33(37(2,35)36)18-24-26(29(34)32-27(20-15-16-20)21-11-8-12-22(30)17-21)23-13-6-7-14-25(23)31-28(24)19-9-4-3-5-10-19/h3-14,17,20,27H,15-16,18H2,1-2H3,(H,32,34)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50219084
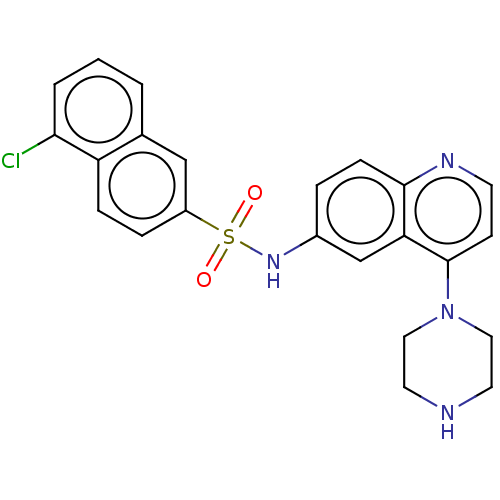
(CHEMBL97596)Show SMILES Clc1cccc2cc(ccc12)S(=O)(=O)Nc1ccc2nccc(N3CCNCC3)c2c1 Show InChI InChI=1S/C23H21ClN4O2S/c24-21-3-1-2-16-14-18(5-6-19(16)21)31(29,30)27-17-4-7-22-20(15-17)23(8-9-26-22)28-12-10-25-11-13-28/h1-9,14-15,25,27H,10-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against human 5-hydroxytryptamine 6 receptor |
Bioorg Med Chem Lett 11: 2843-6 (2001)
BindingDB Entry DOI: 10.7270/Q2TB192H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50219084

(CHEMBL97596)Show SMILES Clc1cccc2cc(ccc12)S(=O)(=O)Nc1ccc2nccc(N3CCNCC3)c2c1 Show InChI InChI=1S/C23H21ClN4O2S/c24-21-3-1-2-16-14-18(5-6-19(16)21)31(29,30)27-17-4-7-22-20(15-17)23(8-9-26-22)28-12-10-25-11-13-28/h1-9,14-15,25,27H,10-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned 5-HT6 receptor in HeLa cells using [3H]- LSD as radioligand |
Bioorg Med Chem Lett 11: 2843-6 (2001)
BindingDB Entry DOI: 10.7270/Q2TB192H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data