

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50368883 (CHEMBL1159458) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039276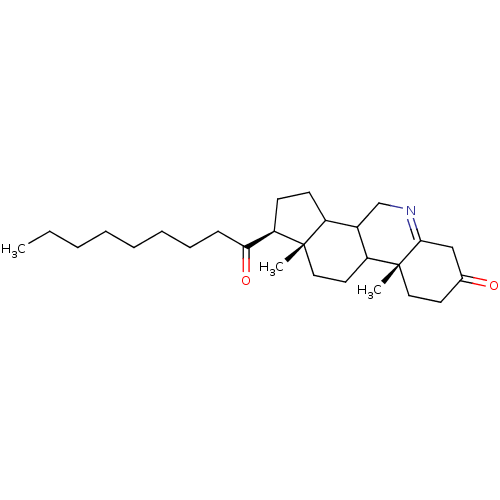 ((1S,9aR,11aS)-9a,11a-Dimethyl-1-nonanoyl-1,2,3,3a,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039277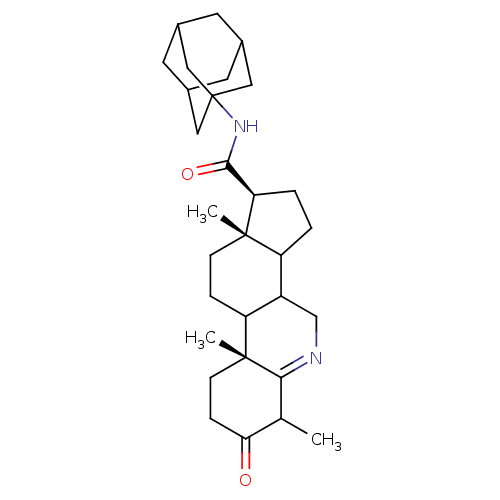 ((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085044 ((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50256480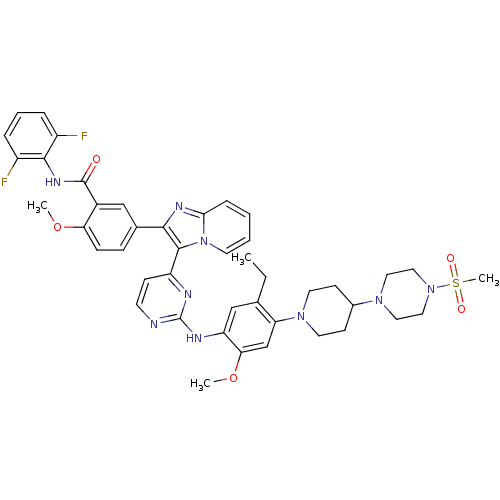 (CHEMBL466397 | N-(2,6-difluorophenyl)-5-(3-(2-(5-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to insulin receptor by liquid scintillation counting | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50418564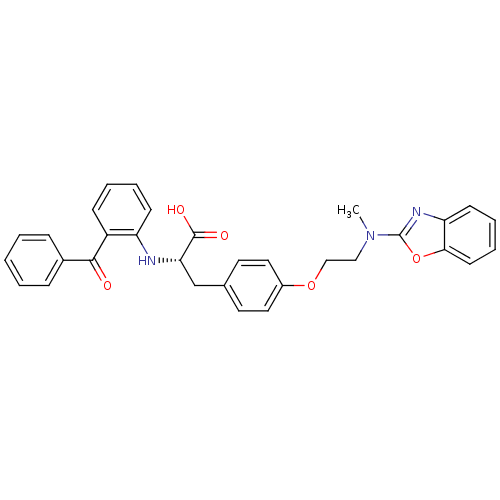 (CHEMBL423026) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256480 (CHEMBL466397 | N-(2,6-difluorophenyl)-5-(3-(2-(5-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to IGF1R by liquid scintillation counting | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50256478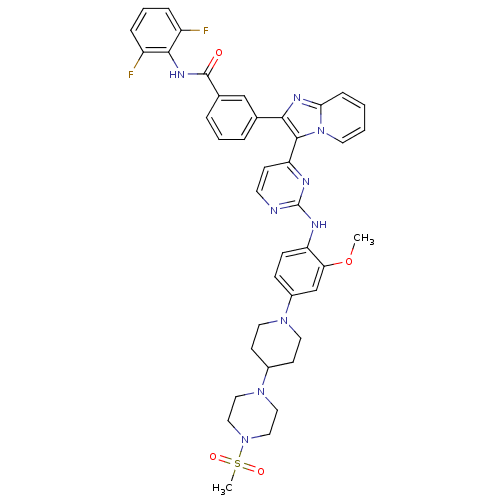 (CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to insulin receptor by liquid scintillation counting | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039315 ((1S,9aR,11aS)-5,9a,11a-Trimethyl-1-(3-methyl-butyr...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039273 ((1S,9aR,11aS)-6-Bromo-9a,11a-dimethyl-1-(3-methyl-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50418568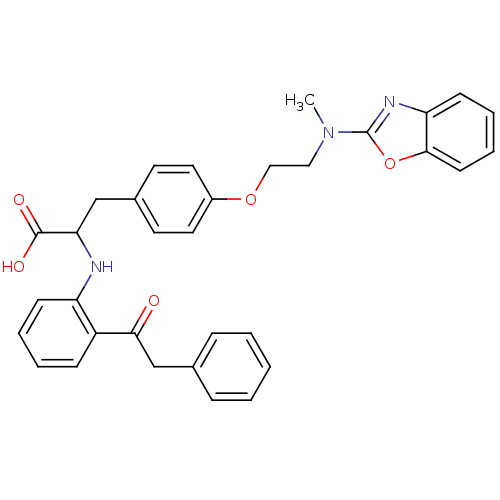 (CHEMBL146822) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039316 ((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50000012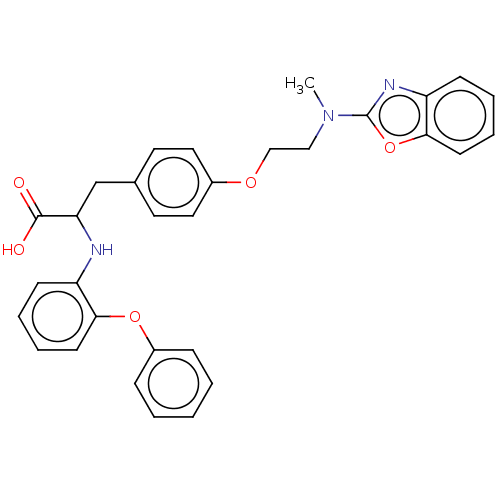 (CHEMBL147826) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039299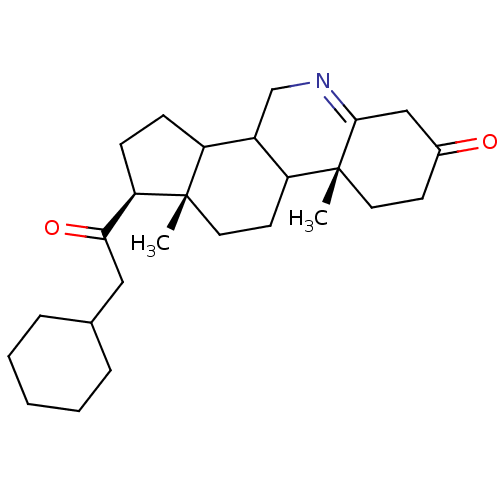 ((1S,9aR,11aS)-1-(2-Cyclohexyl-acetyl)-9a,11a-dimet...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50064451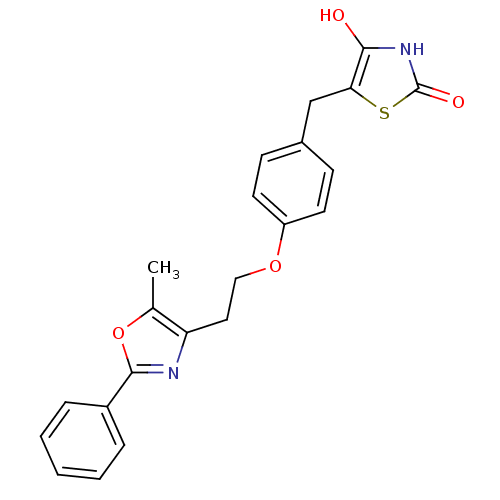 (5-{4-[2-(5-Methyl-2-phenyl-oxazol-4-yl)-ethoxy]-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039324 ((1S,9aR,11aS)-6-Bromo-9a,11a-dimethyl-7-oxo-2,3,3a...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039320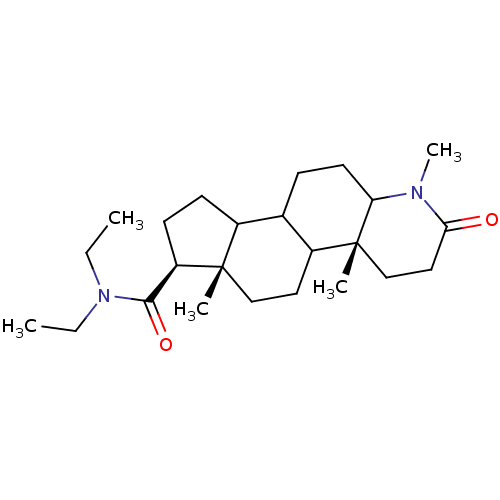 ((4aR,6aS,7S)-1,4a,6a-Trimethyl-2-oxo-hexadecahydro...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50418575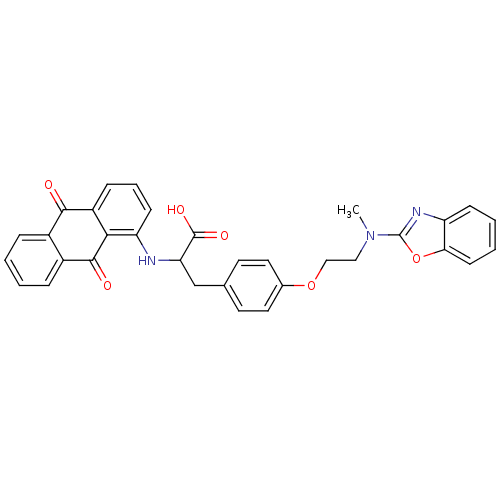 (CHEMBL358137) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50418563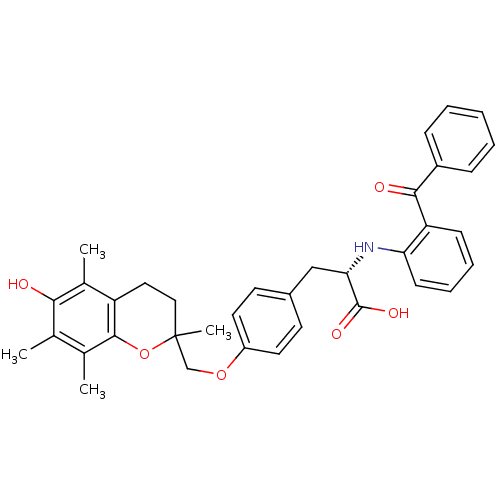 (CHEMBL148301) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256478 (CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to IGF1R by liquid scintillation counting | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50394562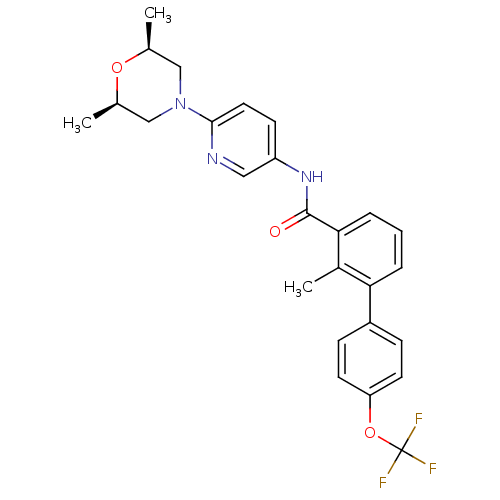 (ERISMODEGIB | LDE225 | N-(6-((2S,6R)-2,6-dimethylm...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | DrugBank Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]cyclopamine from wild type Smo expressed in U2OS cells after 2 hrs by scintillation counting | Bioorg Med Chem 20: 6751-7 (2012) Article DOI: 10.1016/j.bmc.2012.09.030 BindingDB Entry DOI: 10.7270/Q22N53CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039312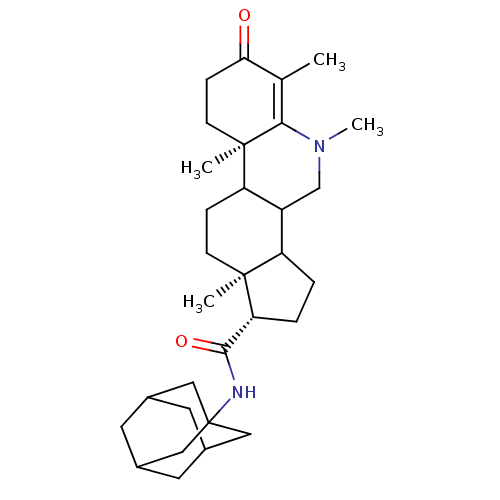 ((1S,9aR,11aS)-5,6,9a,11a-Tetramethyl-7-oxo-2,3,3a,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039288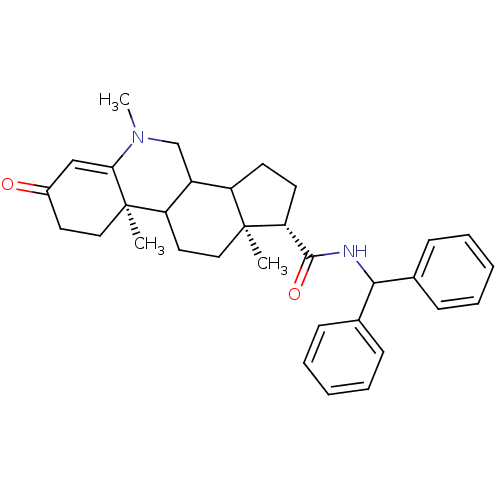 ((1S,9aR,11aS)-5,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50418571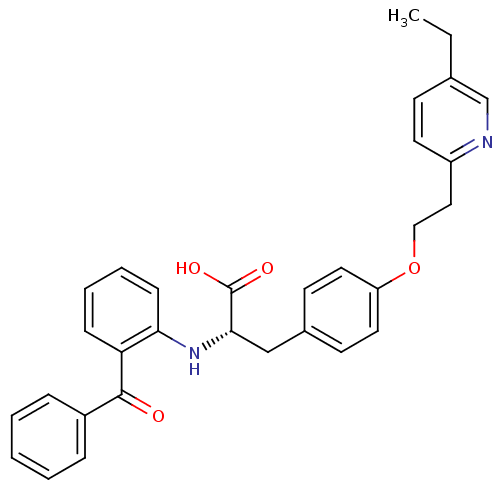 (CHEMBL346219) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50043607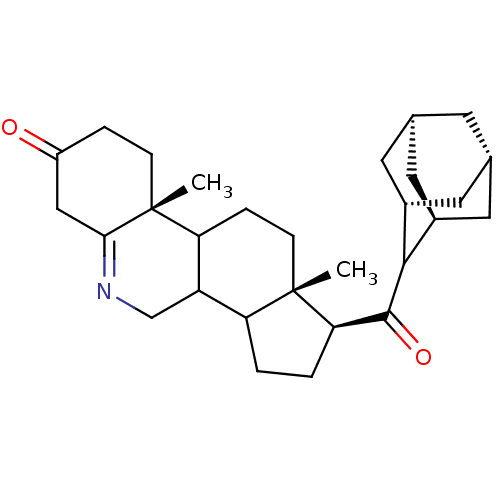 (1-(Adamantane-2-carbonyl)-9a,11a-dimethyl-1,2,3,3a...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-alpha reductase-1 at a concentration of 5 microL after preincubation for 10 minutes | J Med Chem 36: 4313-5 (1994) BindingDB Entry DOI: 10.7270/Q2PK0F76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039286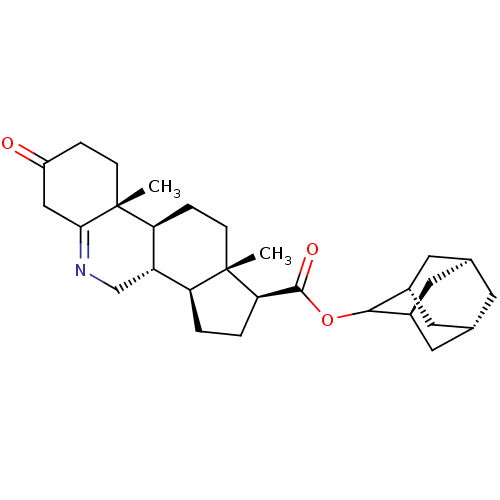 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039268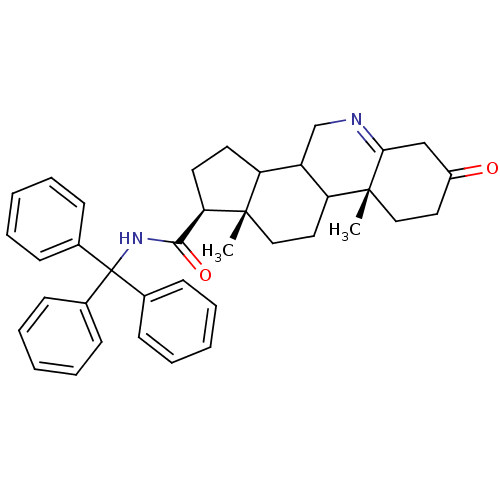 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039268 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-alpha reductase-1 at a concentration of 5 microL after preincubation for 10 minutes | J Med Chem 36: 4313-5 (1994) BindingDB Entry DOI: 10.7270/Q2PK0F76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039321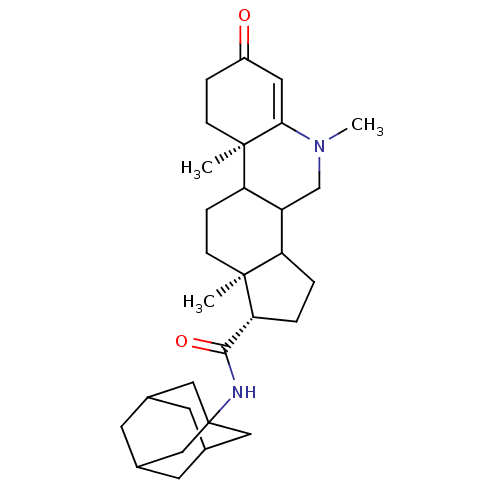 ((1S,9aR,11aS)-5,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039256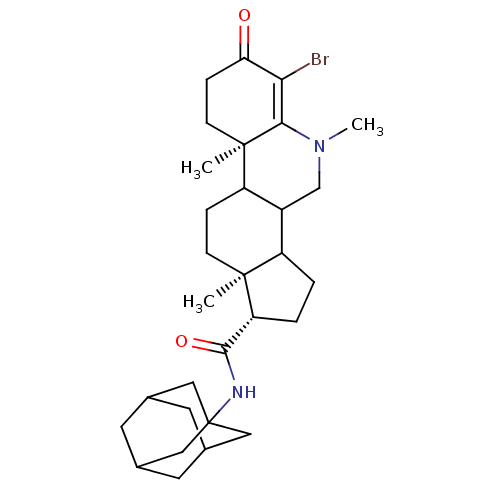 ((1S,9aR,11aS)-6-Bromo-5,9a,11a-trimethyl-7-oxo-2,3...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039257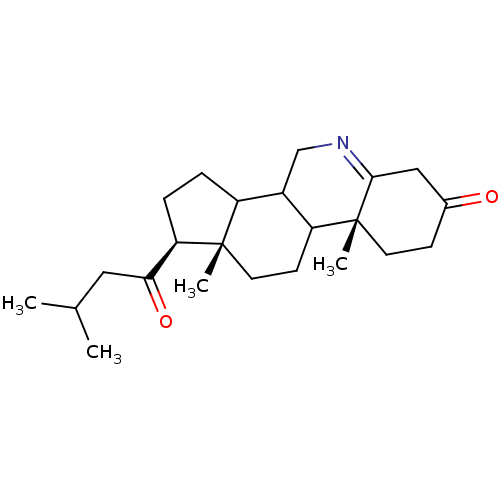 ((1S,9aR,11aS)-9a,11a-Dimethyl-1-(3-methyl-butyryl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-alpha reductase-1 at a concentration of 5 microL after preincubation for 10 minutes | J Med Chem 36: 4313-5 (1994) BindingDB Entry DOI: 10.7270/Q2PK0F76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039257 ((1S,9aR,11aS)-9a,11a-Dimethyl-1-(3-methyl-butyryl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039314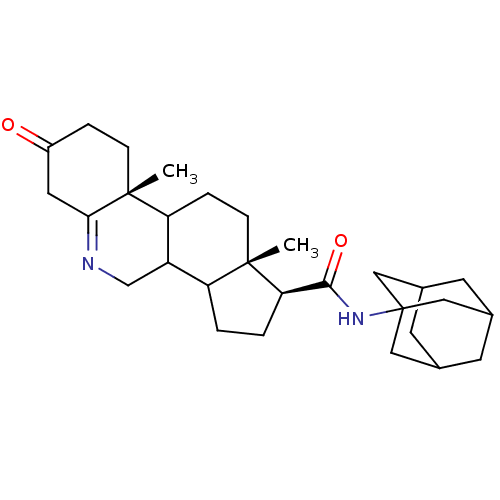 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50043608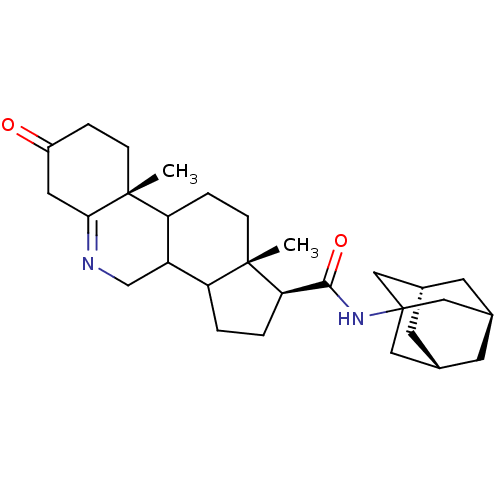 (9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,7,8,9,9a,9b,10...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-alpha reductase-1 at a concentration of 5 microL after preincubation for 10 minutes | J Med Chem 36: 4313-5 (1994) BindingDB Entry DOI: 10.7270/Q2PK0F76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039304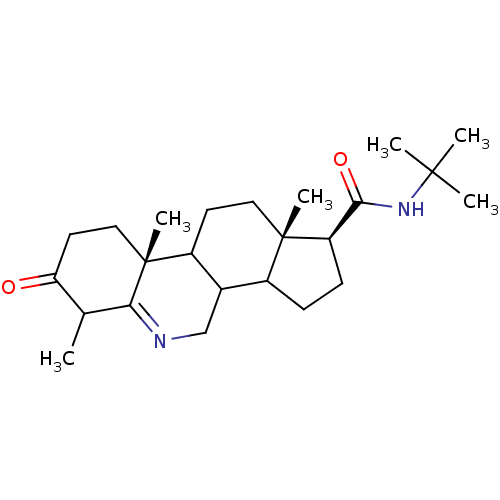 ((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50418557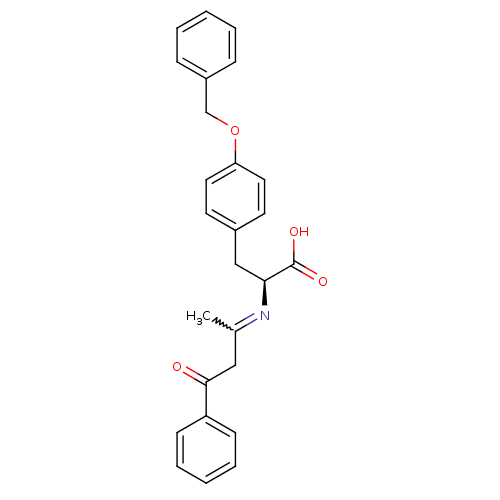 (CHEMBL1785028) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50418557 (CHEMBL1785028) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039318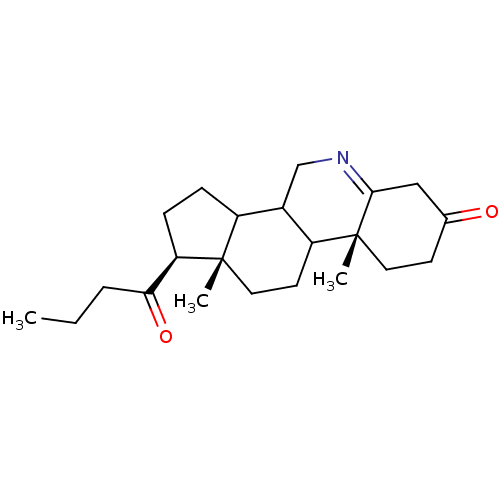 ((1S,9aR,11aS)-1-Butyryl-9a,11a-dimethyl-1,2,3,3a,3...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50232973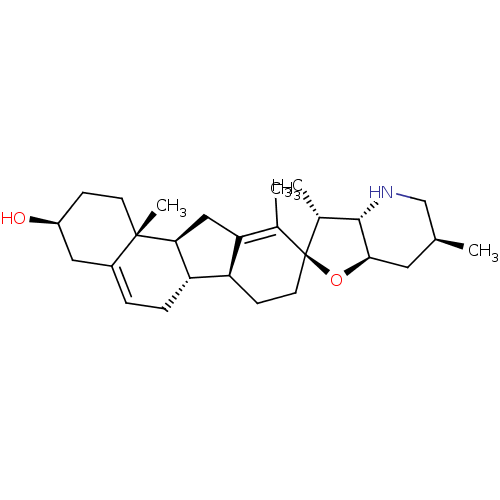 (CHEMBL254129 | CYCLOPAMINE) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]cyclopamine from wild type Smo expressed in U2OS cells after 2 hrs by scintillation counting | Bioorg Med Chem 20: 6751-7 (2012) Article DOI: 10.1016/j.bmc.2012.09.030 BindingDB Entry DOI: 10.7270/Q22N53CV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039295 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-alpha reductase-1 at a concentration of 5 microL after preincubation for 10 minutes | J Med Chem 36: 4313-5 (1994) BindingDB Entry DOI: 10.7270/Q2PK0F76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039295 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039311 ((1S,9aR,11aS)-9a,11a-Dimethyl-1-(naphthalene-1-car...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50249522 (2-chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 16.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]cyclopamine from wild type Smo expressed in U2OS cells after 2 hrs by scintillation counting | Bioorg Med Chem 20: 6751-7 (2012) Article DOI: 10.1016/j.bmc.2012.09.030 BindingDB Entry DOI: 10.7270/Q22N53CV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039302 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039302 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-alpha reductase-1 at a concentration of 5 microL after preincubation for 10 minutes | J Med Chem 36: 4313-5 (1994) BindingDB Entry DOI: 10.7270/Q2PK0F76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039259 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039294 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039294 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-alpha reductase-1 at a concentration of 5 microL after preincubation for 10 minutes | J Med Chem 36: 4313-5 (1994) BindingDB Entry DOI: 10.7270/Q2PK0F76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039259 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-alpha reductase-1 at a concentration of 5 microL after preincubation for 10 minutes | J Med Chem 36: 4313-5 (1994) BindingDB Entry DOI: 10.7270/Q2PK0F76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50049244 (5-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 705 total ) | Next | Last >> |