 Found 821 hits with Last Name = 'butler' and Initial = 's'
Found 821 hits with Last Name = 'butler' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM85357
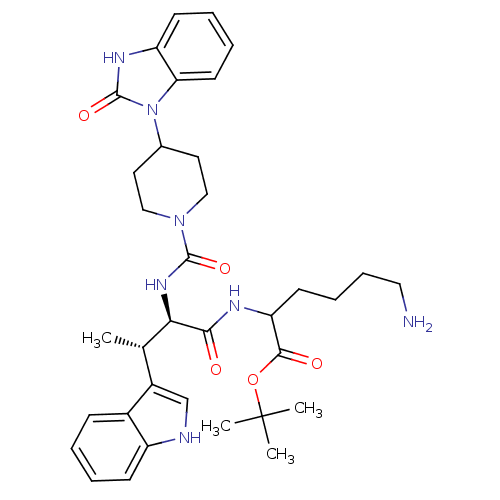
(2-[[(2R,3S)-2-[[4-[(2-Oxo-2,3-dihydro-1H-benzimida...)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O)C(=O)NC(CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N7O5/c1-22(25-21-37-26-12-6-5-11-24(25)26)30(31(43)38-28(14-9-10-18-36)32(44)47-35(2,3)4)40-33(45)41-19-16-23(17-20-41)42-29-15-8-7-13-27(29)39-34(42)46/h5-8,11-13,15,21-23,28,30,37H,9-10,14,16-20,36H2,1-4H3,(H,38,43)(H,39,46)(H,40,45)/t22-,28?,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81766
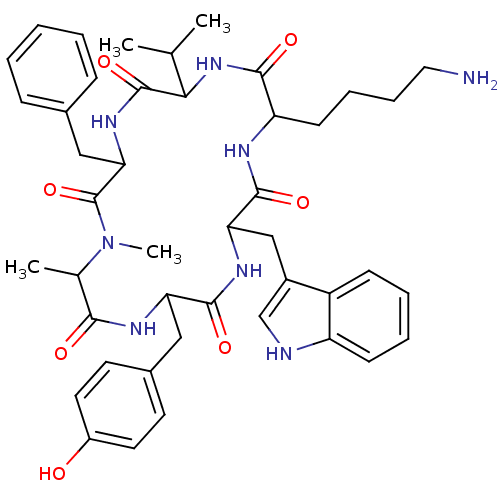
(CAS_3086456 | MK 678 | NSC_3086456)Show SMILES CC(C)C1NC(=O)C(CCCCN)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(C)N(C)C(=O)C(Cc2ccccc2)NC1=O Show InChI InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334454

(CHEMBL1643895 | Ramosetron | US9045501, Ramosetron)Show SMILES Cn1cc(C(=O)[C@@H]2CCc3nc[nH]c3C2)c2ccccc12 |r| Show InChI InChI=1S/C17H17N3O/c1-20-9-13(12-4-2-3-5-16(12)20)17(21)11-6-7-14-15(8-11)19-10-18-14/h2-5,9-11H,6-8H2,1H3,(H,18,19)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50059090
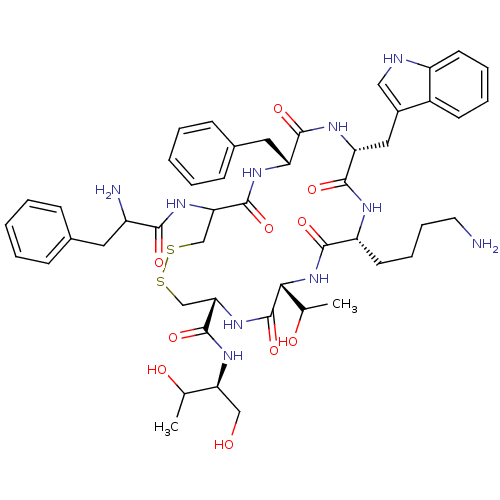
(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES CC(O)[C@H](CO)NC(=O)[C@@H]1CSSCC(NC(=O)C(N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@H](C(C)O)C(=O)N1 Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28?,29?,34?,36-,37-,38-,39+,40?,41+,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064778
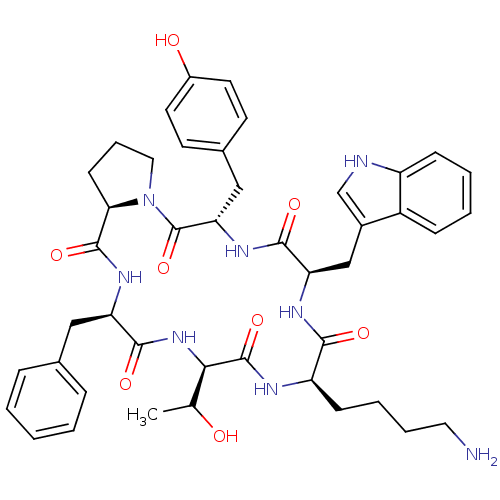
((5S,8R,11R,14R,17R,19aR)-11-(4-Amino-butyl)-17-ben...)Show SMILES CC(O)[C@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCCN)NC1=O Show InChI InChI=1S/C44H54N8O8/c1-26(53)38-43(59)47-33(14-7-8-20-45)39(55)48-35(24-29-25-46-32-13-6-5-12-31(29)32)40(56)50-36(23-28-16-18-30(54)19-17-28)44(60)52-21-9-15-37(52)42(58)49-34(41(57)51-38)22-27-10-3-2-4-11-27/h2-6,10-13,16-19,25-26,33-38,46,53-54H,7-9,14-15,20-24,45H2,1H3,(H,47,59)(H,48,55)(H,49,58)(H,50,56)(H,51,57)/t26?,33-,34-,35-,36+,37-,38-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM93624
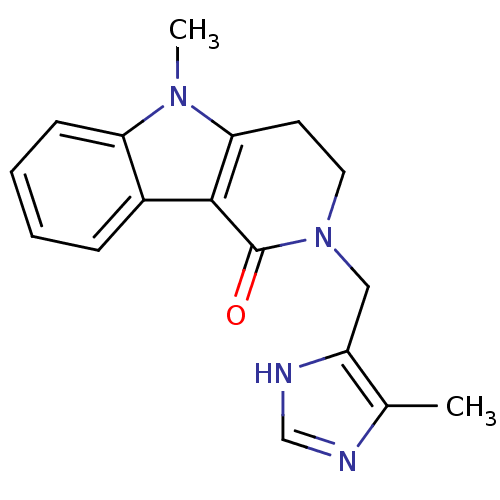
(5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-3,4...)Show InChI InChI=1S/C17H18N4O/c1-11-13(19-10-18-11)9-21-8-7-15-16(17(21)22)12-5-3-4-6-14(12)20(15)2/h3-6,10H,7-9H2,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334453

((S)-5-methyl-2-(quinuclidin-3-yl)-2,3-dihydropyrro...)Show SMILES Cn1cc2CN([C@@H]3CN4CCC3CC4)C(=O)c3cccc1c23 |r,wD:6.5,(4.39,-34,;3.85,-32.56,;4.88,-30.82,;3.72,-29.78,;3.73,-28.24,;2.39,-27.47,;2.38,-25.93,;1.05,-25.17,;1.05,-23.63,;2.39,-22.86,;3.72,-23.63,;3.72,-25.16,;2.21,-24.66,;2.53,-24.02,;1.06,-28.24,;-.28,-27.45,;1.06,-29.78,;-.27,-30.55,;-.28,-32.09,;1.06,-32.86,;2.39,-32.09,;2.39,-30.55,)| Show InChI InChI=1S/C18H21N3O/c1-19-9-13-10-21(16-11-20-7-5-12(16)6-8-20)18(22)14-3-2-4-15(19)17(13)14/h2-4,9,12,16H,5-8,10-11H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334442
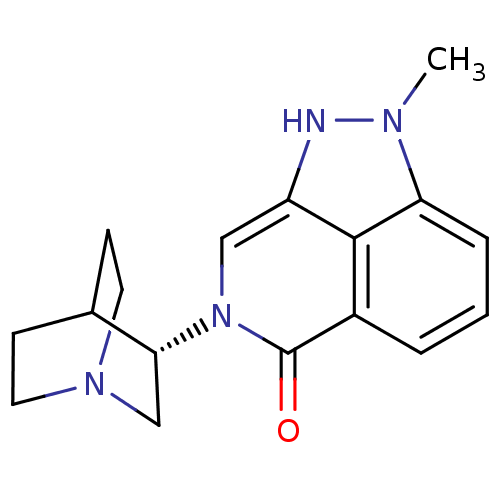
((S)-2-methyl-7-(quinuclidin-3-yl)-7,8-dihydropyraz...)Show SMILES Cn1[nH]c2cn([C@@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wD:6.5,(21.53,-4.9,;20.98,-3.46,;22.01,-1.72,;20.85,-.68,;20.86,.86,;19.52,1.63,;19.51,3.17,;18.18,3.93,;18.18,5.47,;19.52,6.24,;20.85,5.47,;20.85,3.94,;19.34,4.44,;19.66,5.08,;18.19,.86,;16.85,1.65,;18.19,-.68,;16.86,-1.45,;16.85,-2.99,;18.19,-3.76,;19.52,-2.99,;19.52,-1.45,)| Show InChI InChI=1S/C17H20N4O/c1-19-14-4-2-3-12-16(14)13(18-19)9-21(17(12)22)15-10-20-7-5-11(15)6-8-20/h2-4,9,11,15,18H,5-8,10H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334452
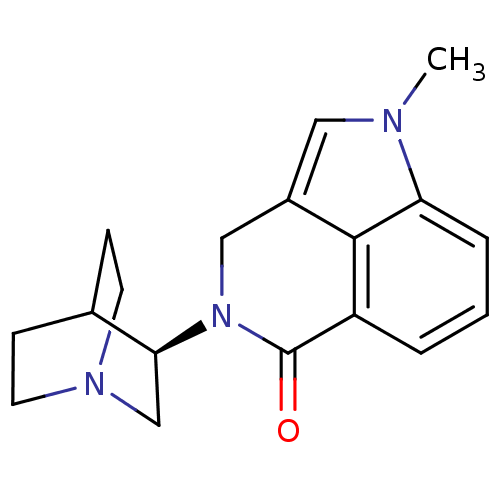
((R)-5-methyl-2-(quinuclidin-3-yl)-2,3-dihydropyrro...)Show SMILES Cn1cc2CN([C@H]3CN4CCC3CC4)C(=O)c3cccc1c23 |r,wU:6.5,(-3.65,-33.57,;-4.2,-32.13,;-3.17,-30.38,;-4.32,-29.35,;-4.32,-27.8,;-5.66,-27.04,;-5.66,-25.5,;-7,-24.74,;-6.99,-23.19,;-5.66,-22.42,;-4.33,-23.2,;-4.33,-24.73,;-5.83,-24.22,;-5.51,-23.59,;-6.98,-27.8,;-8.33,-27.02,;-6.99,-29.35,;-8.32,-30.12,;-8.32,-31.66,;-6.98,-32.43,;-5.66,-31.66,;-5.65,-30.12,)| Show InChI InChI=1S/C18H21N3O/c1-19-9-13-10-21(16-11-20-7-5-12(16)6-8-20)18(22)14-3-2-4-15(19)17(13)14/h2-4,9,12,16H,5-8,10-11H2,1H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334445
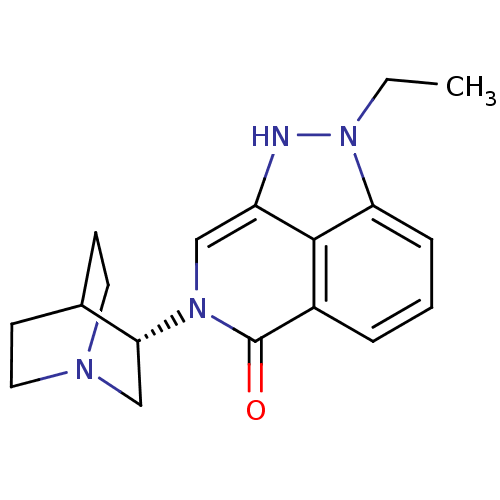
((S)-2-ethyl-7-(quinuclidin-3-yl)-7,8-dihydropyrazo...)Show SMILES CCn1[nH]c2cn([C@@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wD:7.6,(47.4,-4.88,;48.37,-3.68,;47.82,-2.25,;48.85,-.5,;47.7,.54,;47.7,2.08,;46.36,2.85,;46.36,4.39,;45.02,5.15,;45.03,6.69,;46.36,7.46,;47.69,6.69,;47.69,5.16,;46.19,5.66,;46.5,6.3,;45.04,2.08,;43.69,2.87,;45.03,.54,;43.7,-.23,;43.7,-1.78,;45.04,-2.55,;46.36,-1.78,;46.36,-.23,)| Show InChI InChI=1S/C18H22N4O/c1-2-22-15-5-3-4-13-17(15)14(19-22)10-21(18(13)23)16-11-20-8-6-12(16)7-9-20/h3-5,10,12,16,19H,2,6-9,11H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334439
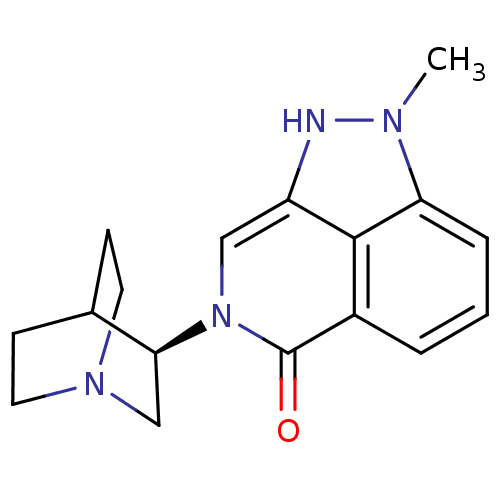
((R)-2-methyl-7-(quinuclidin-3-yl)-7,8-dihydropyraz...)Show SMILES Cn1[nH]c2cn([C@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wU:6.5,(12.29,-5.2,;11.75,-3.77,;12.77,-2.02,;11.62,-.98,;11.62,.56,;10.28,1.33,;10.28,2.87,;8.95,3.63,;8.95,5.17,;10.28,5.94,;11.61,5.17,;11.61,3.64,;10.11,4.14,;10.43,4.78,;8.96,.56,;7.61,1.35,;8.96,-.99,;7.62,-1.76,;7.62,-3.3,;8.96,-4.07,;10.29,-3.3,;10.29,-1.75,)| Show InChI InChI=1S/C17H20N4O/c1-19-14-4-2-3-12-16(14)13(18-19)9-21(17(12)22)15-10-20-7-5-11(15)6-8-20/h2-4,9,11,15,18H,5-8,10H2,1H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334444
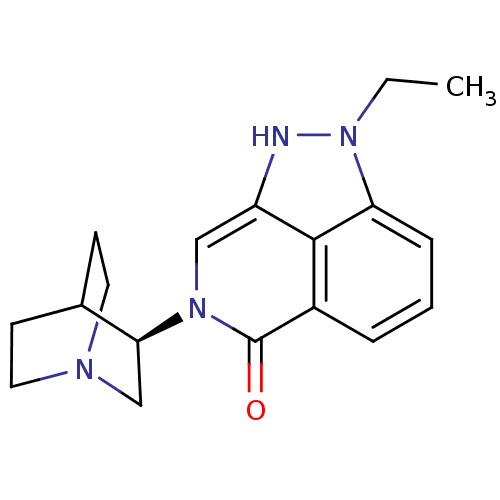
((R)-2-ethyl-7-(quinuclidin-3-yl)-7,8-dihydropyrazo...)Show SMILES CCn1[nH]c2cn([C@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wU:7.6,(39.2,-5.65,;40.17,-4.46,;39.63,-3.02,;40.65,-1.27,;39.5,-.24,;39.5,1.31,;38.16,2.07,;38.16,3.61,;36.83,4.37,;36.83,5.92,;38.16,6.69,;39.49,5.91,;39.49,4.38,;37.99,4.89,;38.31,5.52,;36.84,1.31,;35.49,2.09,;36.84,-.24,;35.5,-1.01,;35.5,-2.55,;36.84,-3.32,;38.16,-2.55,;38.17,-1.01,)| Show InChI InChI=1S/C18H22N4O/c1-2-22-15-5-3-4-13-17(15)14(19-22)10-21(18(13)23)16-11-20-8-6-12(16)7-9-20/h3-5,10,12,16,19H,2,6-9,11H2,1H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334443

((R)-4-fluoro-2-methyl-7-(quinuclidin-3-yl)-7,8-dih...)Show SMILES Cn1[nH]c2cn([C@H]3CN4CCC3CC4)c(=O)c3cc(F)cc1c23 |r,wU:6.5,(31.37,-5.57,;30.82,-4.13,;31.85,-2.38,;30.7,-1.35,;30.7,.2,;29.36,.96,;29.36,2.5,;28.02,3.26,;28.03,4.81,;29.36,5.58,;30.69,4.8,;30.69,3.27,;29.19,3.77,;29.51,4.41,;28.04,.2,;26.69,.98,;28.03,-1.35,;26.7,-2.12,;26.7,-3.66,;25.37,-4.43,;28.04,-4.43,;29.36,-3.66,;29.37,-2.12,)| Show InChI InChI=1S/C17H19FN4O/c1-20-14-7-11(18)6-12-16(14)13(19-20)8-22(17(12)23)15-9-21-4-2-10(15)3-5-21/h6-8,10,15,19H,2-5,9H2,1H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334447
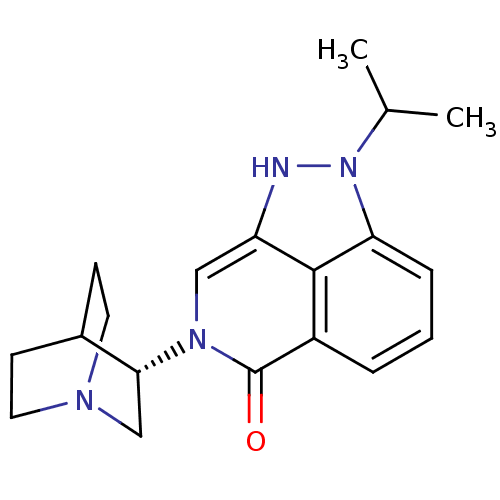
((S)-2-isopropyl-7-(quinuclidin-3-yl)-7,8-dihydropy...)Show SMILES CC(C)n1[nH]c2cn([C@@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wD:8.7,(5.55,-19.44,;6.52,-18.25,;8.03,-18.49,;5.97,-16.81,;7,-15.06,;5.85,-14.02,;5.85,-12.48,;4.51,-11.71,;4.5,-10.18,;3.17,-9.41,;3.17,-7.87,;4.51,-7.1,;5.84,-7.87,;5.84,-9.4,;4.33,-8.9,;4.65,-8.27,;3.18,-12.48,;1.84,-11.7,;3.18,-14.03,;1.85,-14.8,;1.84,-16.34,;3.18,-17.11,;4.51,-16.34,;4.51,-14.8,)| Show InChI InChI=1S/C19H24N4O/c1-12(2)23-16-5-3-4-14-18(16)15(20-23)10-22(19(14)24)17-11-21-8-6-13(17)7-9-21/h3-5,10,12-13,17,20H,6-9,11H2,1-2H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334448

((S)-2-isobutyl-7-(quinuclidin-3-yl)-7,8-dihydropyr...)Show SMILES CC(C)Cn1[nH]c2cn([C@@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wD:9.8,(15.89,-21.07,;15.34,-19.64,;13.82,-19.39,;16.31,-18.44,;15.77,-17.01,;16.79,-15.26,;15.64,-14.22,;15.64,-12.68,;14.3,-11.91,;14.3,-10.37,;12.97,-9.61,;12.97,-8.07,;14.3,-7.3,;15.63,-8.07,;15.63,-9.6,;14.13,-9.1,;14.45,-8.46,;12.98,-12.68,;11.63,-11.89,;12.98,-14.22,;11.64,-14.99,;11.64,-16.54,;12.98,-17.31,;14.31,-16.54,;14.31,-14.99,)| Show InChI InChI=1S/C20H26N4O/c1-13(2)10-24-17-5-3-4-15-19(17)16(21-24)11-23(20(15)25)18-12-22-8-6-14(18)7-9-22/h3-5,11,13-14,18,21H,6-10,12H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334446

((R)-2-isopropyl-7-(quinuclidin-3-yl)-7,8-dihydropy...)Show SMILES CC(C)n1[nH]c2cn([C@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wU:8.7,(-3.99,-20.22,;-3.02,-19.03,;-1.5,-19.27,;-3.56,-17.59,;-2.53,-15.84,;-3.69,-14.8,;-3.68,-13.26,;-5.02,-12.49,;-5.03,-10.95,;-6.36,-10.19,;-6.36,-8.65,;-5.02,-7.88,;-3.69,-8.65,;-3.69,-10.18,;-5.2,-9.68,;-4.88,-9.04,;-6.35,-13.26,;-7.7,-12.48,;-6.35,-14.81,;-7.69,-15.58,;-7.69,-17.12,;-6.35,-17.89,;-5.02,-17.12,;-5.02,-15.58,)| Show InChI InChI=1S/C19H24N4O/c1-12(2)23-16-5-3-4-14-18(16)15(20-23)10-22(19(14)24)17-11-21-8-6-13(17)7-9-21/h3-5,10,12-13,17,20H,6-9,11H2,1-2H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334440

((R)-7-(quinuclidin-3-yl)-7,8-dihydropyrazolo[3,4,5...)Show SMILES O=c1n(cc2[nH][nH]c3cccc1c23)[C@H]1CN2CCC1CC2 |r,wU:13.15,(-7.86,1.16,;-6.52,.38,;-5.19,1.14,;-3.85,.37,;-3.86,-1.17,;-2.7,-2.21,;-3.73,-3.95,;-5.19,-3.48,;-6.52,-4.25,;-7.86,-3.48,;-7.85,-1.94,;-6.52,-1.17,;-5.19,-1.94,;-5.2,2.68,;-6.53,3.44,;-6.53,4.98,;-5.19,5.75,;-3.86,4.98,;-3.86,3.45,;-5.37,3.95,;-5.05,4.59,)| Show InChI InChI=1S/C16H18N4O/c21-16-11-2-1-3-12-15(11)13(18-17-12)8-20(16)14-9-19-6-4-10(14)5-7-19/h1-3,8,10,14,17-18H,4-7,9H2/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528308
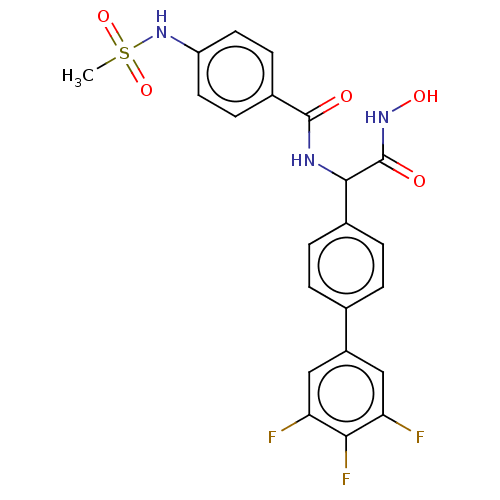
(CHEMBL4460082)Show SMILES CS(=O)(=O)Nc1ccc(cc1)C(=O)NC(C(=O)NO)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C22H18F3N3O5S/c1-34(32,33)28-16-8-6-14(7-9-16)21(29)26-20(22(30)27-31)13-4-2-12(3-5-13)15-10-17(23)19(25)18(24)11-15/h2-11,20,28,31H,1H3,(H,26,29)(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334441

((S)-7-(quinuclidin-3-yl)-7,8-dihydropyrazolo[3,4,5...)Show SMILES O=c1n(cc2[nH][nH]c3cccc1c23)[C@@H]1CN2CCC1CC2 |r,wD:13.15,(-.61,1.94,;.74,1.15,;2.06,1.92,;3.4,1.15,;3.4,-.39,;4.55,-1.43,;3.52,-3.18,;2.06,-2.71,;.74,-3.48,;-.6,-2.71,;-.6,-1.16,;.73,-.4,;2.07,-1.16,;2.06,3.46,;.72,4.22,;.73,5.76,;2.06,6.53,;3.39,5.76,;3.39,4.23,;1.89,4.73,;2.21,5.37,)| Show InChI InChI=1S/C16H18N4O/c21-16-11-2-1-3-12-15(11)13(18-17-12)8-20(16)14-9-19-6-4-10(14)5-7-19/h1-3,8,10,14,17-18H,4-7,9H2/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334449
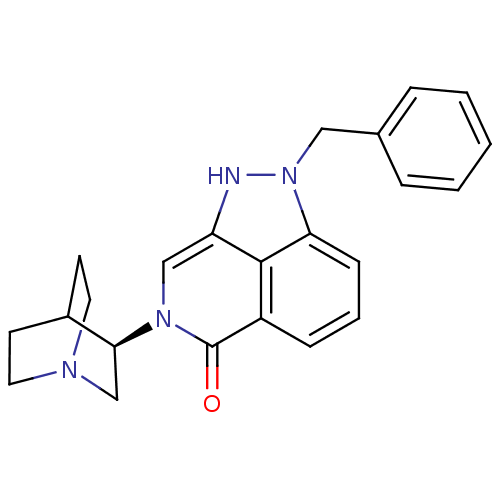
((R)-2-benzyl-7-(quinuclidin-3-yl)-7,8-dihydropyraz...)Show SMILES O=c1n(cc2[nH]n(Cc3ccccc3)c3cccc1c23)[C@H]1CN2CCC1CC2 |r,wU:20.23,(21.21,-11.04,;22.56,-11.82,;23.88,-11.05,;25.22,-11.82,;25.22,-13.37,;26.37,-14.4,;25.34,-16.15,;26.1,-17.48,;25.33,-18.81,;23.79,-18.79,;23.01,-20.12,;23.77,-21.46,;25.32,-21.46,;26.09,-20.13,;23.88,-15.68,;22.56,-16.45,;21.22,-15.68,;21.22,-14.14,;22.56,-13.37,;23.89,-14.14,;23.88,-9.52,;22.55,-8.75,;22.55,-7.21,;23.88,-6.44,;25.21,-7.21,;25.21,-8.75,;23.71,-8.24,;24.03,-7.61,)| Show InChI InChI=1S/C23H24N4O/c28-23-18-7-4-8-20-22(18)19(24-27(20)13-16-5-2-1-3-6-16)14-26(23)21-15-25-11-9-17(21)10-12-25/h1-8,14,17,21,24H,9-13,15H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334451
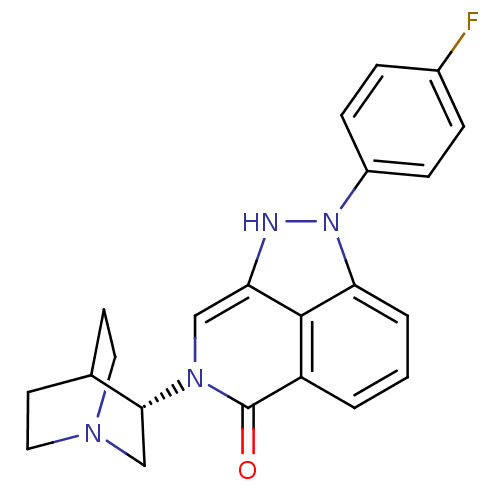
((S)-2-(4-fluorophenyl)-7-(quinuclidin-3-yl)-7,8-di...)Show SMILES Fc1ccc(cc1)-n1[nH]c2cn([C@@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wD:12.12,(46.59,-23.52,;46.04,-22.08,;44.52,-21.83,;43.97,-20.4,;44.95,-19.21,;46.46,-19.44,;47.01,-20.88,;44.4,-17.77,;45.43,-16.02,;44.28,-14.98,;44.28,-13.44,;42.94,-12.67,;42.94,-11.13,;41.6,-10.37,;41.61,-8.83,;42.94,-8.06,;44.27,-8.83,;44.27,-10.36,;42.77,-9.86,;43.09,-9.22,;41.62,-13.44,;40.27,-12.65,;41.61,-14.99,;40.28,-15.76,;40.28,-17.3,;41.62,-18.07,;42.94,-17.3,;42.95,-15.75,)| Show InChI InChI=1S/C22H21FN4O/c23-15-4-6-16(7-5-15)27-19-3-1-2-17-21(19)18(24-27)12-26(22(17)28)20-13-25-10-8-14(20)9-11-25/h1-7,12,14,20,24H,8-11,13H2/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528311
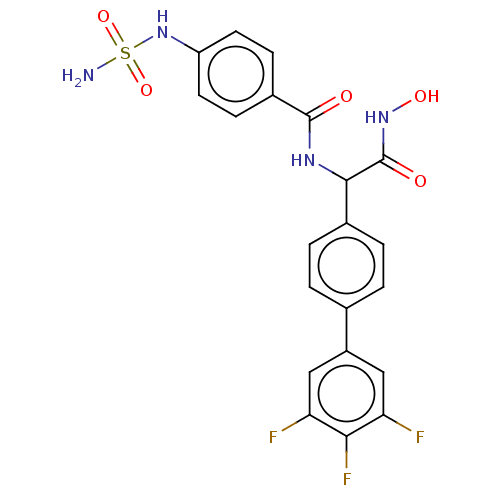
(CHEMBL4448936)Show SMILES NS(=O)(=O)Nc1ccc(cc1)C(=O)NC(C(=O)NO)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C21H17F3N4O5S/c22-16-9-14(10-17(23)18(16)24)11-1-3-12(4-2-11)19(21(30)27-31)26-20(29)13-5-7-15(8-6-13)28-34(25,32)33/h1-10,19,28,31H,(H,26,29)(H,27,30)(H2,25,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50477760
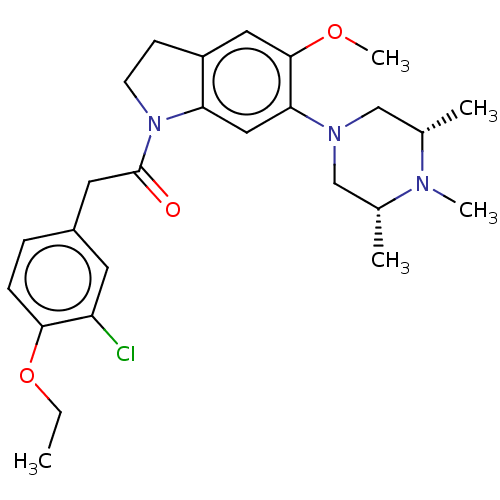
(CHEMBL250973)Show SMILES CCOc1ccc(CC(=O)N2CCc3cc(OC)c(cc23)N2C[C@H](C)N(C)[C@H](C)C2)cc1Cl Show InChI InChI=1S/C26H34ClN3O3/c1-6-33-24-8-7-19(11-21(24)27)12-26(31)30-10-9-20-13-25(32-5)23(14-22(20)30)29-15-17(2)28(4)18(3)16-29/h7-8,11,13-14,17-18H,6,9-10,12,15-16H2,1-5H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at 5HT1B receptor |
Bioorg Med Chem Lett 17: 6584-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.067
BindingDB Entry DOI: 10.7270/Q2MW2KX2 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528345

(CHEMBL4445188)Show SMILES ONC(=O)C(NC(=O)c1ccc2[nH]ncc2c1)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C22H15F3N4O3/c23-16-8-14(9-17(24)19(16)25)11-1-3-12(4-2-11)20(22(31)29-32)27-21(30)13-5-6-18-15(7-13)10-26-28-18/h1-10,20,32H,(H,26,28)(H,27,30)(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528319

(CHEMBL4566015)Show SMILES ONC(=O)C(NC(=O)c1ccc2[nH]nnc2c1)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C21H14F3N5O3/c22-14-7-13(8-15(23)18(14)24)10-1-3-11(4-2-10)19(21(31)28-32)25-20(30)12-5-6-16-17(9-12)27-29-26-16/h1-9,19,32H,(H,25,30)(H,28,31)(H,26,27,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334450
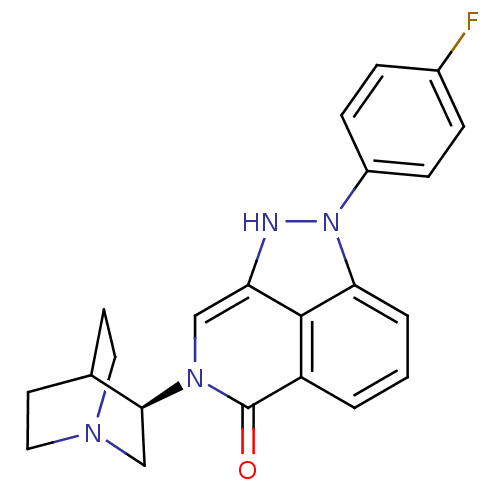
((R)-2-(4-fluorophenyl)-7-(quinuclidin-3-yl)-7,8-di...)Show SMILES Fc1ccc(cc1)-n1[nH]c2cn([C@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wU:12.12,(36.32,-23.94,;35.77,-22.51,;34.25,-22.26,;33.7,-20.83,;34.67,-19.63,;36.19,-19.87,;36.74,-21.31,;34.13,-18.2,;35.16,-16.45,;34,-15.41,;34.01,-13.87,;32.67,-13.1,;32.66,-11.56,;31.33,-10.8,;31.33,-9.25,;32.67,-8.48,;34,-9.26,;34,-10.79,;32.49,-10.29,;32.81,-9.65,;31.34,-13.87,;29.99,-13.08,;31.34,-15.41,;30,-16.18,;30,-17.73,;31.34,-18.5,;32.67,-17.73,;32.67,-16.18,)| Show InChI InChI=1S/C22H21FN4O/c23-15-4-6-16(7-5-15)27-19-3-1-2-17-21(19)18(24-27)12-26(22(17)28)20-13-25-10-8-14(20)9-11-25/h1-7,12,14,20,24H,8-11,13H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528321

(CHEMBL4522953)Show SMILES ONC(=O)C(NC(=O)c1ccc(O)c(F)c1)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C21H14F4N2O4/c22-14-7-12(5-6-17(14)28)20(29)26-19(21(30)27-31)11-3-1-10(2-4-11)13-8-15(23)18(25)16(24)9-13/h1-9,19,28,31H,(H,26,29)(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM85357

(2-[[(2R,3S)-2-[[4-[(2-Oxo-2,3-dihydro-1H-benzimida...)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O)C(=O)NC(CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N7O5/c1-22(25-21-37-26-12-6-5-11-24(25)26)30(31(43)38-28(14-9-10-18-36)32(44)47-35(2,3)4)40-33(45)41-19-16-23(17-20-41)42-29-15-8-7-13-27(29)39-34(42)46/h5-8,11-13,15,21-23,28,30,37H,9-10,14,16-20,36H2,1-4H3,(H,38,43)(H,39,46)(H,40,45)/t22-,28?,30+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528338

(CHEMBL4590563)Show SMILES N\C(=N/O)c1cccc(c1)C(=O)NC(C(=O)NO)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C22H17F3N4O4/c23-16-9-15(10-17(24)18(16)25)11-4-6-12(7-5-11)19(22(31)29-33)27-21(30)14-3-1-2-13(8-14)20(26)28-32/h1-10,19,32-33H,(H2,26,28)(H,27,30)(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528320
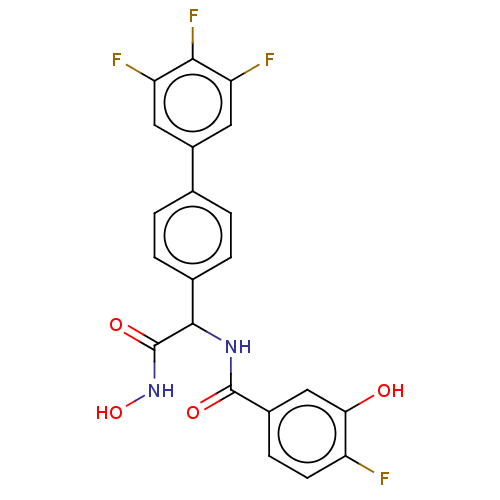
(CHEMBL4578880)Show SMILES ONC(=O)C(NC(=O)c1ccc(F)c(O)c1)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C21H14F4N2O4/c22-14-6-5-12(9-17(14)28)20(29)26-19(21(30)27-31)11-3-1-10(2-4-11)13-7-15(23)18(25)16(24)8-13/h1-9,19,28,31H,(H,26,29)(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528313

(CHEMBL4544937)Show SMILES ONC(=O)C(NC(=O)Cc1ccc(O)c(F)c1)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C22H16F4N2O4/c23-15-7-11(1-6-18(15)29)8-19(30)27-21(22(31)28-32)13-4-2-12(3-5-13)14-9-16(24)20(26)17(25)10-14/h1-7,9-10,21,29,32H,8H2,(H,27,30)(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50059090

(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES CC(O)[C@H](CO)NC(=O)[C@@H]1CSSCC(NC(=O)C(N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@H](C(C)O)C(=O)N1 Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28?,29?,34?,36-,37-,38-,39+,40?,41+,42-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528328
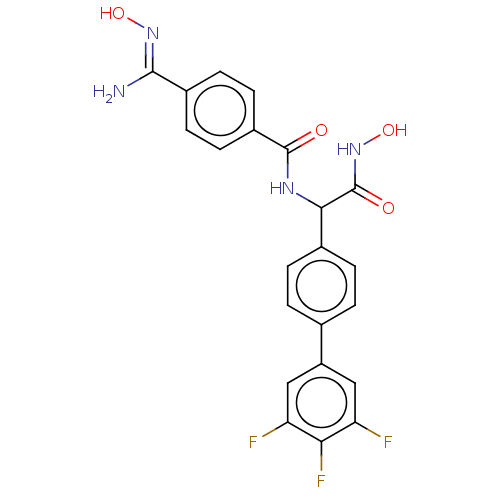
(CHEMBL4589359)Show SMILES N\C(=N/O)c1ccc(cc1)C(=O)NC(C(=O)NO)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C22H17F3N4O4/c23-16-9-15(10-17(24)18(16)25)11-1-3-12(4-2-11)19(22(31)29-33)27-21(30)14-7-5-13(6-8-14)20(26)28-32/h1-10,19,32-33H,(H2,26,28)(H,27,30)(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528310
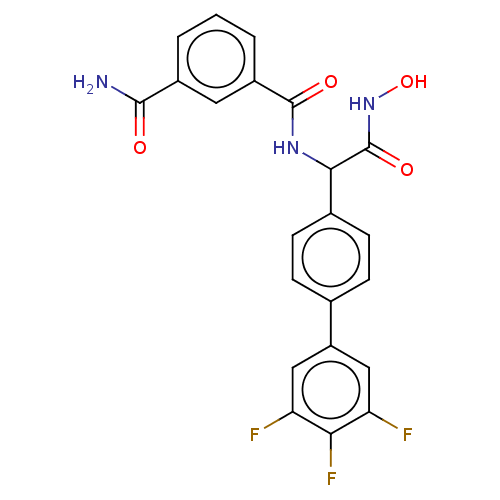
(CHEMBL4455419)Show SMILES NC(=O)c1cccc(c1)C(=O)NC(C(=O)NO)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C22H16F3N3O4/c23-16-9-15(10-17(24)18(16)25)11-4-6-12(7-5-11)19(22(31)28-32)27-21(30)14-3-1-2-13(8-14)20(26)29/h1-10,19,32H,(H2,26,29)(H,27,30)(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM85357

(2-[[(2R,3S)-2-[[4-[(2-Oxo-2,3-dihydro-1H-benzimida...)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O)C(=O)NC(CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N7O5/c1-22(25-21-37-26-12-6-5-11-24(25)26)30(31(43)38-28(14-9-10-18-36)32(44)47-35(2,3)4)40-33(45)41-19-16-23(17-20-41)42-29-15-8-7-13-27(29)39-34(42)46/h5-8,11-13,15,21-23,28,30,37H,9-10,14,16-20,36H2,1-4H3,(H,38,43)(H,39,46)(H,40,45)/t22-,28?,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528327
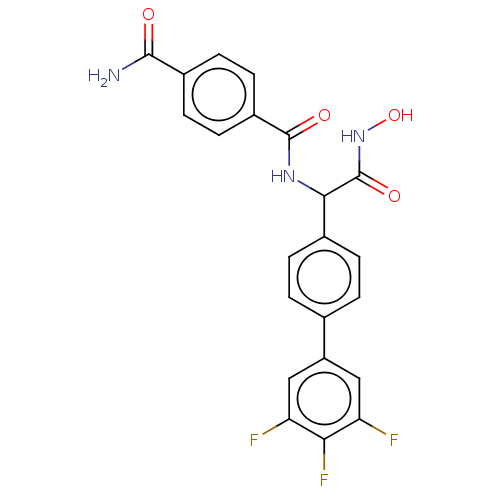
(CHEMBL4586845)Show SMILES NC(=O)c1ccc(cc1)C(=O)NC(C(=O)NO)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C22H16F3N3O4/c23-16-9-15(10-17(24)18(16)25)11-1-3-12(4-2-11)19(22(31)28-32)27-21(30)14-7-5-13(6-8-14)20(26)29/h1-10,19,32H,(H2,26,29)(H,27,30)(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528349

(CHEMBL4441367)Show SMILES ONC(=O)C(NC(=O)c1ccc(O)cc1)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C21H15F3N2O4/c22-16-9-14(10-17(23)18(16)24)11-1-3-12(4-2-11)19(21(29)26-30)25-20(28)13-5-7-15(27)8-6-13/h1-10,19,27,30H,(H,25,28)(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528337
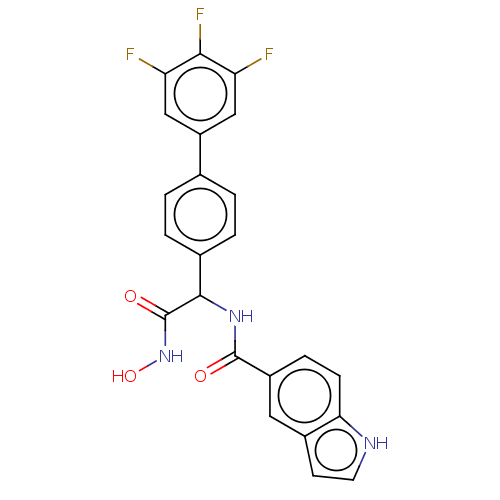
(CHEMBL4446709)Show SMILES ONC(=O)C(NC(=O)c1ccc2[nH]ccc2c1)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C23H16F3N3O3/c24-17-10-16(11-18(25)20(17)26)12-1-3-13(4-2-12)21(23(31)29-32)28-22(30)15-5-6-19-14(9-15)7-8-27-19/h1-11,21,27,32H,(H,28,30)(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
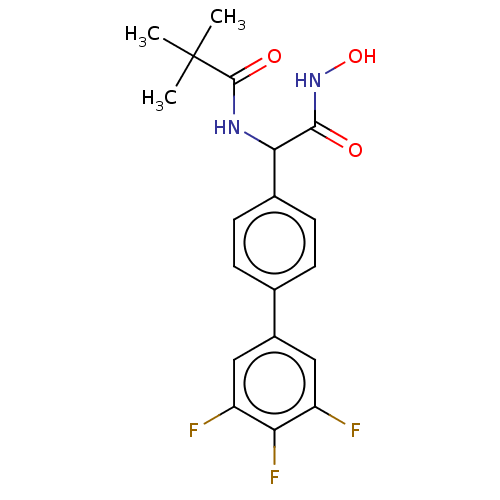
(Homo sapiens (Human)) | BDBM50528335
(CHEMBL3765455)Show SMILES CC(C)(C)C(=O)NC(C(=O)NO)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C19H19F3N2O3/c1-19(2,3)18(26)23-16(17(25)24-27)11-6-4-10(5-7-11)12-8-13(20)15(22)14(21)9-12/h4-9,16,27H,1-3H3,(H,23,26)(H,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
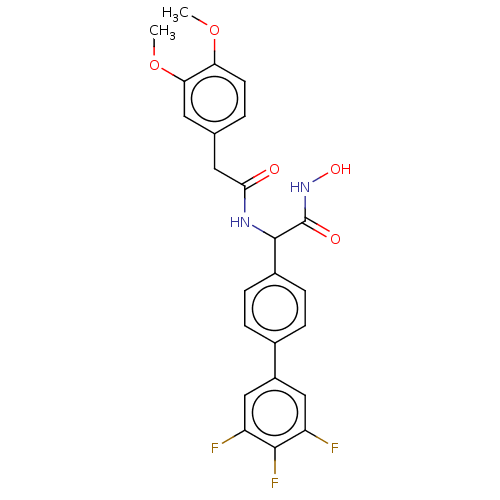
(Homo sapiens (Human)) | BDBM50528314
(CHEMBL4572202)Show SMILES COc1ccc(CC(=O)NC(C(=O)NO)c2ccc(cc2)-c2cc(F)c(F)c(F)c2)cc1OC Show InChI InChI=1S/C24H21F3N2O5/c1-33-19-8-3-13(9-20(19)34-2)10-21(30)28-23(24(31)29-32)15-6-4-14(5-7-15)16-11-17(25)22(27)18(26)12-16/h3-9,11-12,23,32H,10H2,1-2H3,(H,28,30)(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528342

(CHEMBL4435512)Show SMILES Nc1cccc(c1)C(=O)NC(C(=O)NO)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C21H16F3N3O3/c22-16-9-14(10-17(23)18(16)24)11-4-6-12(7-5-11)19(21(29)27-30)26-20(28)13-2-1-3-15(25)8-13/h1-10,19,30H,25H2,(H,26,28)(H,27,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50059090

(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES CC(O)[C@H](CO)NC(=O)[C@@H]1CSSCC(NC(=O)C(N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@H](C(C)O)C(=O)N1 Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28?,29?,34?,36-,37-,38-,39+,40?,41+,42-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528343

(CHEMBL4554326)Show SMILES ONC(=O)C(NC(=O)Cc1ccc(O)c(O)c1)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C22H17F3N2O5/c23-15-9-14(10-16(24)20(15)25)12-2-4-13(5-3-12)21(22(31)27-32)26-19(30)8-11-1-6-17(28)18(29)7-11/h1-7,9-10,21,28-29,32H,8H2,(H,26,30)(H,27,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528309

(CHEMBL4588933)Show SMILES ONC(=O)C(NC(=O)Cc1ccc(F)c(O)c1)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C22H16F4N2O4/c23-15-6-1-11(7-18(15)29)8-19(30)27-21(22(31)28-32)13-4-2-12(3-5-13)14-9-16(24)20(26)17(25)10-14/h1-7,9-10,21,29,32H,8H2,(H,27,30)(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50528307
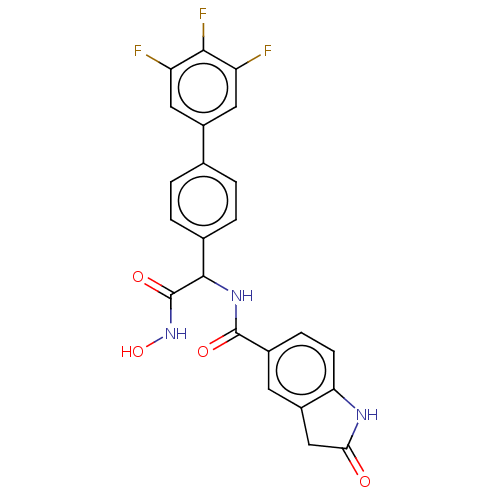
(CHEMBL4593147)Show SMILES ONC(=O)C(NC(=O)c1ccc2NC(=O)Cc2c1)c1ccc(cc1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C23H16F3N3O4/c24-16-8-14(9-17(25)20(16)26)11-1-3-12(4-2-11)21(23(32)29-33)28-22(31)13-5-6-18-15(7-13)10-19(30)27-18/h1-9,21,33H,10H2,(H,27,30)(H,28,31)(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... |
J Med Chem 62: 7185-7209 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00757
BindingDB Entry DOI: 10.7270/Q2KK9G8J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data