

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M17 leucyl aminopeptidase (Plasmodium falciparum 3D7) | BDBM50497553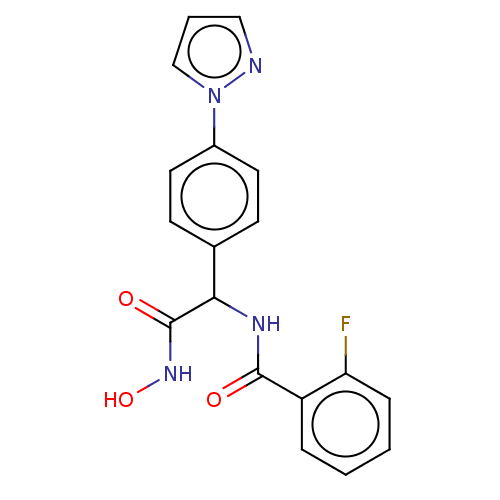 (CHEMBL3359696) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay | J Med Chem 57: 9168-83 (2014) Article DOI: 10.1021/jm501323a BindingDB Entry DOI: 10.7270/Q2QN69RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50162774 (ABT-199 | US11420968, Example ABT-199 | Venetoclax) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity to Bcl-2 (unknown origin) by TR-FRET assay | J Med Chem 60: 821-838 (2017) Article DOI: 10.1021/acs.jmedchem.5b01888 BindingDB Entry DOI: 10.7270/Q2ZC85BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M17 leucyl aminopeptidase (Plasmodium falciparum 3D7) | BDBM50497551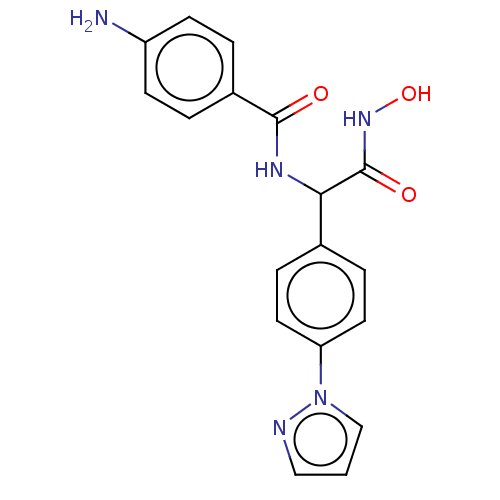 (CHEMBL3359700) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay | J Med Chem 57: 9168-83 (2014) Article DOI: 10.1021/jm501323a BindingDB Entry DOI: 10.7270/Q2QN69RJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| M17 leucyl aminopeptidase (Plasmodium falciparum 3D7) | BDBM50497552 (CHEMBL3359698) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay | J Med Chem 57: 9168-83 (2014) Article DOI: 10.1021/jm501323a BindingDB Entry DOI: 10.7270/Q2QN69RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M17 leucyl aminopeptidase (Plasmodium falciparum 3D7) | BDBM50497556 (CHEMBL3359699) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay | J Med Chem 57: 9168-83 (2014) Article DOI: 10.1021/jm501323a BindingDB Entry DOI: 10.7270/Q2QN69RJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| M17 leucyl aminopeptidase (Plasmodium falciparum 3D7) | BDBM50497548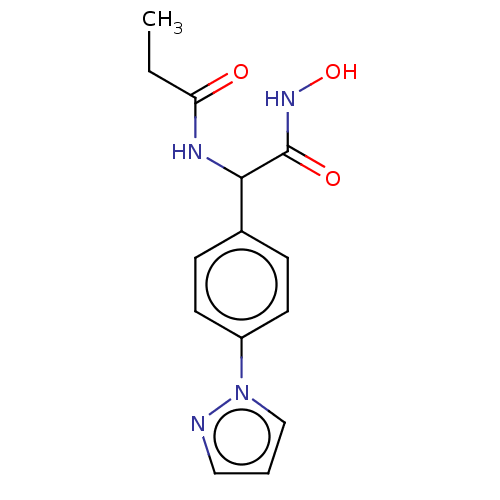 (CHEMBL3359691) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay | J Med Chem 57: 9168-83 (2014) Article DOI: 10.1021/jm501323a BindingDB Entry DOI: 10.7270/Q2QN69RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M17 leucyl aminopeptidase (Plasmodium falciparum 3D7) | BDBM50497549 (CHEMBL3359690) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay | J Med Chem 57: 9168-83 (2014) Article DOI: 10.1021/jm501323a BindingDB Entry DOI: 10.7270/Q2QN69RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M17 leucyl aminopeptidase (Plasmodium falciparum 3D7) | BDBM50497543 (CHEMBL3359693) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay | J Med Chem 57: 9168-83 (2014) Article DOI: 10.1021/jm501323a BindingDB Entry DOI: 10.7270/Q2QN69RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M17 leucyl aminopeptidase (Plasmodium falciparum 3D7) | BDBM50497544 (CHEMBL3359688) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay | J Med Chem 57: 9168-83 (2014) Article DOI: 10.1021/jm501323a BindingDB Entry DOI: 10.7270/Q2QN69RJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| M17 leucyl aminopeptidase (Plasmodium falciparum 3D7) | BDBM50497545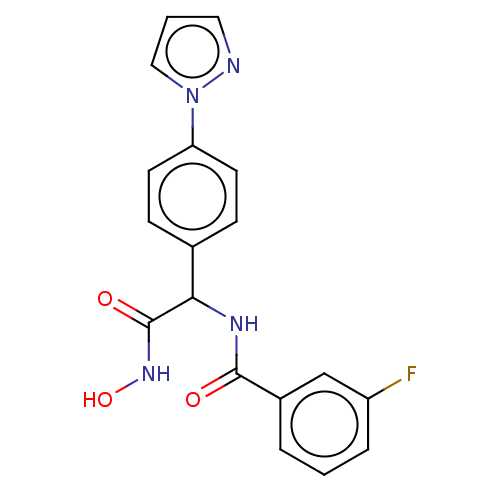 (CHEMBL3359697) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay | J Med Chem 57: 9168-83 (2014) Article DOI: 10.1021/jm501323a BindingDB Entry DOI: 10.7270/Q2QN69RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M17 leucyl aminopeptidase (Plasmodium falciparum 3D7) | BDBM50497547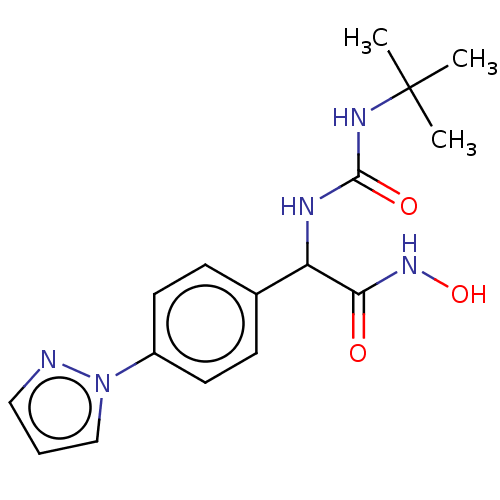 (CHEMBL3359694) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay | J Med Chem 57: 9168-83 (2014) Article DOI: 10.1021/jm501323a BindingDB Entry DOI: 10.7270/Q2QN69RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M17 leucyl aminopeptidase (Plasmodium falciparum 3D7) | BDBM50497557 (CHEMBL3359692) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay | J Med Chem 57: 9168-83 (2014) Article DOI: 10.1021/jm501323a BindingDB Entry DOI: 10.7270/Q2QN69RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50099273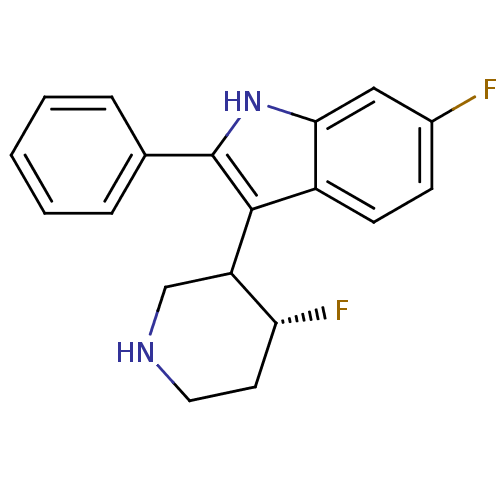 (6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-phenyl-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50095027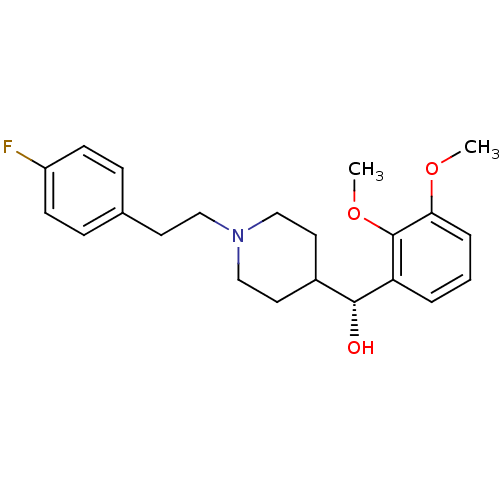 ((2,3-Dimethoxy-phenyl)-{1-[2-(4-fluoro-phenyl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M17 leucyl aminopeptidase (Plasmodium falciparum 3D7) | BDBM50497546 (CHEMBL3359695) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay | J Med Chem 57: 9168-83 (2014) Article DOI: 10.1021/jm501323a BindingDB Entry DOI: 10.7270/Q2QN69RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50108690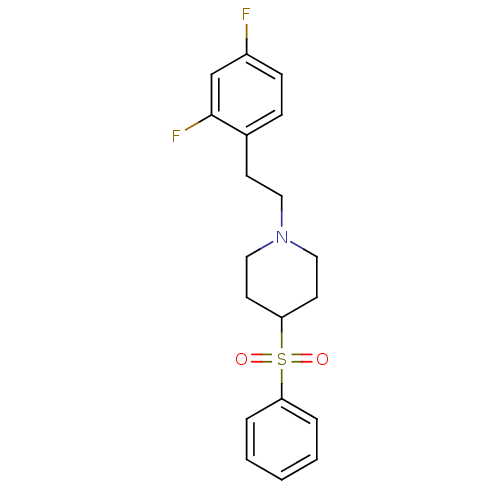 (1-(2,4-difluorophenethyl)-4-(phenylsulfonyl)piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM25121 (4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity to PLK1 (unknown origin) expressed in HEK293 cells after 1 hr by proprietary competition assay | ACS Med Chem Lett 6: 764-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00084 BindingDB Entry DOI: 10.7270/Q2FF3V4D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50108689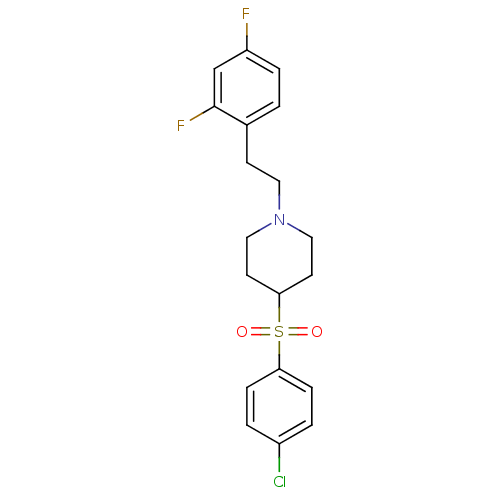 (4-(4-Chloro-benzenesulfonyl)-1-[2-(2,4-difluoro-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50095027 ((2,3-Dimethoxy-phenyl)-{1-[2-(4-fluoro-phenyl)-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108690 (1-(2,4-difluorophenethyl)-4-(phenylsulfonyl)piperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108688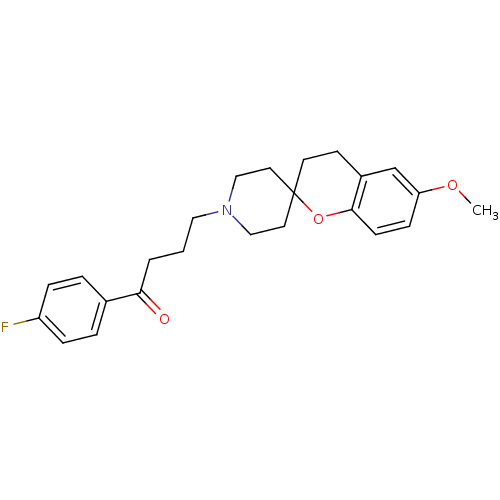 (1-(4-fluorophenyl)-4-[6-methoxyspiro[3,4-dihydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50108688 (1-(4-fluorophenyl)-4-[6-methoxyspiro[3,4-dihydro-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50036756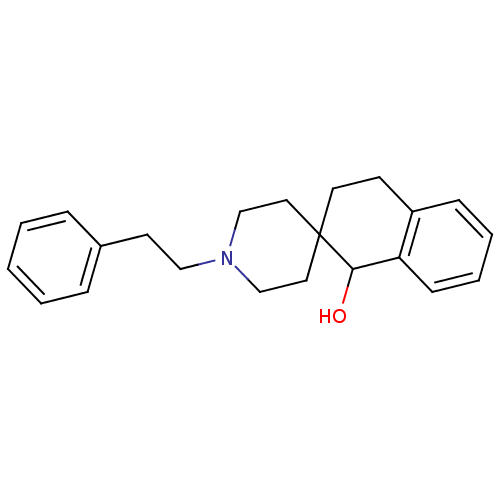 (1'-phenethylspiro[1,2,3,4-tetrahydronaphthalene-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108701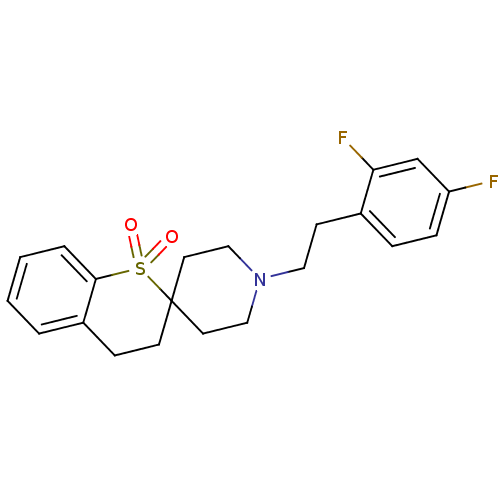 (1'-[2-(2,4-Difluorophenyl)ethyl]-3,4-dihydrospiro[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108699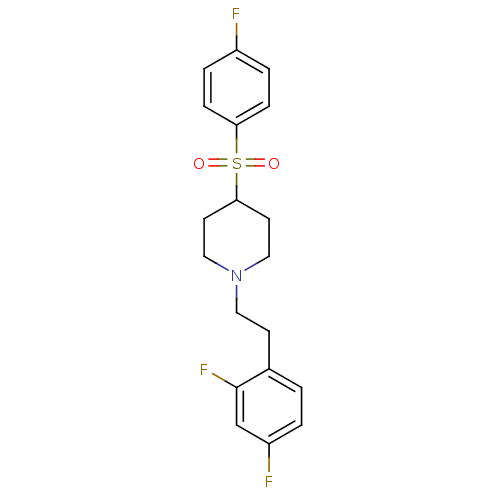 (1-(2,4-difluorophenethyl)-4-(4-fluorophenylsulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50112336 (CHEMBL3609305) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity to PLK1 (unknown origin) expressed in HEK293 cells after 1 hr by proprietary competition assay | ACS Med Chem Lett 6: 764-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00084 BindingDB Entry DOI: 10.7270/Q2FF3V4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108696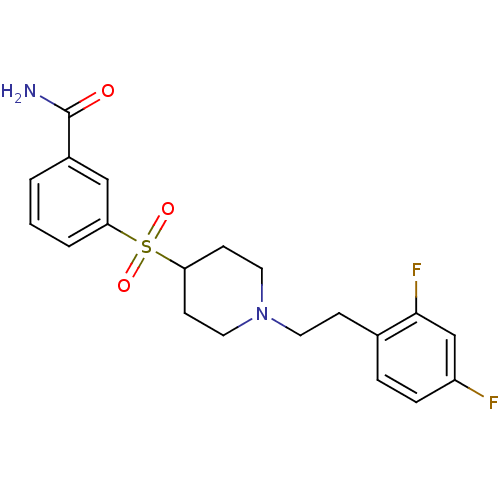 (3-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50108705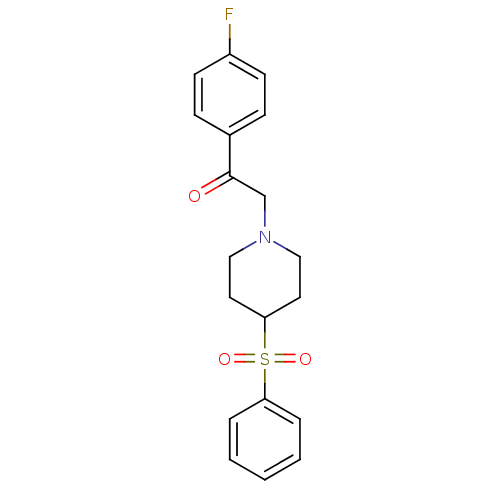 (1-(4-Fluorophenyl)-2-[4-(phenylsulfonyl)-1-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50112340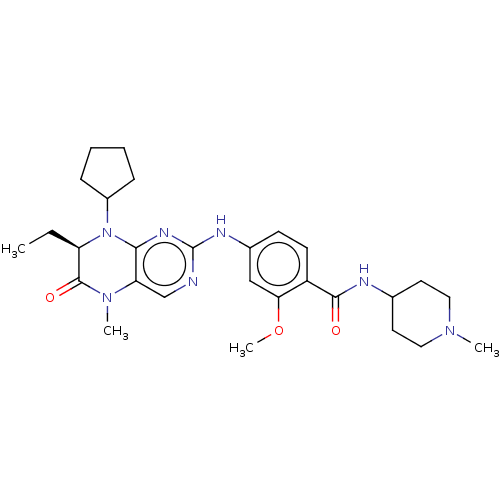 (CHEMBL3609310) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity to PLK1 (unknown origin) expressed in HEK293 cells after 1 hr by proprietary competition assay | ACS Med Chem Lett 6: 764-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00084 BindingDB Entry DOI: 10.7270/Q2FF3V4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108706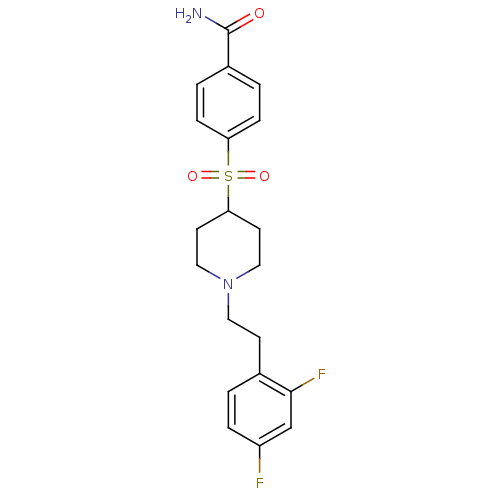 (4-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108694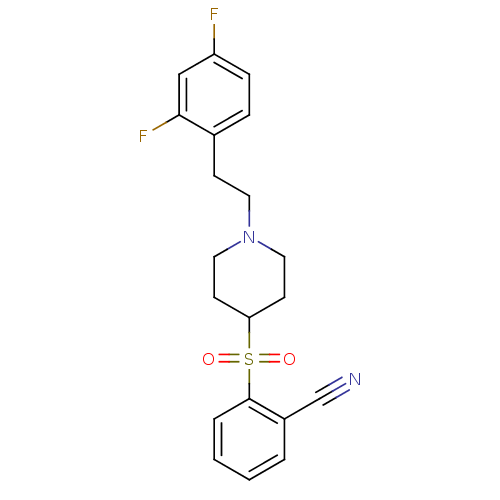 (2-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50108707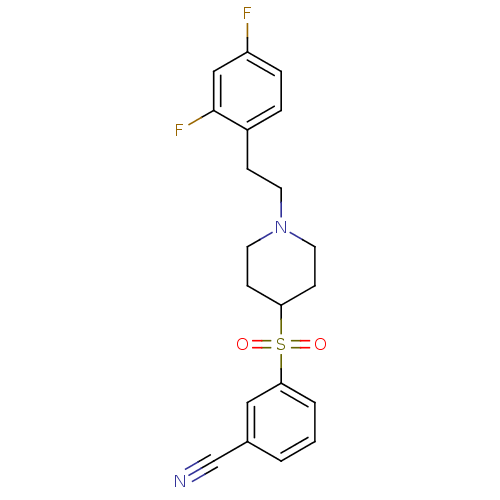 (3-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50036756 (1'-phenethylspiro[1,2,3,4-tetrahydronaphthalene-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50108701 (1'-[2-(2,4-Difluorophenyl)ethyl]-3,4-dihydrospiro[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108707 (3-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50108700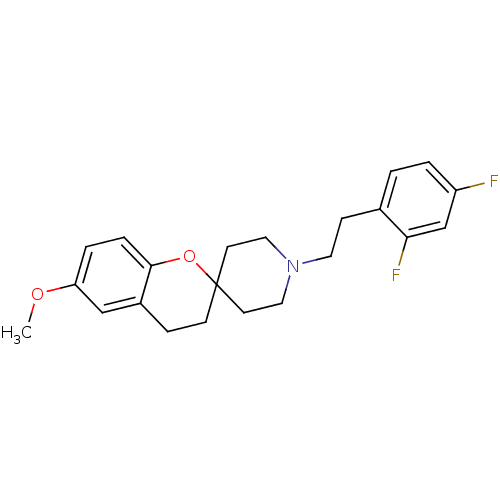 (1'-(2,4-difluorophenethyl)-6-methoxyspiro[3,4-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M1 family aminopeptidase (Plasmodium falciparum (isolate FcB1 / Columbia)) | BDBM50497543 (CHEMBL3359693) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum M1 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay | J Med Chem 57: 9168-83 (2014) Article DOI: 10.1021/jm501323a BindingDB Entry DOI: 10.7270/Q2QN69RJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50108697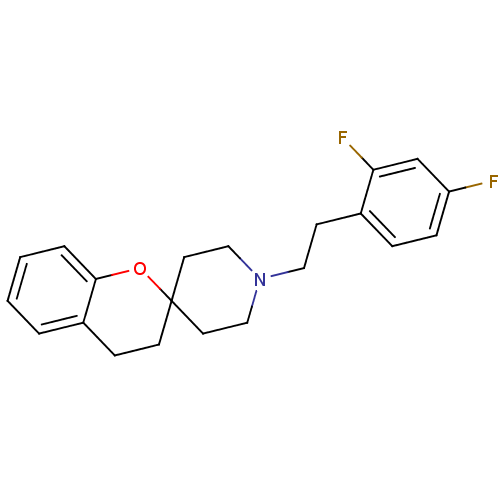 (1'-(2,4-difluorophenethyl)spiro[3,4-dihydro-2H-chr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M1 family aminopeptidase (Plasmodium falciparum (isolate FcB1 / Columbia)) | BDBM50497544 (CHEMBL3359688) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum M1 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay | J Med Chem 57: 9168-83 (2014) Article DOI: 10.1021/jm501323a BindingDB Entry DOI: 10.7270/Q2QN69RJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50112344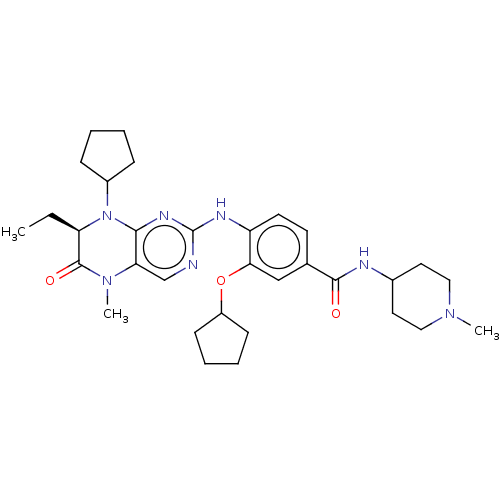 (CHEMBL3609314) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity to PLK1 (unknown origin) expressed in HEK293 cells after 1 hr by proprietary competition assay | ACS Med Chem Lett 6: 764-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00084 BindingDB Entry DOI: 10.7270/Q2FF3V4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50112331 (CHEMBL3609300) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity to PLK1 (unknown origin) expressed in HEK293 cells after 1 hr by proprietary competition assay | ACS Med Chem Lett 6: 764-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00084 BindingDB Entry DOI: 10.7270/Q2FF3V4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM21447 (4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of Bcl-2 (unknown origin) | J Med Chem 60: 821-838 (2017) Article DOI: 10.1021/acs.jmedchem.5b01888 BindingDB Entry DOI: 10.7270/Q2ZC85BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21447 (4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of Bcl-xL (unknown origin) | J Med Chem 60: 821-838 (2017) Article DOI: 10.1021/acs.jmedchem.5b01888 BindingDB Entry DOI: 10.7270/Q2ZC85BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50266959 (CHEMBL4085804) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of Bcl-2 (unknown origin) by fluorescence polarization assay | J Med Chem 60: 821-838 (2017) Article DOI: 10.1021/acs.jmedchem.5b01888 BindingDB Entry DOI: 10.7270/Q2ZC85BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 2 (Homo sapiens (Human)) | BDBM21447 (4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of Bcl-w (unknown origin) | J Med Chem 60: 821-838 (2017) Article DOI: 10.1021/acs.jmedchem.5b01888 BindingDB Entry DOI: 10.7270/Q2ZC85BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50266959 (CHEMBL4085804) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of Bcl-xL (unknown origin) by fluorescence polarization assay | J Med Chem 60: 821-838 (2017) Article DOI: 10.1021/acs.jmedchem.5b01888 BindingDB Entry DOI: 10.7270/Q2ZC85BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50108706 (4-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108703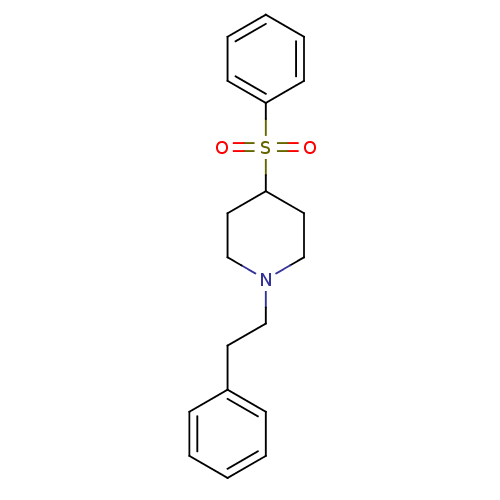 (1-(2-Phenylethyl)-4-(phenylsulfonyl)piperidine | 4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50173700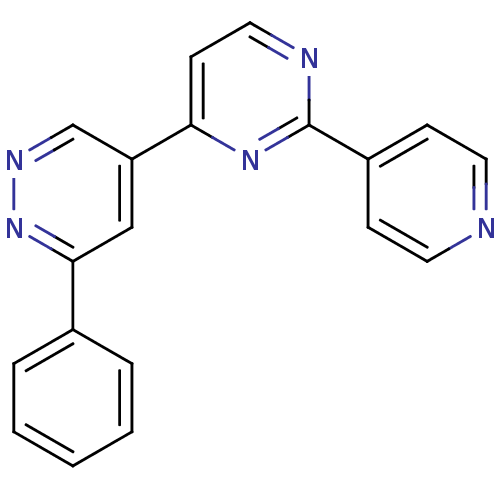 (3-Phenyl-5-(2-pyridin-4-yl-pyrimidin-4-yl)-pyridaz...) | UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-Ro- 15-1788 binding from human recombinant Gamma-aminobutyric-acid A receptor alpha1-beta3-gamma2 expressed in mouse fibroblast ... | J Med Chem 48: 6004-11 (2005) Article DOI: 10.1021/jm050249x BindingDB Entry DOI: 10.7270/Q26H4GZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108689 (4-(4-Chloro-benzenesulfonyl)-1-[2-(2,4-difluoro-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2508 total ) | Next | Last >> |