

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21190 (4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A2A receptor | Bioorg Med Chem Lett 15: 4809-13 (2005) Article DOI: 10.1016/j.bmcl.2005.07.052 BindingDB Entry DOI: 10.7270/Q24Q7TJV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50173189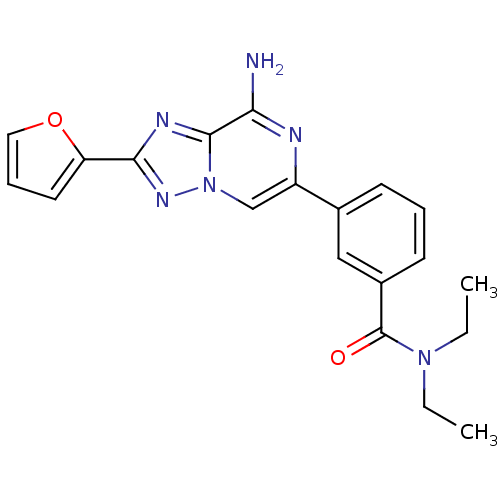 (3-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A2a receptor of rat brain homogenates using [3H]-ZM-241,385 compared to SCH-58261 (Ki=37 nM) | Bioorg Med Chem Lett 15: 4809-13 (2005) Article DOI: 10.1016/j.bmcl.2005.07.052 BindingDB Entry DOI: 10.7270/Q24Q7TJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50173176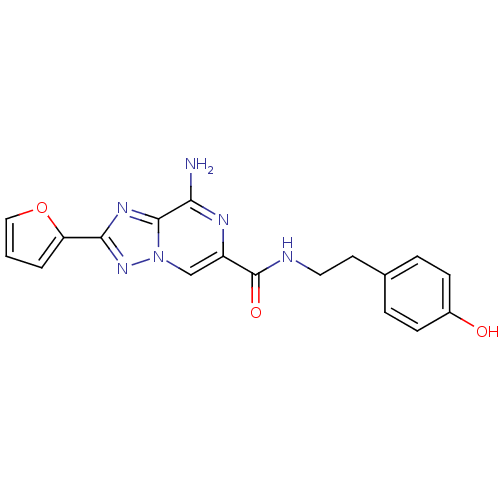 (8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyrazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A2a receptor of rat brain homogenates using [3H]-ZM-241,385 compared to SCH-58261 (Ki=37 nM) | Bioorg Med Chem Lett 15: 4809-13 (2005) Article DOI: 10.1016/j.bmcl.2005.07.052 BindingDB Entry DOI: 10.7270/Q24Q7TJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50173186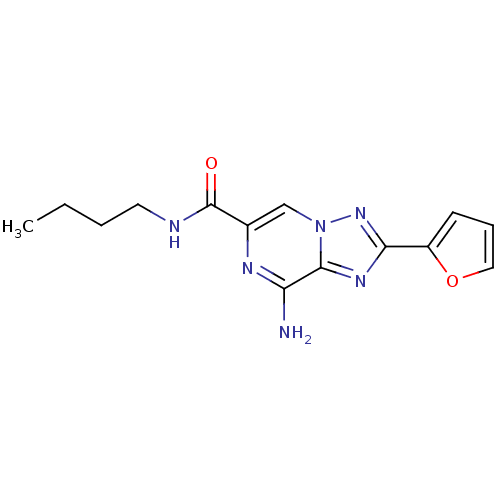 (8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyrazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A2a receptor of rat brain homogenates using [3H]-ZM-241,385 compared to SCH-58261 (Ki=37 nM) | Bioorg Med Chem Lett 15: 4809-13 (2005) Article DOI: 10.1016/j.bmcl.2005.07.052 BindingDB Entry DOI: 10.7270/Q24Q7TJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50159713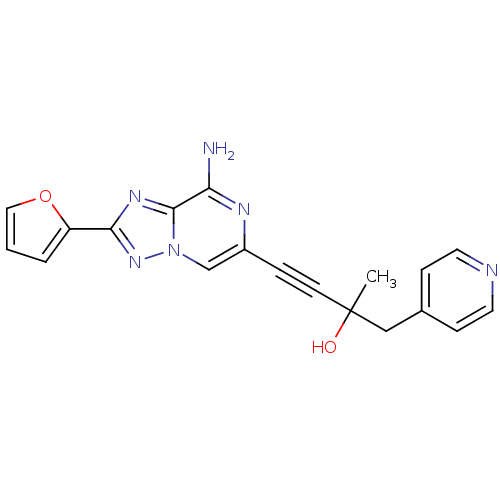 (4-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A2A receptor of rat brain tissues using [3H]-ZM-241,385 as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50076285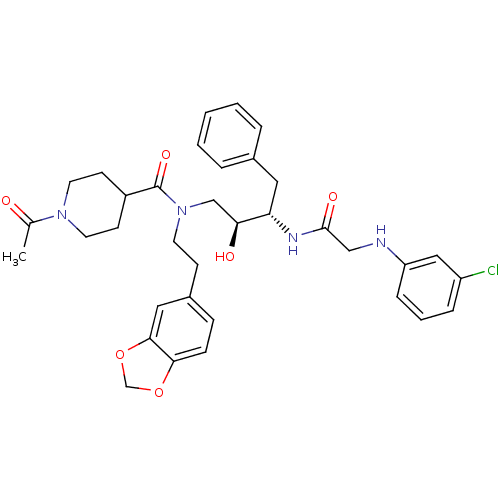 (1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against human liver cathepsin D | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50076294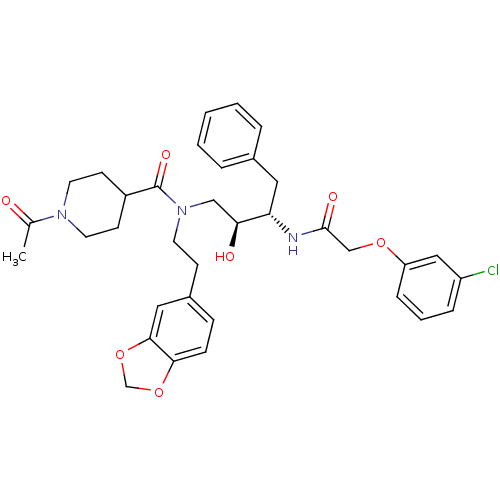 (1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against human liver cathepsin D | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076292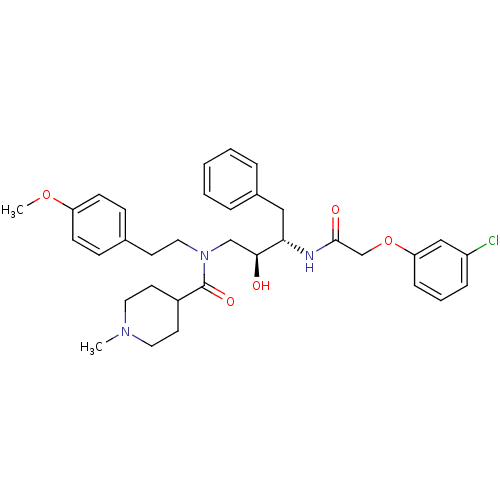 (1-Methyl-piperidine-4-carboxylic acid {(2S,3S)-3-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50173201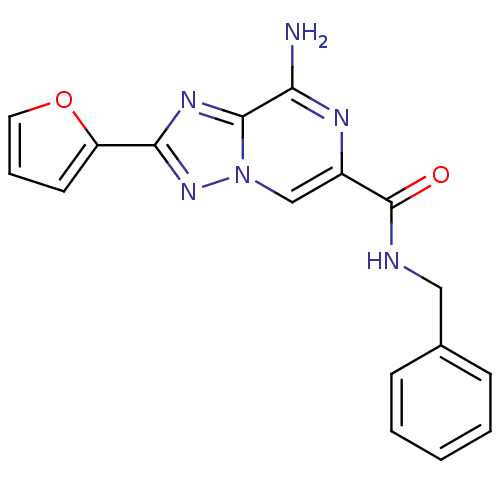 (8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyrazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A2a receptor of rat brain homogenates using [3H]-ZM-241,385 compared to SCH-58261 (Ki=37 nM) | Bioorg Med Chem Lett 15: 4809-13 (2005) Article DOI: 10.1016/j.bmcl.2005.07.052 BindingDB Entry DOI: 10.7270/Q24Q7TJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076291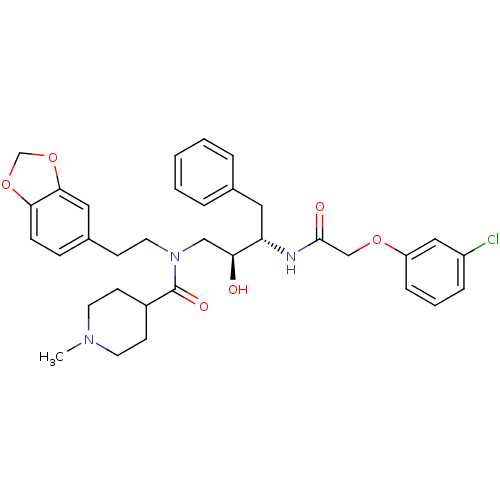 (1-Methyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50048466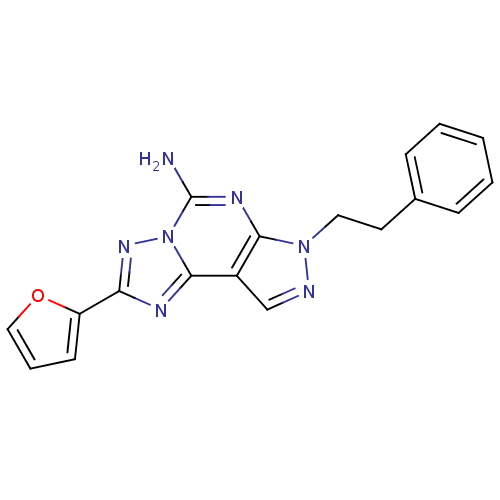 (2-(furan-2-yl)-7-phenethyl-7H-pyrazolo[4,3-e][1,2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A2A receptor | Bioorg Med Chem Lett 15: 4809-13 (2005) Article DOI: 10.1016/j.bmcl.2005.07.052 BindingDB Entry DOI: 10.7270/Q24Q7TJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50159711 (1-[3-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A1 receptor of rat cerebral cortex using [3H]-DPCPX as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076285 (1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50173179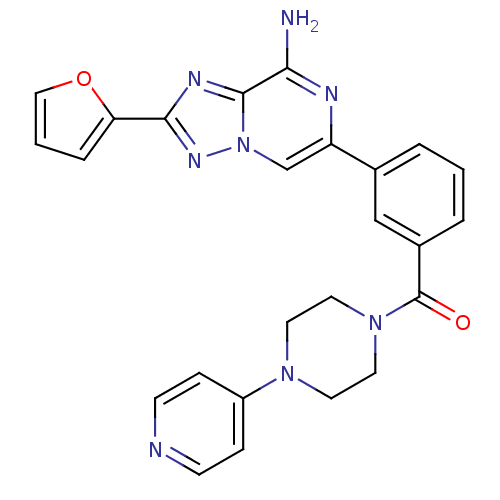 (CHEMBL196104 | [3-(8-Amino-2-furan-2-yl-[1,2,4]tri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A1 receptor of rat cerebral cortex using [3H]-DPCPX compared to SCH-58261 (Ki=390 nM) | Bioorg Med Chem Lett 15: 4809-13 (2005) Article DOI: 10.1016/j.bmcl.2005.07.052 BindingDB Entry DOI: 10.7270/Q24Q7TJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50159712 (1-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A2A receptor of rat brain tissues using [3H]-ZM-241,385 as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119488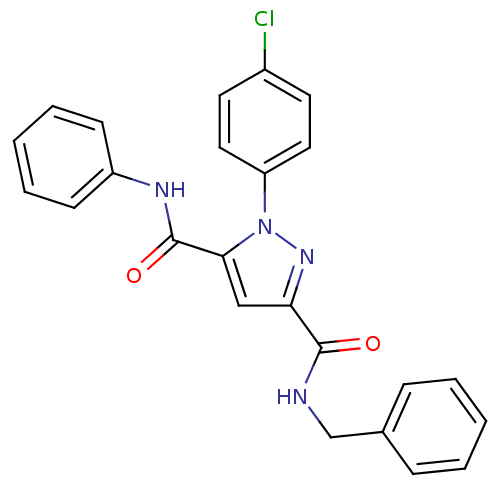 (CHEMBL143628 | X1-(4-Chloro-phenyl)-1H-pyrazole-3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076294 (1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119489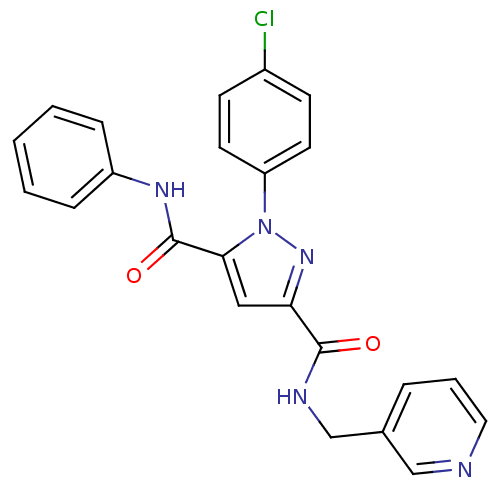 (1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076287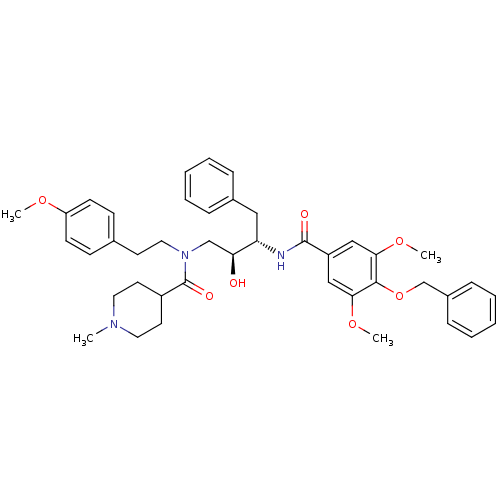 (1-Methyl-piperidine-4-carboxylic acid [(2S,3S)-3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50076293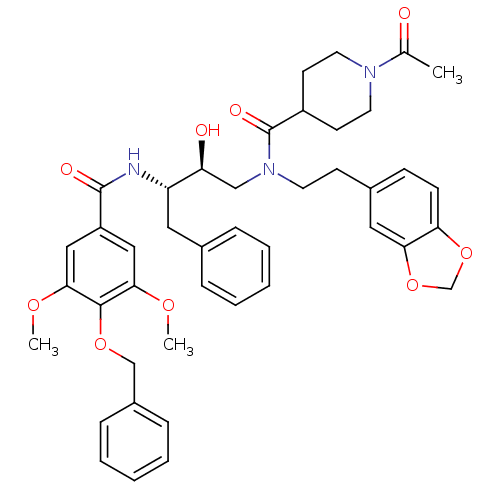 (1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against human liver cathepsin D | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8019 (2-(3-chlorophenoxy)-N-[(2S,3S)-3-hydroxy-4-{N-[2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076293 (1-Acetyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50076291 (1-Methyl-piperidine-4-carboxylic acid (2-benzo[1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against human liver cathepsin D | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119481 (1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50159720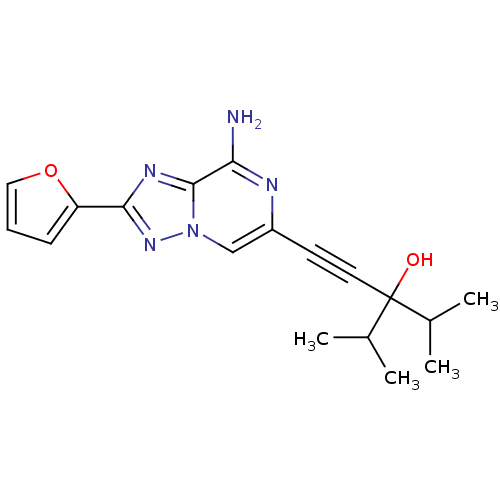 (1-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A2A receptor of rat brain tissues using [3H]-ZM-241,385 as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50159717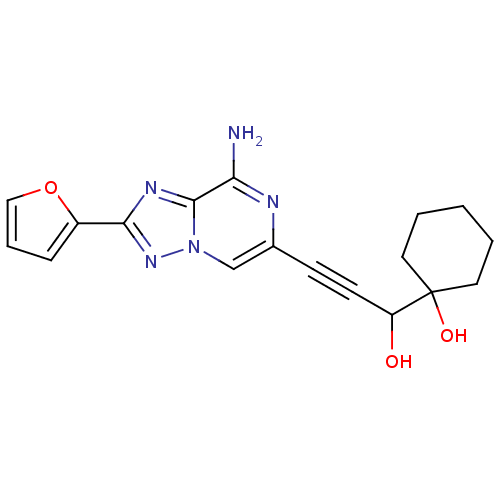 (1-[3-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A2A receptor of rat brain tissues using [3H]-ZM-241,385 as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50159720 (1-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A1 receptor of rat cerebral cortex using [3H]-DPCPX as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119483 (1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50159719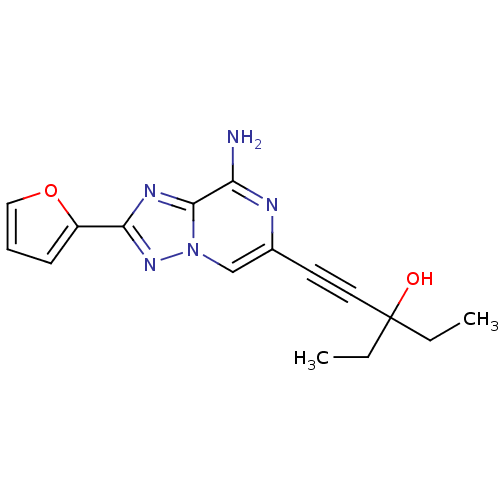 (1-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A2A receptor of rat brain tissues using [3H]-ZM-241,385 as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50076288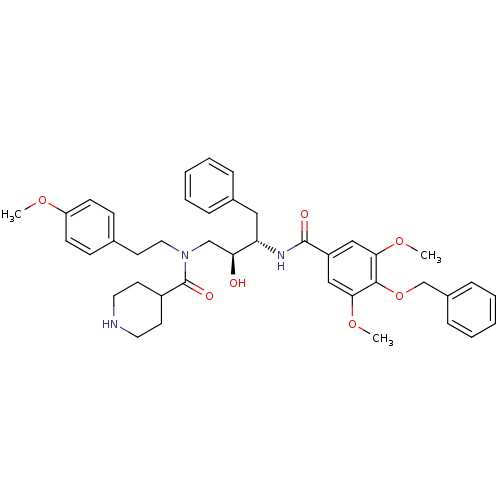 (CHEMBL284955 | Piperidine-4-carboxylic acid [(2S,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against Plasmepsin 2 | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50076292 (1-Methyl-piperidine-4-carboxylic acid {(2S,3S)-3-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against human liver cathepsin D | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50159717 (1-[3-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A1 receptor of rat cerebral cortex using [3H]-DPCPX as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119485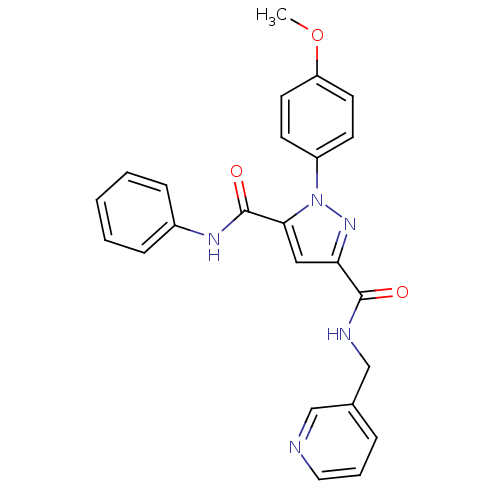 (1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50159732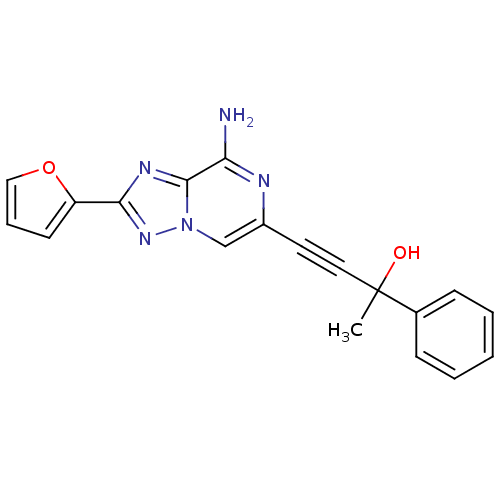 (4-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A2A receptor of rat brain tissues using [3H]-ZM-241,385 as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50159711 (1-[3-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A2A receptor of rat brain tissues using [3H]-ZM-241,385 as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50173201 (8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyrazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A1 receptor of rat cerebral cortex using [3H]-DPCPX compared to SCH-58261 (Ki=390 nM) | Bioorg Med Chem Lett 15: 4809-13 (2005) Article DOI: 10.1016/j.bmcl.2005.07.052 BindingDB Entry DOI: 10.7270/Q24Q7TJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50076286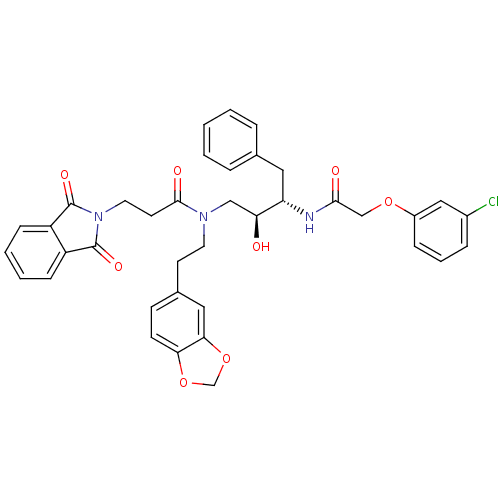 (CHEMBL34051 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Compound was tested for the inhibitory activity against human liver cathepsin D | J Med Chem 42: 1428-40 (1999) Article DOI: 10.1021/jm980641t BindingDB Entry DOI: 10.7270/Q2MS3RZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50173188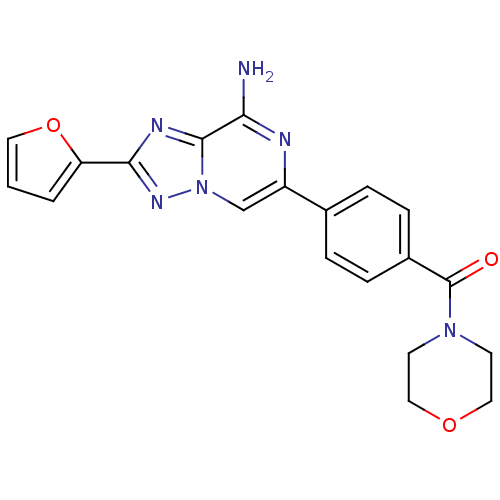 (CHEMBL383568 | [4-(8-Amino-2-furan-2-yl-[1,2,4]tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A2a receptor of rat brain homogenates using [3H]-ZM-241,385 compared to SCH-58261 (Ki=37 nM) | Bioorg Med Chem Lett 15: 4809-13 (2005) Article DOI: 10.1016/j.bmcl.2005.07.052 BindingDB Entry DOI: 10.7270/Q24Q7TJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50159712 (1-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A1 receptor of rat cerebral cortex using [3H]-DPCPX as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50159715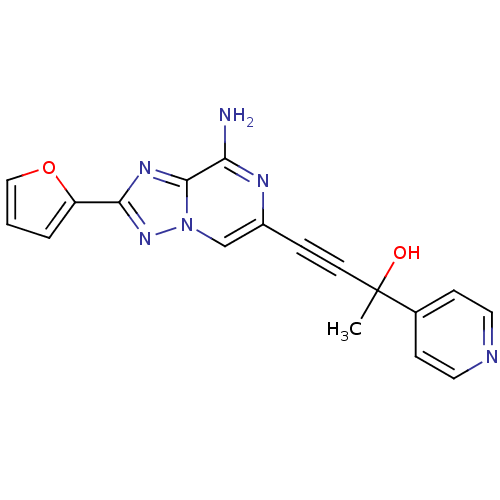 (4-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A2A receptor of rat brain tissues using [3H]-ZM-241,385 as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119490 (1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50173200 (8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyrazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A2a receptor of rat brain homogenates using [3H]-ZM-241,385 compared to SCH-58261 (Ki=37 nM) | Bioorg Med Chem Lett 15: 4809-13 (2005) Article DOI: 10.1016/j.bmcl.2005.07.052 BindingDB Entry DOI: 10.7270/Q24Q7TJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50173182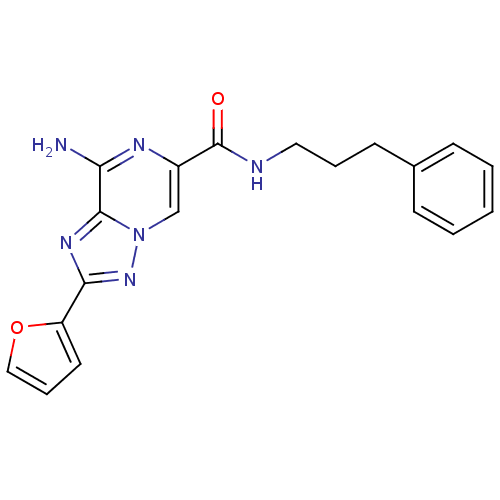 (8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyrazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A2a receptor of rat brain homogenates using [3H]-ZM-241,385 compared to SCH-58261 (Ki=37 nM) | Bioorg Med Chem Lett 15: 4809-13 (2005) Article DOI: 10.1016/j.bmcl.2005.07.052 BindingDB Entry DOI: 10.7270/Q24Q7TJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50159724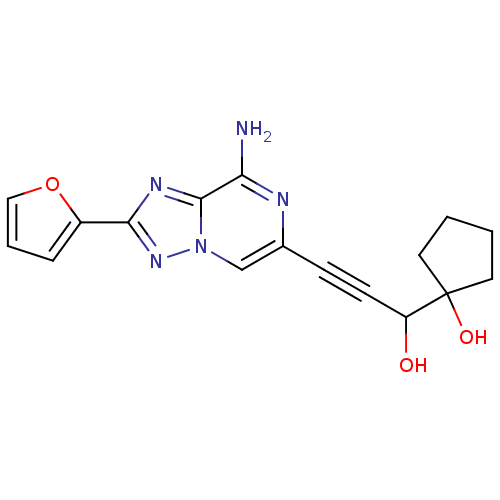 (1-[3-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A2A receptor of rat brain tissues using [3H]-ZM-241,385 as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119492 (1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50159733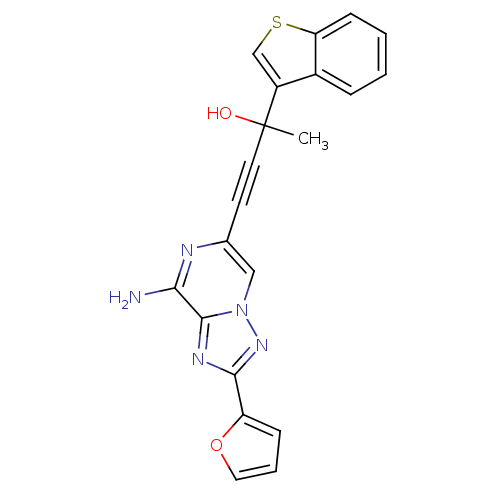 (4-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A2A receptor of rat brain tissues using [3H]-ZM-241,385 as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50159719 (1-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A1 receptor of rat cerebral cortex using [3H]-DPCPX as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50159716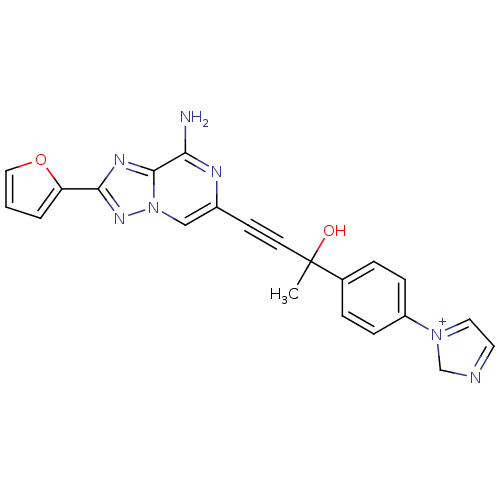 (4-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A2A receptor of rat brain tissues using [3H]-ZM-241,385 as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50159734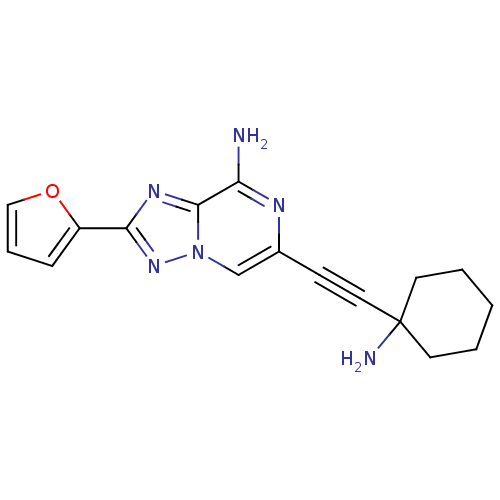 (6-(1-Amino-cyclohexylethynyl)-2-furan-2-yl-[1,2,4]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Binding affinity towards Adenosine A1 receptor of rat cerebral cortex using [3H]-DPCPX as radioligand | Bioorg Med Chem Lett 15: 511-5 (2005) Article DOI: 10.1016/j.bmcl.2004.11.062 BindingDB Entry DOI: 10.7270/Q2319VCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50173192 (4-(8-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-a]pyra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A1 receptor of rat cerebral cortex using [3H]-DPCPX compared to SCH-58261 (Ki=390 nM) | Bioorg Med Chem Lett 15: 4809-13 (2005) Article DOI: 10.1016/j.bmcl.2005.07.052 BindingDB Entry DOI: 10.7270/Q24Q7TJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4821 total ) | Next | Last >> |