 Found 918 hits with Last Name = 'oishi' and Initial = 's'
Found 918 hits with Last Name = 'oishi' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM126083

(US8779142, 102)Show SMILES CCc1cc(ccc1-n1nc(C(C)C)c2c(ccnc12)-n1cnc(c1)-c1cnn(C)c1)C(N)=O Show InChI InChI=1S/C25H26N8O/c1-5-16-10-17(24(26)34)6-7-20(16)33-25-22(23(30-33)15(2)3)21(8-9-27-25)32-13-19(28-14-32)18-11-29-31(4)12-18/h6-15H,5H2,1-4H3,(H2,26,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human HSP90-beta by fluorescence polarization competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01715
BindingDB Entry DOI: 10.7270/Q2Z323H2 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM126083

(US8779142, 102)Show SMILES CCc1cc(ccc1-n1nc(C(C)C)c2c(ccnc12)-n1cnc(c1)-c1cnn(C)c1)C(N)=O Show InChI InChI=1S/C25H26N8O/c1-5-16-10-17(24(26)34)6-7-20(16)33-25-22(23(30-33)15(2)3)21(8-9-27-25)32-13-19(28-14-32)18-11-29-31(4)12-18/h6-15H,5H2,1-4H3,(H2,26,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FITC-GDA binding to human HSP90-alpha incubated for 2 hrs followed by FITC-GDA addition and measured after 5 hrs by fluorescence polari... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01715
BindingDB Entry DOI: 10.7270/Q2Z323H2 |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50175277

(CHEMBL3809675)Show SMILES Cl.CCCCCCCCCCCCc1ccc(cc1)-c1noc(n1)C1(CC1)C(N)=N Show InChI InChI=1S/C24H36N4O.ClH/c1-2-3-4-5-6-7-8-9-10-11-12-19-13-15-20(16-14-19)21-27-23(29-28-21)24(17-18-24)22(25)26;/h13-16H,2-12,17-18H2,1H3,(H3,25,26);1H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SphK1 expressed in baculovirus infected sf9 cells using D-erythro-sphingosine as substrate in presence of [gamma-33P]... |
Bioorg Med Chem 25: 3046-3052 (2017)
Article DOI: 10.1016/j.bmc.2017.03.059
BindingDB Entry DOI: 10.7270/Q2XD144Z |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM174110
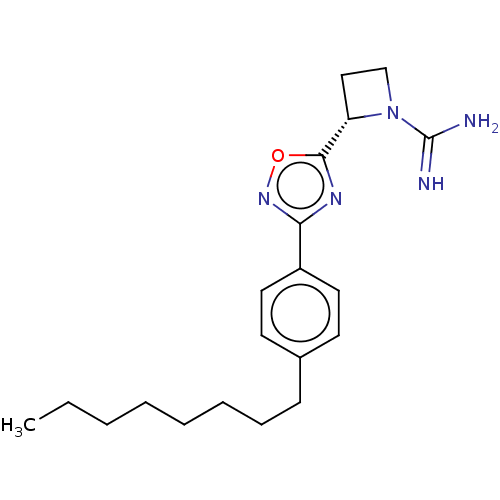
((S)-2-(3-(4-octylphenyl)-1,2,4- oxadiazol-5-yl)aze...)Show SMILES CCCCCCCCc1ccc(cc1)-c1noc(n1)[C@@H]1CCN1C(N)=N |r| Show InChI InChI=1S/C20H29N5O/c1-2-3-4-5-6-7-8-15-9-11-16(12-10-15)18-23-19(26-24-18)17-13-14-25(17)20(21)22/h9-12,17H,2-8,13-14H2,1H3,(H3,21,22)/t17-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SphK2 expressed in baculovirus infected sf9 cells using D-erythro-sphingosine as substrate after 20 mins in presence ... |
Bioorg Med Chem 25: 3046-3052 (2017)
Article DOI: 10.1016/j.bmc.2017.03.059
BindingDB Entry DOI: 10.7270/Q2XD144Z |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM174110

((S)-2-(3-(4-octylphenyl)-1,2,4- oxadiazol-5-yl)aze...)Show SMILES CCCCCCCCc1ccc(cc1)-c1noc(n1)[C@@H]1CCN1C(N)=N |r| Show InChI InChI=1S/C20H29N5O/c1-2-3-4-5-6-7-8-15-9-11-16(12-10-15)18-23-19(26-24-18)17-13-14-25(17)20(21)22/h9-12,17H,2-8,13-14H2,1H3,(H3,21,22)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SphK1 expressed in baculovirus infected sf9 cells using D-erythro-sphingosine as substrate after 20 mins in presence ... |
Bioorg Med Chem 25: 3046-3052 (2017)
Article DOI: 10.1016/j.bmc.2017.03.059
BindingDB Entry DOI: 10.7270/Q2XD144Z |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50582399
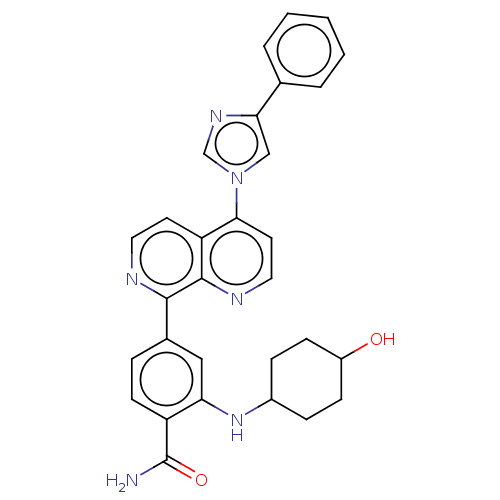
(CHEMBL5094983)Show SMILES NC(=O)c1ccc(cc1NC1CCC(O)CC1)-c1nccc2c(ccnc12)-n1cnc(c1)-c1ccccc1 |(-1.67,-8.76,;-.34,-7.99,;1,-8.76,;-.34,-6.45,;-1.67,-5.69,;-1.67,-4.14,;-.34,-3.37,;.99,-4.14,;.99,-5.68,;2.32,-6.45,;3.66,-5.68,;3.66,-4.14,;4.99,-3.37,;6.32,-4.14,;7.66,-3.37,;6.32,-5.68,;4.99,-6.45,;-.34,-1.83,;1,-1.06,;.99,.48,;-.34,1.25,;-1.67,.48,;-3.01,1.25,;-4.33,.47,;-4.33,-1.05,;-3,-1.82,;-1.67,-1.06,;-3.01,2.79,;-1.88,3.82,;-2.5,5.24,;-4,5.09,;-4.34,3.56,;-5.08,6.18,;-6.57,5.78,;-7.66,6.87,;-7.26,8.36,;-5.78,8.76,;-4.68,7.68,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FITC-GDA binding to human HSP90-alpha incubated for 2 hrs followed by FITC-GDA addition and measured after 5 hrs by fluorescence polari... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01715
BindingDB Entry DOI: 10.7270/Q2Z323H2 |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Mus musculus (Mouse)) | BDBM50175277

(CHEMBL3809675)Show SMILES Cl.CCCCCCCCCCCCc1ccc(cc1)-c1noc(n1)C1(CC1)C(N)=N Show InChI InChI=1S/C24H36N4O.ClH/c1-2-3-4-5-6-7-8-9-10-11-12-19-13-15-20(16-14-19)21-27-23(29-28-21)24(17-18-24)22(25)26;/h13-16H,2-12,17-18H2,1H3,(H3,25,26);1H | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse SphK2 expressed in baculovirus infected sf9 cells using D-erythro-sphingosine as substrate in presence of [gamma-33P]... |
Bioorg Med Chem 25: 3046-3052 (2017)
Article DOI: 10.1016/j.bmc.2017.03.059
BindingDB Entry DOI: 10.7270/Q2XD144Z |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50582399

(CHEMBL5094983)Show SMILES NC(=O)c1ccc(cc1NC1CCC(O)CC1)-c1nccc2c(ccnc12)-n1cnc(c1)-c1ccccc1 |(-1.67,-8.76,;-.34,-7.99,;1,-8.76,;-.34,-6.45,;-1.67,-5.69,;-1.67,-4.14,;-.34,-3.37,;.99,-4.14,;.99,-5.68,;2.32,-6.45,;3.66,-5.68,;3.66,-4.14,;4.99,-3.37,;6.32,-4.14,;7.66,-3.37,;6.32,-5.68,;4.99,-6.45,;-.34,-1.83,;1,-1.06,;.99,.48,;-.34,1.25,;-1.67,.48,;-3.01,1.25,;-4.33,.47,;-4.33,-1.05,;-3,-1.82,;-1.67,-1.06,;-3.01,2.79,;-1.88,3.82,;-2.5,5.24,;-4,5.09,;-4.34,3.56,;-5.08,6.18,;-6.57,5.78,;-7.66,6.87,;-7.26,8.36,;-5.78,8.76,;-4.68,7.68,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human HSP90-beta by fluorescence polarization competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01715
BindingDB Entry DOI: 10.7270/Q2Z323H2 |
More data for this
Ligand-Target Pair | |
Endoplasmin
(Homo sapiens (Human)) | BDBM126083

(US8779142, 102)Show SMILES CCc1cc(ccc1-n1nc(C(C)C)c2c(ccnc12)-n1cnc(c1)-c1cnn(C)c1)C(N)=O Show InChI InChI=1S/C25H26N8O/c1-5-16-10-17(24(26)34)6-7-20(16)33-25-22(23(30-33)15(2)3)21(8-9-27-25)32-13-19(28-14-32)18-11-29-31(4)12-18/h6-15H,5H2,1-4H3,(H2,26,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to GRP94 (unknown origin) by fluorescence polarization competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01715
BindingDB Entry DOI: 10.7270/Q2Z323H2 |
More data for this
Ligand-Target Pair | |
Heat shock protein 75 kDa, mitochondrial
(Homo sapiens (Human)) | BDBM126083

(US8779142, 102)Show SMILES CCc1cc(ccc1-n1nc(C(C)C)c2c(ccnc12)-n1cnc(c1)-c1cnn(C)c1)C(N)=O Show InChI InChI=1S/C25H26N8O/c1-5-16-10-17(24(26)34)6-7-20(16)33-25-22(23(30-33)15(2)3)21(8-9-27-25)32-13-19(28-14-32)18-11-29-31(4)12-18/h6-15H,5H2,1-4H3,(H2,26,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to TRAP1 (unknown origin) by fluorescence polarization competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01715
BindingDB Entry DOI: 10.7270/Q2Z323H2 |
More data for this
Ligand-Target Pair | |
Endoplasmin
(Homo sapiens (Human)) | BDBM50582399

(CHEMBL5094983)Show SMILES NC(=O)c1ccc(cc1NC1CCC(O)CC1)-c1nccc2c(ccnc12)-n1cnc(c1)-c1ccccc1 |(-1.67,-8.76,;-.34,-7.99,;1,-8.76,;-.34,-6.45,;-1.67,-5.69,;-1.67,-4.14,;-.34,-3.37,;.99,-4.14,;.99,-5.68,;2.32,-6.45,;3.66,-5.68,;3.66,-4.14,;4.99,-3.37,;6.32,-4.14,;7.66,-3.37,;6.32,-5.68,;4.99,-6.45,;-.34,-1.83,;1,-1.06,;.99,.48,;-.34,1.25,;-1.67,.48,;-3.01,1.25,;-4.33,.47,;-4.33,-1.05,;-3,-1.82,;-1.67,-1.06,;-3.01,2.79,;-1.88,3.82,;-2.5,5.24,;-4,5.09,;-4.34,3.56,;-5.08,6.18,;-6.57,5.78,;-7.66,6.87,;-7.26,8.36,;-5.78,8.76,;-4.68,7.68,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to GRP94 (unknown origin) by fluorescence polarization competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01715
BindingDB Entry DOI: 10.7270/Q2Z323H2 |
More data for this
Ligand-Target Pair | |
Heat shock protein 75 kDa, mitochondrial
(Homo sapiens (Human)) | BDBM50582399

(CHEMBL5094983)Show SMILES NC(=O)c1ccc(cc1NC1CCC(O)CC1)-c1nccc2c(ccnc12)-n1cnc(c1)-c1ccccc1 |(-1.67,-8.76,;-.34,-7.99,;1,-8.76,;-.34,-6.45,;-1.67,-5.69,;-1.67,-4.14,;-.34,-3.37,;.99,-4.14,;.99,-5.68,;2.32,-6.45,;3.66,-5.68,;3.66,-4.14,;4.99,-3.37,;6.32,-4.14,;7.66,-3.37,;6.32,-5.68,;4.99,-6.45,;-.34,-1.83,;1,-1.06,;.99,.48,;-.34,1.25,;-1.67,.48,;-3.01,1.25,;-4.33,.47,;-4.33,-1.05,;-3,-1.82,;-1.67,-1.06,;-3.01,2.79,;-1.88,3.82,;-2.5,5.24,;-4,5.09,;-4.34,3.56,;-5.08,6.18,;-6.57,5.78,;-7.66,6.87,;-7.26,8.36,;-5.78,8.76,;-4.68,7.68,)| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to TRAP1 (unknown origin) by fluorescence polarization competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01715
BindingDB Entry DOI: 10.7270/Q2Z323H2 |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26349

((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-amino-3-(4-hydroxyp...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:83.86,72.75,12.20,29.34,37.50,51.58,59.62,wD:4.4,23.26,(23.93,-21.81,;22.46,-22.29,;22.15,-23.79,;21.33,-21.26,;21.65,-19.76,;20.51,-18.74,;19.04,-19.2,;18.73,-20.72,;17.9,-18.17,;16.44,-18.65,;15.29,-17.62,;15.61,-16.12,;13.83,-18.1,;12.69,-17.06,;13.01,-15.57,;11.86,-14.54,;12.18,-13.03,;13.65,-12.55,;14.79,-13.59,;14.47,-15.08,;13.51,-19.6,;12.05,-20.08,;10.9,-19.05,;11.73,-21.57,;12.87,-22.61,;14.34,-22.12,;10.27,-22.05,;9.94,-23.55,;11.09,-24.58,;8.49,-24.03,;7.34,-23,;7.67,-21.49,;6.52,-20.46,;9.12,-21.02,;8.16,-25.53,;6.7,-26.01,;5.56,-24.98,;6.38,-27.51,;4.92,-27.99,;4.6,-29.49,;5.63,-30.63,;4.86,-31.97,;3.35,-31.65,;2.11,-32.56,;.71,-31.92,;.55,-30.4,;1.79,-29.49,;3.2,-30.12,;7.53,-28.54,;8.98,-28.07,;9.31,-26.56,;10.13,-29.1,;9.81,-30.6,;8.35,-31.08,;8.02,-32.58,;7.2,-30.05,;11.59,-28.62,;12.74,-29.65,;12.42,-31.16,;14.21,-29.18,;14.52,-27.67,;15.34,-30.2,;16.8,-29.73,;17.12,-28.22,;18.58,-27.75,;19.72,-28.77,;21.19,-28.3,;19.41,-30.28,;17.94,-30.75,;23.11,-19.28,;23.42,-17.77,;24.24,-20.31,;25.71,-19.83,;26.03,-18.32,;27.49,-17.85,;27.81,-16.34,;29.28,-15.87,;29.59,-14.36,;28.45,-13.34,;31.06,-13.9,;26.85,-20.86,;26.53,-22.37,;28.32,-20.39,;29.46,-21.42,;30.93,-20.94,;32.07,-21.97,;33.53,-21.49,;34.68,-22.52,;34.35,-24.03,;32.89,-24.5,;31.75,-23.47,;29.14,-22.92,;27.68,-23.4,;30.28,-23.95,)| Show InChI InChI=1S/C63H83N17O14/c1-34(2)24-45(58(90)74-43(18-11-23-70-63(68)69)57(89)75-44(54(67)86)26-35-12-5-3-6-13-35)73-53(85)32-72-56(88)46(27-36-14-7-4-8-15-36)77-62(94)50(33-81)80-61(93)49(30-52(66)84)79-59(91)47(28-38-31-71-42-17-10-9-16-40(38)42)78-60(92)48(29-51(65)83)76-55(87)41(64)25-37-19-21-39(82)22-20-37/h3-10,12-17,19-22,31,34,41,43-50,71,81-82H,11,18,23-30,32-33,64H2,1-2H3,(H2,65,83)(H2,66,84)(H2,67,86)(H,72,88)(H,73,85)(H,74,90)(H,75,89)(H,76,87)(H,77,94)(H,78,92)(H,79,91)(H,80,93)(H4,68,69,70)/t41-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]kisspeptin-15 from GPR54 |
ACS Med Chem Lett 2: 53-57 (2011)
Article DOI: 10.1021/ml1002053
BindingDB Entry DOI: 10.7270/Q2K07586 |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26339
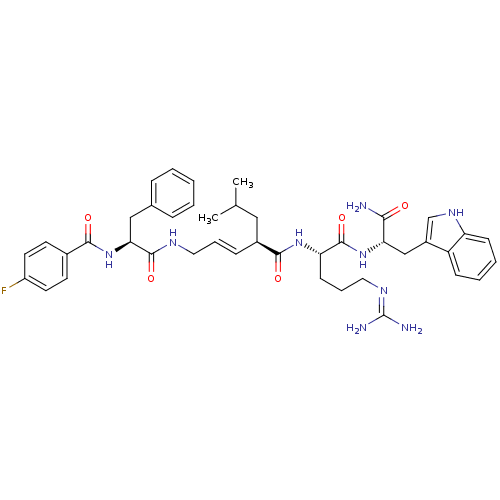
((2R,3E)-N-[(1S)-4-carbamimidamido-1-{[(1S)-1-carba...)Show SMILES CC(C)C[C@H](\C=C\CNC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(F)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,wU:32.33,wD:11.19,4.4,43.44,(10.76,-1.42,;10.76,.12,;12.1,.89,;9.43,.89,;9.43,2.43,;8.09,3.2,;6.76,2.43,;5.43,3.2,;4.09,2.43,;2.76,3.2,;2.76,4.74,;1.43,2.43,;1.43,.89,;.66,-.45,;-.87,-.66,;-1.45,-2.09,;-.5,-3.3,;1.02,-3.09,;1.6,-1.66,;.09,3.2,;-1.24,2.43,;-1.24,.89,;-2.57,3.2,;-2.57,4.74,;-3.91,5.51,;-5.24,4.74,;-6.58,5.51,;-5.24,3.2,;-3.91,2.43,;10.76,3.2,;10.76,4.74,;12.1,2.43,;13.43,3.2,;13.43,4.74,;12.1,5.51,;12.1,7.05,;10.76,7.82,;10.76,9.36,;12.1,10.13,;9.43,10.13,;14.76,2.43,;14.76,.89,;16.1,3.2,;17.43,2.43,;17.43,.89,;18.76,.12,;18.15,-1.3,;19.3,-2.32,;20.63,-1.53,;22.1,-2,;23.23,-.96,;22.9,.55,;21.43,1.01,;20.3,-.03,;18.76,3.2,;20.1,2.43,;18.76,4.74,)| Show InChI InChI=1S/C42H52FN9O5/c1-26(2)22-29(12-8-20-47-40(56)36(23-27-10-4-3-5-11-27)52-38(54)28-16-18-31(43)19-17-28)39(55)50-34(15-9-21-48-42(45)46)41(57)51-35(37(44)53)24-30-25-49-33-14-7-6-13-32(30)33/h3-8,10-14,16-19,25-26,29,34-36,49H,9,15,20-24H2,1-2H3,(H2,44,53)(H,47,56)(H,50,55)(H,51,57)(H,52,54)(H4,45,46,48)/b12-8+/t29-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Kyoto University
| Assay Description
Membranes were incubated with [125I] kisspeptin-15 and increasing concentrations of test compound in binding buffer. The reaction mixtures were dilut... |
J Med Chem 51: 7645-9 (2008)
Article DOI: 10.1021/jm800930w
BindingDB Entry DOI: 10.7270/Q2ZS2TTP |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM107307
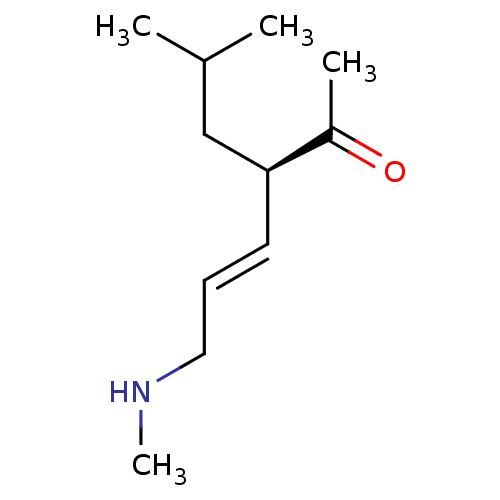
(US8592379, 19)Show InChI InChI=1S/C11H21NO/c1-9(2)8-11(10(3)13)6-5-7-12-4/h5-6,9,11-12H,7-8H2,1-4H3/b6-5+/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University; Takeda Pharmaceutical Company Limited
US Patent
| Assay Description
Binding inhibition assay using human GPR54. |
US Patent US8592379 (2013)
BindingDB Entry DOI: 10.7270/Q2N0156N |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26349

((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-amino-3-(4-hydroxyp...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:83.86,72.75,12.20,29.34,37.50,51.58,59.62,wD:4.4,23.26,(23.93,-21.81,;22.46,-22.29,;22.15,-23.79,;21.33,-21.26,;21.65,-19.76,;20.51,-18.74,;19.04,-19.2,;18.73,-20.72,;17.9,-18.17,;16.44,-18.65,;15.29,-17.62,;15.61,-16.12,;13.83,-18.1,;12.69,-17.06,;13.01,-15.57,;11.86,-14.54,;12.18,-13.03,;13.65,-12.55,;14.79,-13.59,;14.47,-15.08,;13.51,-19.6,;12.05,-20.08,;10.9,-19.05,;11.73,-21.57,;12.87,-22.61,;14.34,-22.12,;10.27,-22.05,;9.94,-23.55,;11.09,-24.58,;8.49,-24.03,;7.34,-23,;7.67,-21.49,;6.52,-20.46,;9.12,-21.02,;8.16,-25.53,;6.7,-26.01,;5.56,-24.98,;6.38,-27.51,;4.92,-27.99,;4.6,-29.49,;5.63,-30.63,;4.86,-31.97,;3.35,-31.65,;2.11,-32.56,;.71,-31.92,;.55,-30.4,;1.79,-29.49,;3.2,-30.12,;7.53,-28.54,;8.98,-28.07,;9.31,-26.56,;10.13,-29.1,;9.81,-30.6,;8.35,-31.08,;8.02,-32.58,;7.2,-30.05,;11.59,-28.62,;12.74,-29.65,;12.42,-31.16,;14.21,-29.18,;14.52,-27.67,;15.34,-30.2,;16.8,-29.73,;17.12,-28.22,;18.58,-27.75,;19.72,-28.77,;21.19,-28.3,;19.41,-30.28,;17.94,-30.75,;23.11,-19.28,;23.42,-17.77,;24.24,-20.31,;25.71,-19.83,;26.03,-18.32,;27.49,-17.85,;27.81,-16.34,;29.28,-15.87,;29.59,-14.36,;28.45,-13.34,;31.06,-13.9,;26.85,-20.86,;26.53,-22.37,;28.32,-20.39,;29.46,-21.42,;30.93,-20.94,;32.07,-21.97,;33.53,-21.49,;34.68,-22.52,;34.35,-24.03,;32.89,-24.5,;31.75,-23.47,;29.14,-22.92,;27.68,-23.4,;30.28,-23.95,)| Show InChI InChI=1S/C63H83N17O14/c1-34(2)24-45(58(90)74-43(18-11-23-70-63(68)69)57(89)75-44(54(67)86)26-35-12-5-3-6-13-35)73-53(85)32-72-56(88)46(27-36-14-7-4-8-15-36)77-62(94)50(33-81)80-61(93)49(30-52(66)84)79-59(91)47(28-38-31-71-42-17-10-9-16-40(38)42)78-60(92)48(29-51(65)83)76-55(87)41(64)25-37-19-21-39(82)22-20-37/h3-10,12-17,19-22,31,34,41,43-50,71,81-82H,11,18,23-30,32-33,64H2,1-2H3,(H2,65,83)(H2,66,84)(H2,67,86)(H,72,88)(H,73,85)(H,74,90)(H,75,89)(H,76,87)(H,77,94)(H,78,92)(H,79,91)(H,80,93)(H4,68,69,70)/t41-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Kyoto University
| Assay Description
Membranes were incubated with [125I] kisspeptin-15 and increasing concentrations of test compound in binding buffer. The reaction mixtures were dilut... |
J Med Chem 51: 7645-9 (2008)
Article DOI: 10.1021/jm800930w
BindingDB Entry DOI: 10.7270/Q2ZS2TTP |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26339

((2R,3E)-N-[(1S)-4-carbamimidamido-1-{[(1S)-1-carba...)Show SMILES CC(C)C[C@H](\C=C\CNC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(F)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,wU:32.33,wD:11.19,4.4,43.44,(10.76,-1.42,;10.76,.12,;12.1,.89,;9.43,.89,;9.43,2.43,;8.09,3.2,;6.76,2.43,;5.43,3.2,;4.09,2.43,;2.76,3.2,;2.76,4.74,;1.43,2.43,;1.43,.89,;.66,-.45,;-.87,-.66,;-1.45,-2.09,;-.5,-3.3,;1.02,-3.09,;1.6,-1.66,;.09,3.2,;-1.24,2.43,;-1.24,.89,;-2.57,3.2,;-2.57,4.74,;-3.91,5.51,;-5.24,4.74,;-6.58,5.51,;-5.24,3.2,;-3.91,2.43,;10.76,3.2,;10.76,4.74,;12.1,2.43,;13.43,3.2,;13.43,4.74,;12.1,5.51,;12.1,7.05,;10.76,7.82,;10.76,9.36,;12.1,10.13,;9.43,10.13,;14.76,2.43,;14.76,.89,;16.1,3.2,;17.43,2.43,;17.43,.89,;18.76,.12,;18.15,-1.3,;19.3,-2.32,;20.63,-1.53,;22.1,-2,;23.23,-.96,;22.9,.55,;21.43,1.01,;20.3,-.03,;18.76,3.2,;20.1,2.43,;18.76,4.74,)| Show InChI InChI=1S/C42H52FN9O5/c1-26(2)22-29(12-8-20-47-40(56)36(23-27-10-4-3-5-11-27)52-38(54)28-16-18-31(43)19-17-28)39(55)50-34(15-9-21-48-42(45)46)41(57)51-35(37(44)53)24-30-25-49-33-14-7-6-13-32(30)33/h3-8,10-14,16-19,25-26,29,34-36,49H,9,15,20-24H2,1-2H3,(H2,44,53)(H,47,56)(H,50,55)(H,51,57)(H,52,54)(H4,45,46,48)/b12-8+/t29-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]kisspeptin-15 from GPR54 |
ACS Med Chem Lett 2: 53-57 (2011)
Article DOI: 10.1021/ml1002053
BindingDB Entry DOI: 10.7270/Q2K07586 |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM107314
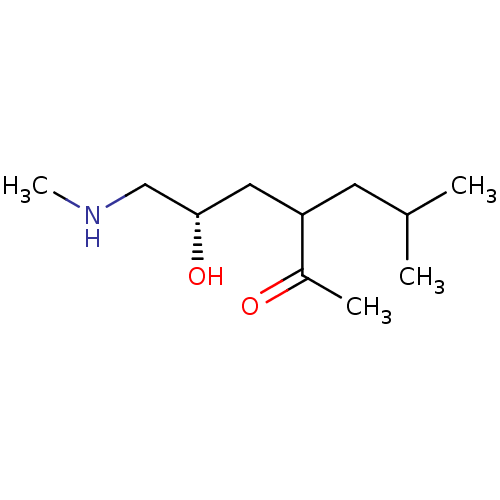
(US8592379, 36 | US8592379, 37)Show InChI InChI=1S/C11H23NO2/c1-8(2)5-10(9(3)13)6-11(14)7-12-4/h8,10-12,14H,5-7H2,1-4H3/t10?,11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University; Takeda Pharmaceutical Company Limited
US Patent
| Assay Description
Binding inhibition assay using human GPR54. |
US Patent US8592379 (2013)
BindingDB Entry DOI: 10.7270/Q2N0156N |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26347
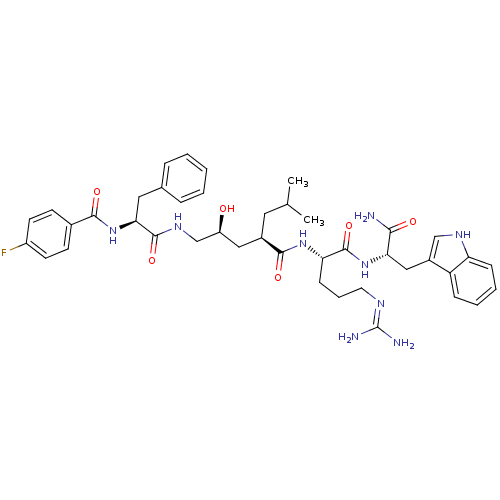
((2R,4S)-N-[(1S)-4-carbamimidamido-1-{[(1S)-1-carba...)Show SMILES CC(C)C[C@H](C[C@H](O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(F)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,wU:33.34,wD:12.20,4.31,44.45,6.6,(10.76,-1.42,;10.76,.12,;12.1,.89,;9.43,.89,;9.43,2.43,;8.09,3.2,;6.76,2.43,;6.76,.89,;5.43,3.2,;4.09,2.43,;2.76,3.2,;2.76,4.74,;1.43,2.43,;1.43,.89,;.66,-.45,;-.87,-.66,;-1.45,-2.09,;-.5,-3.3,;1.02,-3.09,;1.6,-1.66,;.09,3.2,;-1.24,2.43,;-1.24,.89,;-2.57,3.2,;-2.57,4.74,;-3.91,5.51,;-5.24,4.74,;-6.58,5.51,;-5.24,3.2,;-3.91,2.43,;10.76,3.2,;10.76,4.74,;12.1,2.43,;13.43,3.2,;13.43,4.74,;12.1,5.51,;12.1,7.05,;10.76,7.82,;10.76,9.36,;12.1,10.13,;9.43,10.13,;14.76,2.43,;14.76,.89,;16.1,3.2,;17.43,2.43,;17.43,.89,;18.76,.12,;18.15,-1.3,;19.3,-2.32,;20.63,-1.53,;22.1,-2,;23.23,-.96,;22.9,.55,;21.43,1.01,;20.3,-.03,;18.76,3.2,;20.1,2.43,;18.76,4.74,)| Show InChI InChI=1S/C42H54FN9O6/c1-25(2)19-28(21-31(53)24-49-40(57)36(20-26-9-4-3-5-10-26)52-38(55)27-14-16-30(43)17-15-27)39(56)50-34(13-8-18-47-42(45)46)41(58)51-35(37(44)54)22-29-23-48-33-12-7-6-11-32(29)33/h3-7,9-12,14-17,23,25,28,31,34-36,48,53H,8,13,18-22,24H2,1-2H3,(H2,44,54)(H,49,57)(H,50,56)(H,51,58)(H,52,55)(H4,45,46,47)/t28-,31+,34+,35+,36+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Kyoto University
| Assay Description
Membranes were incubated with [125I] kisspeptin-15 and increasing concentrations of test compound in binding buffer. The reaction mixtures were dilut... |
J Med Chem 51: 7645-9 (2008)
Article DOI: 10.1021/jm800930w
BindingDB Entry DOI: 10.7270/Q2ZS2TTP |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26338
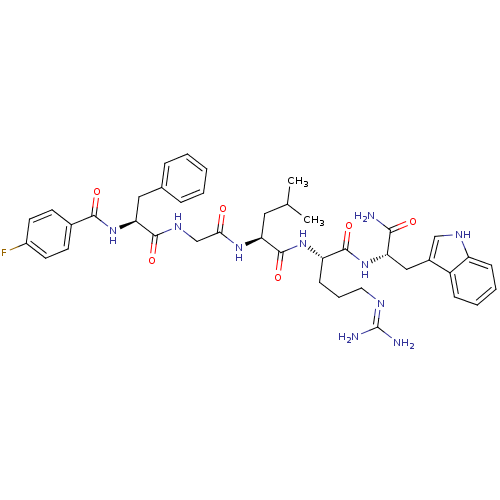
((2S)-N-[(1S)-4-carbamimidamido-1-{[(1S)-1-carbamoy...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(F)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,wU:33.34,wD:12.20,4.4,44.45,(10.76,-1.42,;10.76,.12,;12.1,.89,;9.43,.89,;9.43,2.43,;8.09,3.2,;6.76,2.43,;6.76,.89,;5.43,3.2,;4.09,2.43,;2.76,3.2,;2.76,4.74,;1.43,2.43,;1.43,.89,;.66,-.45,;-.87,-.66,;-1.45,-2.09,;-.5,-3.3,;1.02,-3.09,;1.6,-1.66,;.09,3.2,;-1.24,2.43,;-1.24,.89,;-2.57,3.2,;-2.57,4.74,;-3.91,5.51,;-5.24,4.74,;-6.58,5.51,;-5.24,3.2,;-3.91,2.43,;10.76,3.2,;10.76,4.74,;12.1,2.43,;13.43,3.2,;13.43,4.74,;12.1,5.51,;12.1,7.05,;10.76,7.82,;10.76,9.36,;12.1,10.13,;9.43,10.13,;14.76,2.43,;14.76,.89,;16.1,3.2,;17.43,2.43,;17.43,.89,;18.76,.12,;18.15,-1.3,;19.3,-2.32,;20.63,-1.53,;22.1,-2,;23.23,-.96,;22.9,.55,;21.43,1.01,;20.3,-.03,;18.76,3.2,;20.1,2.43,;18.76,4.74,)| Show InChI InChI=1S/C41H51FN10O6/c1-24(2)19-33(49-35(53)23-48-38(56)34(20-25-9-4-3-5-10-25)52-37(55)26-14-16-28(42)17-15-26)40(58)50-31(13-8-18-46-41(44)45)39(57)51-32(36(43)54)21-27-22-47-30-12-7-6-11-29(27)30/h3-7,9-12,14-17,22,24,31-34,47H,8,13,18-21,23H2,1-2H3,(H2,43,54)(H,48,56)(H,49,53)(H,50,58)(H,51,57)(H,52,55)(H4,44,45,46)/t31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Kyoto University
| Assay Description
Membranes were incubated with [125I] kisspeptin-15 and increasing concentrations of test compound in binding buffer. The reaction mixtures were dilut... |
J Med Chem 51: 7645-9 (2008)
Article DOI: 10.1021/jm800930w
BindingDB Entry DOI: 10.7270/Q2ZS2TTP |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM107306
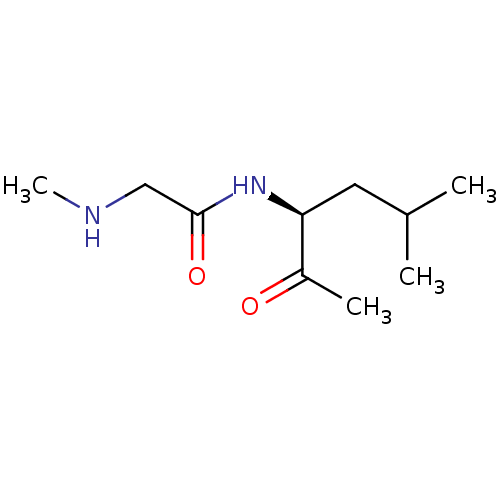
(US8592379, 1)Show InChI InChI=1S/C10H20N2O2/c1-7(2)5-9(8(3)13)12-10(14)6-11-4/h7,9,11H,5-6H2,1-4H3,(H,12,14)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University; Takeda Pharmaceutical Company Limited
US Patent
| Assay Description
Binding inhibition assay using human GPR54. |
US Patent US8592379 (2013)
BindingDB Entry DOI: 10.7270/Q2N0156N |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26338

((2S)-N-[(1S)-4-carbamimidamido-1-{[(1S)-1-carbamoy...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(F)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,wU:33.34,wD:12.20,4.4,44.45,(10.76,-1.42,;10.76,.12,;12.1,.89,;9.43,.89,;9.43,2.43,;8.09,3.2,;6.76,2.43,;6.76,.89,;5.43,3.2,;4.09,2.43,;2.76,3.2,;2.76,4.74,;1.43,2.43,;1.43,.89,;.66,-.45,;-.87,-.66,;-1.45,-2.09,;-.5,-3.3,;1.02,-3.09,;1.6,-1.66,;.09,3.2,;-1.24,2.43,;-1.24,.89,;-2.57,3.2,;-2.57,4.74,;-3.91,5.51,;-5.24,4.74,;-6.58,5.51,;-5.24,3.2,;-3.91,2.43,;10.76,3.2,;10.76,4.74,;12.1,2.43,;13.43,3.2,;13.43,4.74,;12.1,5.51,;12.1,7.05,;10.76,7.82,;10.76,9.36,;12.1,10.13,;9.43,10.13,;14.76,2.43,;14.76,.89,;16.1,3.2,;17.43,2.43,;17.43,.89,;18.76,.12,;18.15,-1.3,;19.3,-2.32,;20.63,-1.53,;22.1,-2,;23.23,-.96,;22.9,.55,;21.43,1.01,;20.3,-.03,;18.76,3.2,;20.1,2.43,;18.76,4.74,)| Show InChI InChI=1S/C41H51FN10O6/c1-24(2)19-33(49-35(53)23-48-38(56)34(20-25-9-4-3-5-10-25)52-37(55)26-14-16-28(42)17-15-26)40(58)50-31(13-8-18-46-41(44)45)39(57)51-32(36(43)54)21-27-22-47-30-12-7-6-11-29(27)30/h3-7,9-12,14-17,22,24,31-34,47H,8,13,18-21,23H2,1-2H3,(H2,43,54)(H,48,56)(H,49,53)(H,50,58)(H,51,57)(H,52,55)(H4,44,45,46)/t31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]kisspeptin-15 from GPR54 |
ACS Med Chem Lett 2: 53-57 (2011)
Article DOI: 10.1021/ml1002053
BindingDB Entry DOI: 10.7270/Q2K07586 |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 2
(Homo sapiens (Human)) | BDBM50336911
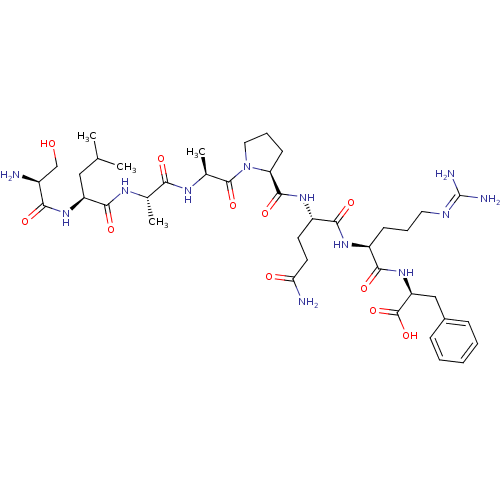
((S)-2-((S)-2-((S)-5-amino-2-((S)-1-((S)-2-((S)-2-(...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C40H64N12O11/c1-21(2)18-28(50-33(56)25(41)20-53)36(59)46-22(3)32(55)47-23(4)38(61)52-17-9-13-30(52)37(60)49-27(14-15-31(42)54)35(58)48-26(12-8-16-45-40(43)44)34(57)51-29(39(62)63)19-24-10-6-5-7-11-24/h5-7,10-11,21-23,25-30,53H,8-9,12-20,41H2,1-4H3,(H2,42,54)(H,46,59)(H,47,55)(H,48,58)(H,49,60)(H,50,56)(H,51,57)(H,62,63)(H4,43,44,45)/t22-,23-,25-,26-,27-,28-,29-,30-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of (D-[125I]Tyr1, MePhe3)-NPFF from NPFFR2 after 2 hrs |
ACS Med Chem Lett 2: 53-57 (2011)
Article DOI: 10.1021/ml1002053
BindingDB Entry DOI: 10.7270/Q2K07586 |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM107308
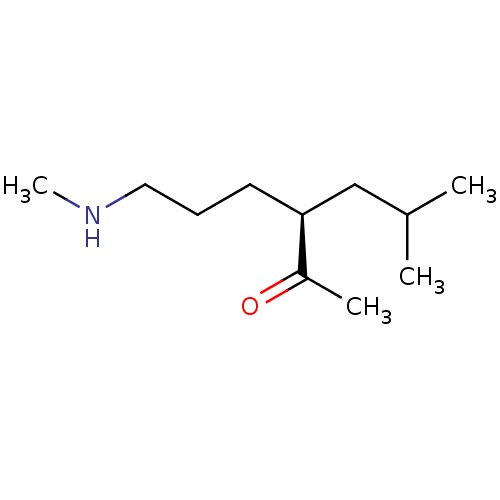
(US8592379, 20)Show InChI InChI=1S/C11H23NO/c1-9(2)8-11(10(3)13)6-5-7-12-4/h9,11-12H,5-8H2,1-4H3/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University; Takeda Pharmaceutical Company Limited
US Patent
| Assay Description
Binding inhibition assay using human GPR54. |
US Patent US8592379 (2013)
BindingDB Entry DOI: 10.7270/Q2N0156N |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26340
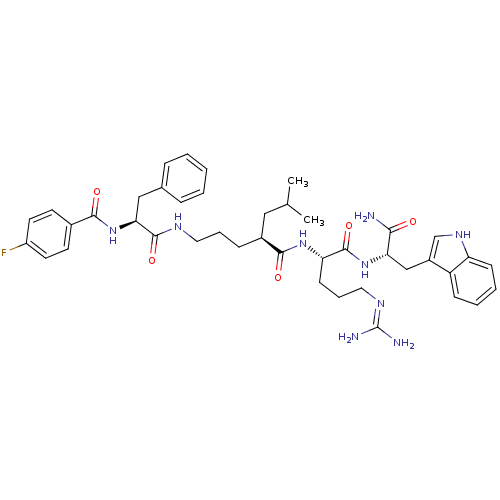
((2S)-N-[(1S)-4-carbamimidamido-1-{[(1S)-1-carbamoy...)Show SMILES CC(C)C[C@H](CCCNC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(F)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,wU:32.33,wD:11.19,4.4,43.44,(10.76,-1.42,;10.76,.12,;12.1,.89,;9.43,.89,;9.43,2.43,;8.09,3.2,;6.76,2.43,;5.43,3.2,;4.09,2.43,;2.76,3.2,;2.76,4.74,;1.43,2.43,;1.43,.89,;.66,-.45,;-.87,-.66,;-1.45,-2.09,;-.5,-3.3,;1.02,-3.09,;1.6,-1.66,;.09,3.2,;-1.24,2.43,;-1.24,.89,;-2.57,3.2,;-2.57,4.74,;-3.91,5.51,;-5.24,4.74,;-6.58,5.51,;-5.24,3.2,;-3.91,2.43,;10.76,3.2,;10.76,4.74,;12.1,2.43,;13.43,3.2,;13.43,4.74,;12.1,5.51,;12.1,7.05,;10.76,7.82,;10.76,9.36,;12.1,10.13,;9.43,10.13,;14.76,2.43,;14.76,.89,;16.1,3.2,;17.43,2.43,;17.43,.89,;18.76,.12,;18.15,-1.3,;19.3,-2.32,;20.63,-1.53,;22.1,-2,;23.23,-.96,;22.9,.55,;21.43,1.01,;20.3,-.03,;18.76,3.2,;20.1,2.43,;18.76,4.74,)| Show InChI InChI=1S/C42H54FN9O5/c1-26(2)22-29(12-8-20-47-40(56)36(23-27-10-4-3-5-11-27)52-38(54)28-16-18-31(43)19-17-28)39(55)50-34(15-9-21-48-42(45)46)41(57)51-35(37(44)53)24-30-25-49-33-14-7-6-13-32(30)33/h3-7,10-11,13-14,16-19,25-26,29,34-36,49H,8-9,12,15,20-24H2,1-2H3,(H2,44,53)(H,47,56)(H,50,55)(H,51,57)(H,52,54)(H4,45,46,48)/t29-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Kyoto University
| Assay Description
Membranes were incubated with [125I] kisspeptin-15 and increasing concentrations of test compound in binding buffer. The reaction mixtures were dilut... |
J Med Chem 51: 7645-9 (2008)
Article DOI: 10.1021/jm800930w
BindingDB Entry DOI: 10.7270/Q2ZS2TTP |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50432242
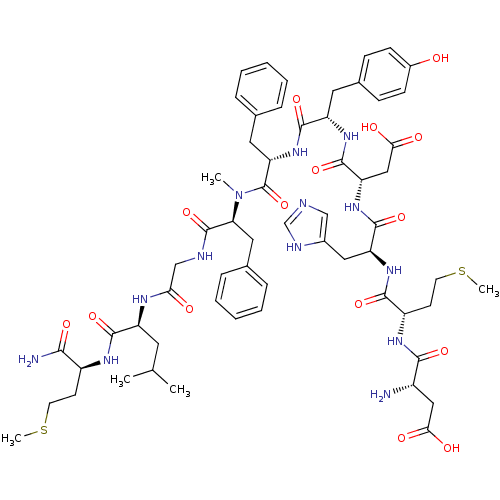
(CHEMBL2347361)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(N)=O |r| Show InChI InChI=1S/C60H81N13O15S2/c1-34(2)24-43(55(83)67-41(52(62)80)20-22-89-4)66-49(75)32-64-59(87)48(27-36-14-10-7-11-15-36)73(3)60(88)47(26-35-12-8-6-9-13-35)72-56(84)44(25-37-16-18-39(74)19-17-37)69-58(86)46(30-51(78)79)71-57(85)45(28-38-31-63-33-65-38)70-54(82)42(21-23-90-5)68-53(81)40(61)29-50(76)77/h6-19,31,33-34,40-48,74H,20-30,32,61H2,1-5H3,(H2,62,80)(H,63,65)(H,64,87)(H,66,75)(H,67,83)(H,68,81)(H,69,86)(H,70,82)(H,71,85)(H,72,84)(H,76,77)(H,78,79)/t40-,41-,42-,43-,44-,45-,46-,47-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of ([125I]-His3, MePhe7)-NKB from NK3R (unknown origin) transfected in CHO cells by gamma counting analysis |
Bioorg Med Chem 21: 2413-7 (2013)
Article DOI: 10.1016/j.bmc.2013.01.036
BindingDB Entry DOI: 10.7270/Q20K29X2 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50432275
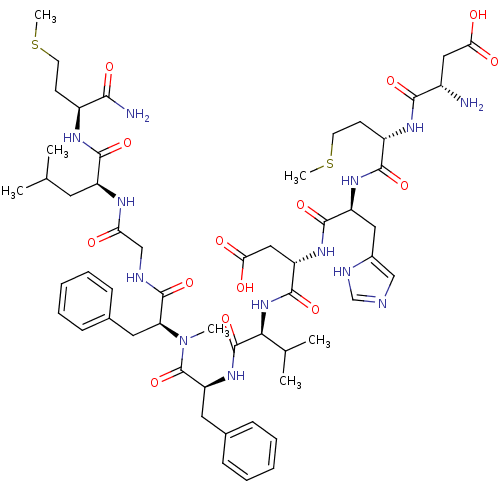
(CHEMBL2347488)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(N)=O |r| Show InChI InChI=1S/C56H81N13O14S2/c1-31(2)22-39(51(78)63-37(48(58)75)18-20-84-6)62-44(70)29-60-54(81)43(24-34-16-12-9-13-17-34)69(5)56(83)42(23-33-14-10-8-11-15-33)67-55(82)47(32(3)4)68-53(80)41(27-46(73)74)66-52(79)40(25-35-28-59-30-61-35)65-50(77)38(19-21-85-7)64-49(76)36(57)26-45(71)72/h8-17,28,30-32,36-43,47H,18-27,29,57H2,1-7H3,(H2,58,75)(H,59,61)(H,60,81)(H,62,70)(H,63,78)(H,64,76)(H,65,77)(H,66,79)(H,67,82)(H,68,80)(H,71,72)(H,73,74)/t36-,37-,38-,39-,40-,41-,42-,43-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of ([125I]-His3, MePhe7)-NKB from NK3R (unknown origin) transfected in CHO cells by gamma counting analysis |
Bioorg Med Chem 21: 2413-7 (2013)
Article DOI: 10.1016/j.bmc.2013.01.036
BindingDB Entry DOI: 10.7270/Q20K29X2 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50180193

(AZD-2624 | AZD2624)Show SMILES CC[C@H](NC(=O)c1c(NS(C)(=O)=O)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H25N3O3S/c1-3-21(18-12-6-4-7-13-18)28-26(30)23-20-16-10-11-17-22(20)27-24(19-14-8-5-9-15-19)25(23)29-33(2,31)32/h4-17,21,29H,3H2,1-2H3,(H,28,30)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [125I]His3-MePhe7)-NKB from human NK3R expressed in CHO cell membranes by topcounting method |
Bioorg Med Chem 24: 3494-500 (2016)
Article DOI: 10.1016/j.bmc.2016.05.054
BindingDB Entry DOI: 10.7270/Q2RR216W |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50432267
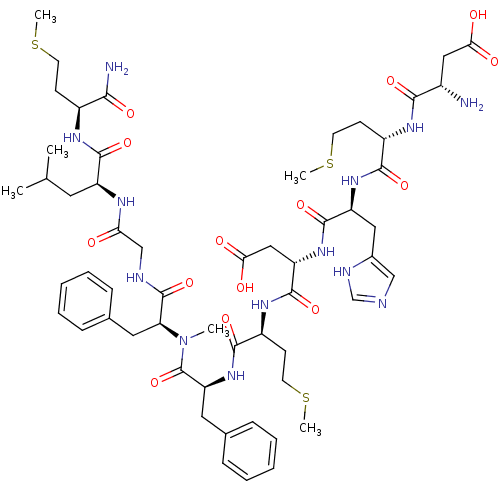
(CHEMBL2347496)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(N)=O |r| Show InChI InChI=1S/C56H81N13O14S3/c1-32(2)23-40(52(79)63-37(48(58)75)17-20-84-4)62-45(70)30-60-55(82)44(25-34-15-11-8-12-16-34)69(3)56(83)43(24-33-13-9-7-10-14-33)68-51(78)39(19-22-86-6)65-54(81)42(28-47(73)74)67-53(80)41(26-35-29-59-31-61-35)66-50(77)38(18-21-85-5)64-49(76)36(57)27-46(71)72/h7-16,29,31-32,36-44H,17-28,30,57H2,1-6H3,(H2,58,75)(H,59,61)(H,60,82)(H,62,70)(H,63,79)(H,64,76)(H,65,81)(H,66,77)(H,67,80)(H,68,78)(H,71,72)(H,73,74)/t36-,37-,38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of ([125I]-His3, MePhe7)-NKB from NK3R (unknown origin) transfected in CHO cells by gamma counting analysis |
Bioorg Med Chem 21: 2413-7 (2013)
Article DOI: 10.1016/j.bmc.2013.01.036
BindingDB Entry DOI: 10.7270/Q20K29X2 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50432241
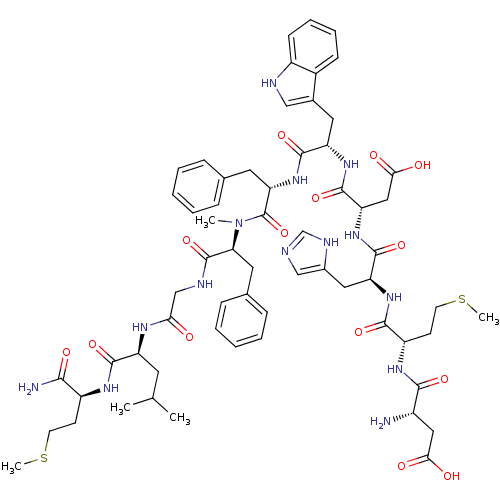
(CHEMBL2347362)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(N)=O |r| Show InChI InChI=1S/C62H82N14O14S2/c1-35(2)24-45(57(85)70-43(54(64)82)20-22-91-4)69-51(77)33-67-61(89)50(26-37-16-10-7-11-17-37)76(3)62(90)49(25-36-14-8-6-9-15-36)75-58(86)46(27-38-31-66-42-19-13-12-18-40(38)42)72-60(88)48(30-53(80)81)74-59(87)47(28-39-32-65-34-68-39)73-56(84)44(21-23-92-5)71-55(83)41(63)29-52(78)79/h6-19,31-32,34-35,41,43-50,66H,20-30,33,63H2,1-5H3,(H2,64,82)(H,65,68)(H,67,89)(H,69,77)(H,70,85)(H,71,83)(H,72,88)(H,73,84)(H,74,87)(H,75,86)(H,78,79)(H,80,81)/t41-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of ([125I]-His3, MePhe7)-NKB from NK3R (unknown origin) transfected in CHO cells by gamma counting analysis |
Bioorg Med Chem 21: 2413-7 (2013)
Article DOI: 10.1016/j.bmc.2013.01.036
BindingDB Entry DOI: 10.7270/Q20K29X2 |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 1
(Homo sapiens (Human)) | BDBM50336911

((S)-2-((S)-2-((S)-5-amino-2-((S)-1-((S)-2-((S)-2-(...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C40H64N12O11/c1-21(2)18-28(50-33(56)25(41)20-53)36(59)46-22(3)32(55)47-23(4)38(61)52-17-9-13-30(52)37(60)49-27(14-15-31(42)54)35(58)48-26(12-8-16-45-40(43)44)34(57)51-29(39(62)63)19-24-10-6-5-7-11-24/h5-7,10-11,21-23,25-30,53H,8-9,12-20,41H2,1-4H3,(H2,42,54)(H,46,59)(H,47,55)(H,48,58)(H,49,60)(H,50,56)(H,51,57)(H,62,63)(H4,43,44,45)/t22-,23-,25-,26-,27-,28-,29-,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of (D-[125I]Tyr1, MePhe3)-NPFF from NPFFR1 after 2 hrs |
ACS Med Chem Lett 2: 53-57 (2011)
Article DOI: 10.1021/ml1002053
BindingDB Entry DOI: 10.7270/Q2K07586 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50432245
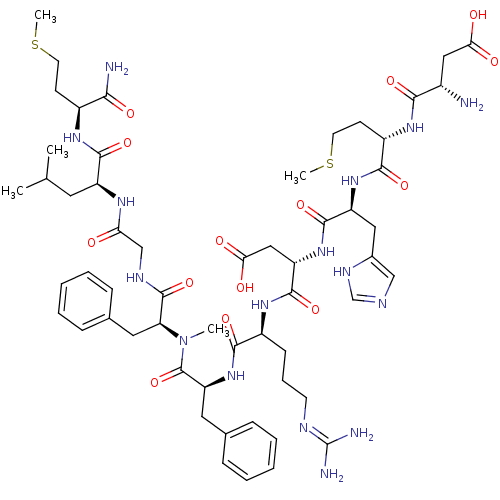
(CHEMBL2347511)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(N)=O |r,wU:80.83,62.71,43.52,20.28,8.12,wD:72.79,54.60,32.41,4.4,(45.8,-105.89,;44.44,-105.1,;44.44,-103.58,;43.09,-102.79,;43.09,-101.27,;41.77,-100.51,;40.41,-101.29,;40.41,-102.81,;39.1,-100.53,;39.1,-98.97,;40.41,-98.21,;40.41,-96.64,;41.73,-98.97,;37.75,-101.31,;36.42,-100.55,;36.42,-98.99,;35.07,-101.33,;33.75,-100.56,;32.4,-101.35,;32.4,-102.87,;31.07,-100.58,;31.07,-99.02,;32.4,-98.26,;33.72,-99,;35.06,-98.23,;35.05,-96.68,;33.71,-95.92,;32.37,-96.7,;29.72,-101.37,;29.72,-102.91,;28.4,-100.61,;28.4,-99.05,;27.05,-101.39,;27.04,-102.91,;26.24,-104.25,;27.02,-105.61,;26.23,-106.93,;24.7,-106.92,;23.93,-105.57,;24.72,-104.24,;25.73,-100.63,;24.37,-101.4,;24.37,-102.93,;23.06,-100.64,;23.06,-99.08,;24.39,-98.31,;24.39,-96.77,;25.72,-96,;25.72,-94.47,;27.05,-93.7,;24.39,-93.7,;21.7,-101.42,;20.38,-100.66,;20.38,-99.1,;19.03,-101.45,;19.03,-102.97,;20.38,-103.75,;21.73,-102.97,;20.38,-105.27,;17.71,-100.68,;16.35,-101.47,;16.35,-102.99,;15.03,-100.7,;15.03,-99.14,;16.36,-98.37,;17.76,-98.98,;18.78,-97.82,;18,-96.49,;16.5,-96.82,;13.68,-101.48,;12.36,-100.72,;12.36,-99.16,;11.01,-101.5,;11.01,-103.03,;12.36,-103.81,;12.36,-105.34,;13.71,-106.11,;9.68,-100.73,;8.34,-101.5,;8.34,-103.05,;7.01,-100.73,;5.67,-101.51,;7.01,-99.19,;8.33,-98.44,;9.66,-99.2,;8.33,-96.87,;44.44,-100.49,;45.76,-101.25,;44.44,-98.92,)| Show InChI InChI=1S/C57H84N16O14S2/c1-32(2)23-40(52(83)67-37(48(59)79)18-21-88-4)66-45(74)30-64-55(86)44(25-34-15-10-7-11-16-34)73(3)56(87)43(24-33-13-8-6-9-14-33)72-50(81)38(17-12-20-63-57(60)61)69-54(85)42(28-47(77)78)71-53(84)41(26-35-29-62-31-65-35)70-51(82)39(19-22-89-5)68-49(80)36(58)27-46(75)76/h6-11,13-16,29,31-32,36-44H,12,17-28,30,58H2,1-5H3,(H2,59,79)(H,62,65)(H,64,86)(H,66,74)(H,67,83)(H,68,80)(H,69,85)(H,70,82)(H,71,84)(H,72,81)(H,75,76)(H,77,78)(H4,60,61,63)/t36-,37-,38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of ([125I]-His3, MePhe7)-NKB from NK3R (unknown origin) transfected in CHO cells by gamma counting analysis |
Bioorg Med Chem 21: 2413-7 (2013)
Article DOI: 10.1016/j.bmc.2013.01.036
BindingDB Entry DOI: 10.7270/Q20K29X2 |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50041978

(CHEMBL3134157)Show SMILES Cc1cc(CS(=O)(=O)c2ccccc2)cc(OCc2ccc(CN3CCC[C@@H]3CO)cc2)c1 |r| Show InChI InChI=1S/C27H31NO4S/c1-21-14-24(20-33(30,31)27-7-3-2-4-8-27)16-26(15-21)32-19-23-11-9-22(10-12-23)17-28-13-5-6-25(28)18-29/h2-4,7-12,14-16,25,29H,5-6,13,17-20H2,1H3/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6-tagged human SphK1 expressed in baculovirus infected sf21 cells using FITC-sphingosine as substrate after 1... |
Bioorg Med Chem 25: 3046-3052 (2017)
Article DOI: 10.1016/j.bmc.2017.03.059
BindingDB Entry DOI: 10.7270/Q2XD144Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50202356

(CHEMBL218806 | cyclo(-D-Tyr-D-MeArg-L-Arg-L-Nal-Gl...)Show SMILES [#6]-[#7]-1-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc3ccccc3c2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]-1=O |r| Show InChI InChI=1S/C37H49N11O6/c1-48-30(9-5-17-43-37(40)41)34(53)47-28(19-22-11-14-26(49)15-12-22)32(51)44-21-31(50)45-29(20-23-10-13-24-6-2-3-7-25(24)18-23)33(52)46-27(35(48)54)8-4-16-42-36(38)39/h2-3,6-7,10-15,18,27-30,49H,4-5,8-9,16-17,19-21H2,1H3,(H,44,51)(H,45,50)(H,46,52)(H,47,53)(H4,38,39,42)(H4,40,41,43)/t27-,28+,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells |
J Med Chem 50: 192-8 (2007)
Article DOI: 10.1021/jm0607350
BindingDB Entry DOI: 10.7270/Q25M65D3 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50299467
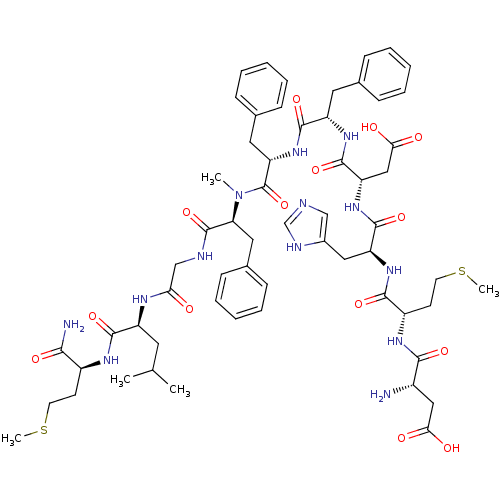
((5S,8S,14S,17S,20S,23S,26S,29S,32S)-26-((1H-imidaz...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(N)=O |r| Show InChI InChI=1S/C60H81N13O14S2/c1-35(2)25-43(55(82)67-41(52(62)79)21-23-88-4)66-49(74)33-64-59(86)48(28-38-19-13-8-14-20-38)73(3)60(87)47(27-37-17-11-7-12-18-37)72-56(83)44(26-36-15-9-6-10-16-36)69-58(85)46(31-51(77)78)71-57(84)45(29-39-32-63-34-65-39)70-54(81)42(22-24-89-5)68-53(80)40(61)30-50(75)76/h6-20,32,34-35,40-48H,21-31,33,61H2,1-5H3,(H2,62,79)(H,63,65)(H,64,86)(H,66,74)(H,67,82)(H,68,80)(H,69,85)(H,70,81)(H,71,84)(H,72,83)(H,75,76)(H,77,78)/t40-,41-,42-,43-,44-,45-,46-,47-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of ([125I]-His3, MePhe7)-NKB from NK3R (unknown origin) transfected in CHO cells by gamma counting analysis |
Bioorg Med Chem 21: 2413-7 (2013)
Article DOI: 10.1016/j.bmc.2013.01.036
BindingDB Entry DOI: 10.7270/Q20K29X2 |
More data for this
Ligand-Target Pair | |
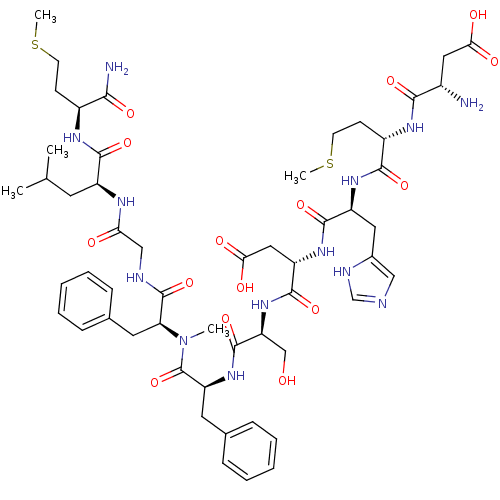
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50432269
(CHEMBL2347494)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(N)=O |r| Show InChI InChI=1S/C54H77N13O15S2/c1-30(2)20-37(49(77)61-35(46(56)74)16-18-83-4)60-43(69)27-58-53(81)42(22-32-14-10-7-11-15-32)67(3)54(82)40(21-31-12-8-6-9-13-31)65-52(80)41(28-68)66-51(79)39(25-45(72)73)64-50(78)38(23-33-26-57-29-59-33)63-48(76)36(17-19-84-5)62-47(75)34(55)24-44(70)71/h6-15,26,29-30,34-42,68H,16-25,27-28,55H2,1-5H3,(H2,56,74)(H,57,59)(H,58,81)(H,60,69)(H,61,77)(H,62,75)(H,63,76)(H,64,78)(H,65,80)(H,66,79)(H,70,71)(H,72,73)/t34-,35-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of ([125I]-His3, MePhe7)-NKB from NK3R (unknown origin) transfected in CHO cells by gamma counting analysis |
Bioorg Med Chem 21: 2413-7 (2013)
Article DOI: 10.1016/j.bmc.2013.01.036
BindingDB Entry DOI: 10.7270/Q20K29X2 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
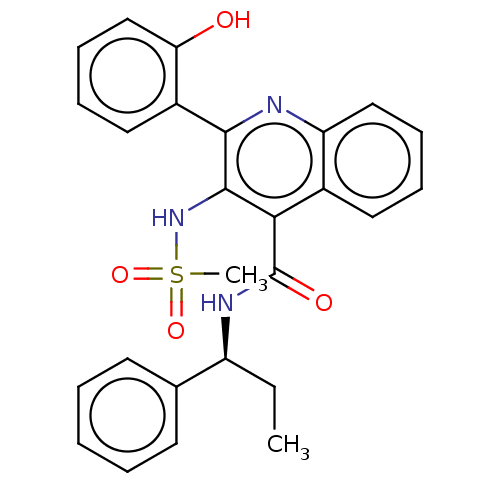
(Homo sapiens (Human)) | BDBM50180171
(CHEMBL3813763)Show SMILES CC[C@H](NC(=O)c1c(NS(C)(=O)=O)c(nc2ccccc12)-c1ccccc1O)c1ccccc1 |r| Show InChI InChI=1S/C26H25N3O4S/c1-3-20(17-11-5-4-6-12-17)28-26(31)23-18-13-7-9-15-21(18)27-24(25(23)29-34(2,32)33)19-14-8-10-16-22(19)30/h4-16,20,29-30H,3H2,1-2H3,(H,28,31)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [125I]His3-MePhe7)-NKB from human NK3R expressed in CHO cell membranes by topcounting method |
Bioorg Med Chem 24: 3494-500 (2016)
Article DOI: 10.1016/j.bmc.2016.05.054
BindingDB Entry DOI: 10.7270/Q2RR216W |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
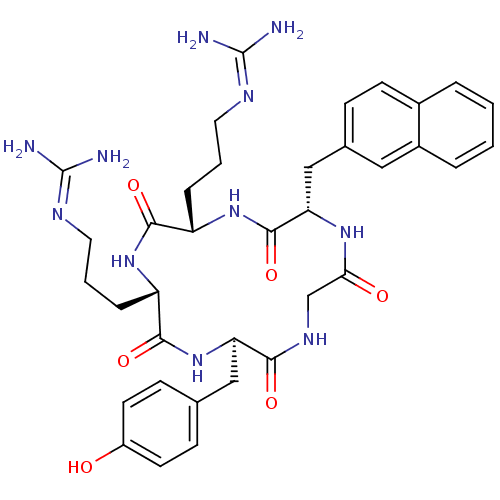
(Homo sapiens (Human)) | BDBM50202350
(CHEMBL219474 | cyclo(-D-Tyr-L-Arg-L-Arg-L-Nal-Gly-...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc3ccccc3c2)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C36H47N11O6/c37-35(38)41-15-3-7-26-32(51)45-27(8-4-16-42-36(39)40)33(52)47-28(18-21-10-13-25(48)14-11-21)31(50)43-20-30(49)44-29(34(53)46-26)19-22-9-12-23-5-1-2-6-24(23)17-22/h1-2,5-6,9-14,17,26-29,48H,3-4,7-8,15-16,18-20H2,(H,43,50)(H,44,49)(H,45,51)(H,46,53)(H,47,52)(H4,37,38,41)(H4,39,40,42)/t26-,27-,28+,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells |
J Med Chem 50: 192-8 (2007)
Article DOI: 10.1021/jm0607350
BindingDB Entry DOI: 10.7270/Q25M65D3 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50432273
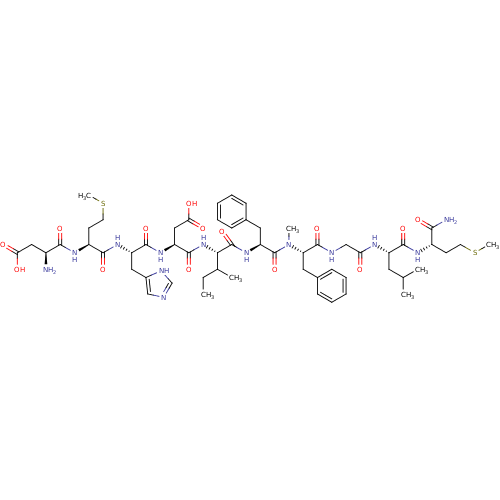
(CHEMBL2347490)Show SMILES CCC(C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(N)=O |r| Show InChI InChI=1S/C57H83N13O14S2/c1-8-33(4)48(69-54(81)42(28-47(74)75)67-53(80)41(26-36-29-60-31-62-36)66-51(78)39(20-22-86-7)65-50(77)37(58)27-46(72)73)56(83)68-43(24-34-15-11-9-12-16-34)57(84)70(5)44(25-35-17-13-10-14-18-35)55(82)61-30-45(71)63-40(23-32(2)3)52(79)64-38(49(59)76)19-21-85-6/h9-18,29,31-33,37-44,48H,8,19-28,30,58H2,1-7H3,(H2,59,76)(H,60,62)(H,61,82)(H,63,71)(H,64,79)(H,65,77)(H,66,78)(H,67,80)(H,68,83)(H,69,81)(H,72,73)(H,74,75)/t33?,37-,38-,39-,40-,41-,42-,43-,44-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of ([125I]-His3, MePhe7)-NKB from NK3R (unknown origin) transfected in CHO cells by gamma counting analysis |
Bioorg Med Chem 21: 2413-7 (2013)
Article DOI: 10.1016/j.bmc.2013.01.036
BindingDB Entry DOI: 10.7270/Q20K29X2 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50385614

(CHEMBL2042118)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#7]=[#6](-[#7])-[#6@H](-[#6]-c2ccc3ccccc3c2)-[#7]-[#6]-1=O |r,w:35.35| Show InChI InChI=1S/C36H48N12O5/c37-31-28(19-22-9-12-23-5-1-2-6-24(23)17-22)48-33(52)27(8-4-16-43-36(40)41)46-32(51)26(7-3-15-42-35(38)39)47-34(53)29(45-30(50)20-44-31)18-21-10-13-25(49)14-11-21/h1-2,5-6,9-14,17,26-29,49H,3-4,7-8,15-16,18-20H2,(H2,37,44)(H,45,50)(H,46,51)(H,47,53)(H,48,52)(H4,38,39,42)(H4,40,41,43)/t26-,27-,28-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-SDF-1alpha from CXCR4 receptor expressed in HEK293 cells after 1 hr by scintillation counting |
ACS Med Chem Lett 2: 477-480 (2011)
Article DOI: 10.1021/ml200047e
BindingDB Entry DOI: 10.7270/Q2D21ZNH |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50166087
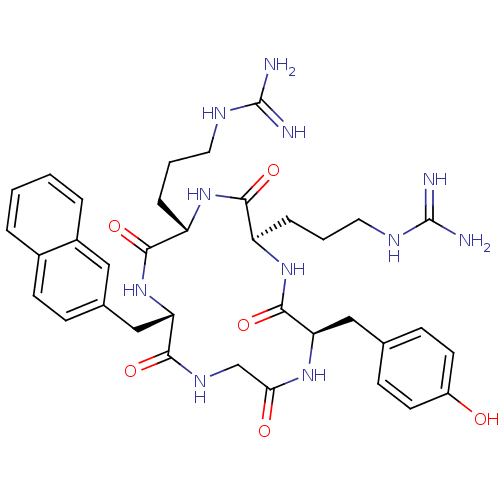
(CHEMBL436097 | N-{3-[(2R,5S,8S,14R)-5-(3-Guanidino...)Show SMILES NC(=N)NCCC[C@@H]1NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)CNC(=O)[C@H](Cc2ccc3ccccc3c2)NC1=O Show InChI InChI=1S/C36H47N11O6/c37-35(38)41-15-3-7-26-32(51)45-27(8-4-16-42-36(39)40)33(52)47-28(19-22-9-12-23-5-1-2-6-24(23)17-22)31(50)43-20-30(49)44-29(34(53)46-26)18-21-10-13-25(48)14-11-21/h1-2,5-6,9-14,17,26-29,48H,3-4,7-8,15-16,18-20H2,(H,43,50)(H,44,49)(H,45,51)(H,46,53)(H,47,52)(H4,37,38,41)(H4,39,40,42)/t26-,27+,28+,29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-SDF-1 binding to C-X-C chemokine receptor type 4 (CXCR4) expressed in CHO cells |
J Med Chem 48: 3280-9 (2005)
Article DOI: 10.1021/jm050009h
BindingDB Entry DOI: 10.7270/Q24X5790 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50432268
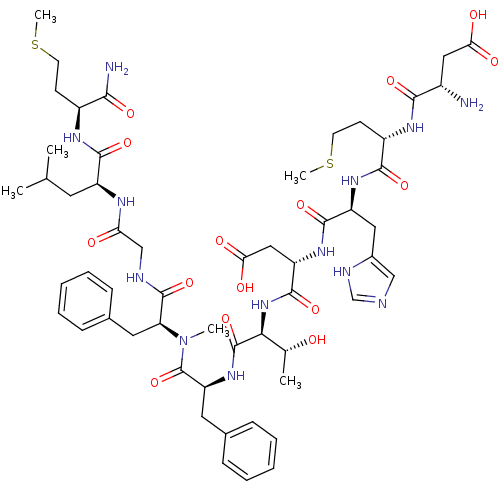
(CHEMBL2347495)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C55H79N13O15S2/c1-30(2)21-38(50(78)62-36(47(57)75)17-19-84-5)61-43(70)28-59-53(81)42(23-33-15-11-8-12-16-33)68(4)55(83)41(22-32-13-9-7-10-14-32)66-54(82)46(31(3)69)67-52(80)40(26-45(73)74)65-51(79)39(24-34-27-58-29-60-34)64-49(77)37(18-20-85-6)63-48(76)35(56)25-44(71)72/h7-16,27,29-31,35-42,46,69H,17-26,28,56H2,1-6H3,(H2,57,75)(H,58,60)(H,59,81)(H,61,70)(H,62,78)(H,63,76)(H,64,77)(H,65,79)(H,66,82)(H,67,80)(H,71,72)(H,73,74)/t31-,35+,36+,37+,38+,39+,40+,41+,42+,46+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of ([125I]-His3, MePhe7)-NKB from NK3R (unknown origin) transfected in CHO cells by gamma counting analysis |
Bioorg Med Chem 21: 2413-7 (2013)
Article DOI: 10.1016/j.bmc.2013.01.036
BindingDB Entry DOI: 10.7270/Q20K29X2 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50432272
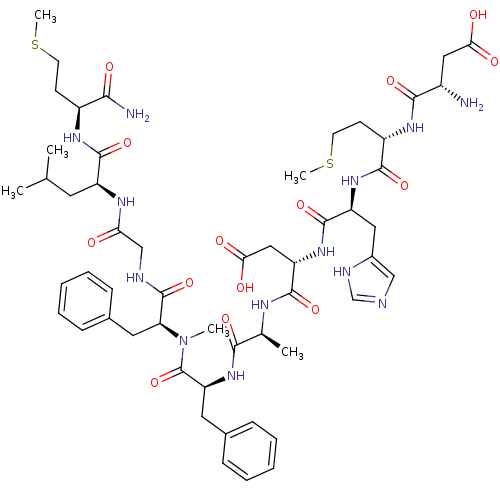
(CHEMBL2347491)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(N)=O |r| Show InChI InChI=1S/C54H77N13O14S2/c1-30(2)21-38(51(78)62-36(46(56)73)17-19-82-5)61-43(68)28-58-53(80)42(23-33-15-11-8-12-16-33)67(4)54(81)41(22-32-13-9-7-10-14-32)66-47(74)31(3)60-50(77)40(26-45(71)72)65-52(79)39(24-34-27-57-29-59-34)64-49(76)37(18-20-83-6)63-48(75)35(55)25-44(69)70/h7-16,27,29-31,35-42H,17-26,28,55H2,1-6H3,(H2,56,73)(H,57,59)(H,58,80)(H,60,77)(H,61,68)(H,62,78)(H,63,75)(H,64,76)(H,65,79)(H,66,74)(H,69,70)(H,71,72)/t31-,35-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of ([125I]-His3, MePhe7)-NKB from NK3R (unknown origin) transfected in CHO cells by gamma counting analysis |
Bioorg Med Chem 21: 2413-7 (2013)
Article DOI: 10.1016/j.bmc.2013.01.036
BindingDB Entry DOI: 10.7270/Q20K29X2 |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26341
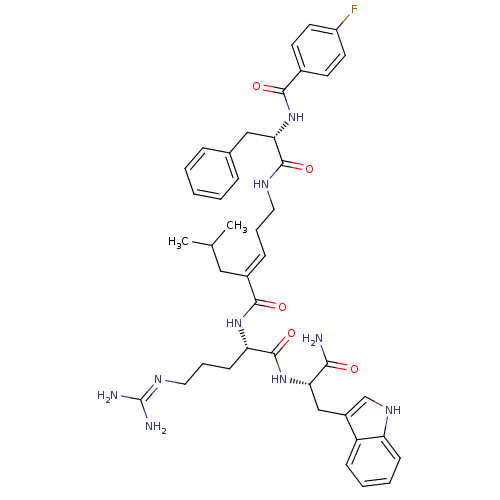
((2E)-N-[(1S)-4-carbamimidamido-1-{[(1S)-1-carbamoy...)Show SMILES CC(C)C\C(=C/CCNC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(F)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,wU:32.33,wD:11.19,43.44,(10.76,-1.42,;10.76,.12,;12.1,.89,;9.43,.89,;9.43,2.43,;8.09,3.2,;6.76,2.43,;5.43,3.2,;4.09,2.43,;2.76,3.2,;2.76,4.74,;1.43,2.43,;1.43,.89,;.66,-.45,;-.87,-.66,;-1.45,-2.09,;-.5,-3.3,;1.02,-3.09,;1.6,-1.66,;.09,3.2,;-1.24,2.43,;-1.24,.89,;-2.57,3.2,;-2.57,4.74,;-3.91,5.51,;-5.24,4.74,;-6.58,5.51,;-5.24,3.2,;-3.91,2.43,;10.76,3.2,;10.76,4.74,;12.1,2.43,;13.43,3.2,;13.43,4.74,;12.1,5.51,;12.1,7.05,;10.76,7.82,;10.76,9.36,;12.1,10.13,;9.43,10.13,;14.76,2.43,;14.76,.89,;16.1,3.2,;17.43,2.43,;17.43,.89,;18.76,.12,;18.15,-1.3,;19.3,-2.32,;20.63,-1.53,;22.1,-2,;23.23,-.96,;22.9,.55,;21.43,1.01,;20.3,-.03,;18.76,3.2,;20.1,2.43,;18.76,4.74,)| Show InChI InChI=1S/C42H52FN9O5/c1-26(2)22-29(12-8-20-47-40(56)36(23-27-10-4-3-5-11-27)52-38(54)28-16-18-31(43)19-17-28)39(55)50-34(15-9-21-48-42(45)46)41(57)51-35(37(44)53)24-30-25-49-33-14-7-6-13-32(30)33/h3-7,10-14,16-19,25-26,34-36,49H,8-9,15,20-24H2,1-2H3,(H2,44,53)(H,47,56)(H,50,55)(H,51,57)(H,52,54)(H4,45,46,48)/b29-12+/t34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Kyoto University
| Assay Description
Membranes were incubated with [125I] kisspeptin-15 and increasing concentrations of test compound in binding buffer. The reaction mixtures were dilut... |
J Med Chem 51: 7645-9 (2008)
Article DOI: 10.1021/jm800930w
BindingDB Entry DOI: 10.7270/Q2ZS2TTP |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha'
(Homo sapiens (Human)) | BDBM50144654

(CHEMBL3760043)Show SMILES COc1ccc(cc1)C(=O)Nc1ncc(s1)-c1ccc(nc1)C(O)=O Show InChI InChI=1S/C17H13N3O4S/c1-24-12-5-2-10(3-6-12)15(21)20-17-19-9-14(25-17)11-4-7-13(16(22)23)18-8-11/h2-9H,1H3,(H,22,23)(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human CK2alpha' (1 to 350 residues) using CK2tide as substrate incubated for 1 hr by off-chip mobility shift assay |
Bioorg Med Chem 24: 1136-41 (2016)
Article DOI: 10.1016/j.bmc.2016.01.043
BindingDB Entry DOI: 10.7270/Q2BK1F7G |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM107309
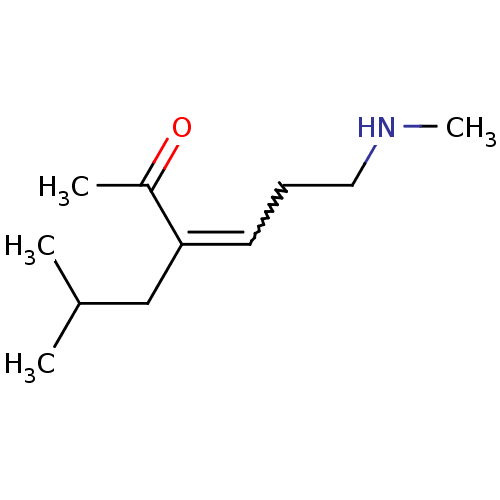
(US8592379, 21)Show InChI InChI=1S/C11H21NO/c1-9(2)8-11(10(3)13)6-5-7-12-4/h6,9,12H,5,7-8H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University; Takeda Pharmaceutical Company Limited
US Patent
| Assay Description
Binding inhibition assay using human GPR54. |
US Patent US8592379 (2013)
BindingDB Entry DOI: 10.7270/Q2N0156N |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50432270
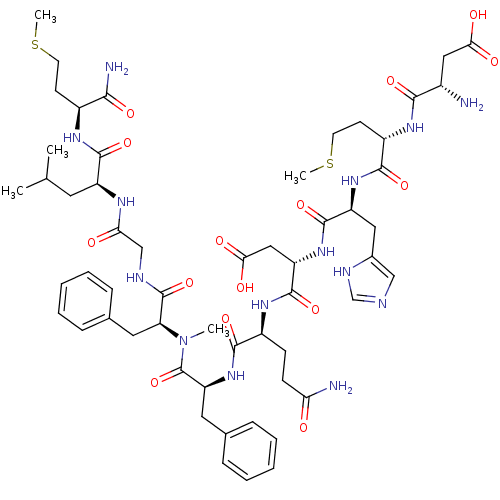
(CHEMBL2347493)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(N)=O |r| Show InChI InChI=1S/C56H80N14O15S2/c1-31(2)22-39(52(81)64-36(48(59)77)18-20-86-4)63-45(72)29-61-55(84)43(24-33-14-10-7-11-15-33)70(3)56(85)42(23-32-12-8-6-9-13-32)69-50(79)37(16-17-44(58)71)66-54(83)41(27-47(75)76)68-53(82)40(25-34-28-60-30-62-34)67-51(80)38(19-21-87-5)65-49(78)35(57)26-46(73)74/h6-15,28,30-31,35-43H,16-27,29,57H2,1-5H3,(H2,58,71)(H2,59,77)(H,60,62)(H,61,84)(H,63,72)(H,64,81)(H,65,78)(H,66,83)(H,67,80)(H,68,82)(H,69,79)(H,73,74)(H,75,76)/t35-,36-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of ([125I]-His3, MePhe7)-NKB from NK3R (unknown origin) transfected in CHO cells by gamma counting analysis |
Bioorg Med Chem 21: 2413-7 (2013)
Article DOI: 10.1016/j.bmc.2013.01.036
BindingDB Entry DOI: 10.7270/Q20K29X2 |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 1
(Homo sapiens (Human)) | BDBM26349

((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-amino-3-(4-hydroxyp...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:83.86,72.75,12.20,29.34,37.50,51.58,59.62,wD:4.4,23.26,(23.93,-21.81,;22.46,-22.29,;22.15,-23.79,;21.33,-21.26,;21.65,-19.76,;20.51,-18.74,;19.04,-19.2,;18.73,-20.72,;17.9,-18.17,;16.44,-18.65,;15.29,-17.62,;15.61,-16.12,;13.83,-18.1,;12.69,-17.06,;13.01,-15.57,;11.86,-14.54,;12.18,-13.03,;13.65,-12.55,;14.79,-13.59,;14.47,-15.08,;13.51,-19.6,;12.05,-20.08,;10.9,-19.05,;11.73,-21.57,;12.87,-22.61,;14.34,-22.12,;10.27,-22.05,;9.94,-23.55,;11.09,-24.58,;8.49,-24.03,;7.34,-23,;7.67,-21.49,;6.52,-20.46,;9.12,-21.02,;8.16,-25.53,;6.7,-26.01,;5.56,-24.98,;6.38,-27.51,;4.92,-27.99,;4.6,-29.49,;5.63,-30.63,;4.86,-31.97,;3.35,-31.65,;2.11,-32.56,;.71,-31.92,;.55,-30.4,;1.79,-29.49,;3.2,-30.12,;7.53,-28.54,;8.98,-28.07,;9.31,-26.56,;10.13,-29.1,;9.81,-30.6,;8.35,-31.08,;8.02,-32.58,;7.2,-30.05,;11.59,-28.62,;12.74,-29.65,;12.42,-31.16,;14.21,-29.18,;14.52,-27.67,;15.34,-30.2,;16.8,-29.73,;17.12,-28.22,;18.58,-27.75,;19.72,-28.77,;21.19,-28.3,;19.41,-30.28,;17.94,-30.75,;23.11,-19.28,;23.42,-17.77,;24.24,-20.31,;25.71,-19.83,;26.03,-18.32,;27.49,-17.85,;27.81,-16.34,;29.28,-15.87,;29.59,-14.36,;28.45,-13.34,;31.06,-13.9,;26.85,-20.86,;26.53,-22.37,;28.32,-20.39,;29.46,-21.42,;30.93,-20.94,;32.07,-21.97,;33.53,-21.49,;34.68,-22.52,;34.35,-24.03,;32.89,-24.5,;31.75,-23.47,;29.14,-22.92,;27.68,-23.4,;30.28,-23.95,)| Show InChI InChI=1S/C63H83N17O14/c1-34(2)24-45(58(90)74-43(18-11-23-70-63(68)69)57(89)75-44(54(67)86)26-35-12-5-3-6-13-35)73-53(85)32-72-56(88)46(27-36-14-7-4-8-15-36)77-62(94)50(33-81)80-61(93)49(30-52(66)84)79-59(91)47(28-38-31-71-42-17-10-9-16-40(38)42)78-60(92)48(29-51(65)83)76-55(87)41(64)25-37-19-21-39(82)22-20-37/h3-10,12-17,19-22,31,34,41,43-50,71,81-82H,11,18,23-30,32-33,64H2,1-2H3,(H2,65,83)(H2,66,84)(H2,67,86)(H,72,88)(H,73,85)(H,74,90)(H,75,89)(H,76,87)(H,77,94)(H,78,92)(H,79,91)(H,80,93)(H4,68,69,70)/t41-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of (D-[125I]Tyr1, MePhe3)-NPFF from NPFFR1 after 2 hrs |
ACS Med Chem Lett 2: 53-57 (2011)
Article DOI: 10.1021/ml1002053
BindingDB Entry DOI: 10.7270/Q2K07586 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50385613

(CHEMBL2042119)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#7]=[#6](-[#7])-[#6@@H](-[#6]-c2ccc3ccccc3c2)-[#7]-[#6]-1=O |r,w:35.35| Show InChI InChI=1S/C36H48N12O5/c37-31-28(19-22-9-12-23-5-1-2-6-24(23)17-22)48-33(52)27(8-4-16-43-36(40)41)46-32(51)26(7-3-15-42-35(38)39)47-34(53)29(45-30(50)20-44-31)18-21-10-13-25(49)14-11-21/h1-2,5-6,9-14,17,26-29,49H,3-4,7-8,15-16,18-20H2,(H2,37,44)(H,45,50)(H,46,51)(H,47,53)(H,48,52)(H4,38,39,42)(H4,40,41,43)/t26-,27-,28+,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-SDF-1alpha from CXCR4 receptor expressed in HEK293 cells after 1 hr by scintillation counting |
ACS Med Chem Lett 2: 477-480 (2011)
Article DOI: 10.1021/ml200047e
BindingDB Entry DOI: 10.7270/Q2D21ZNH |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50432259
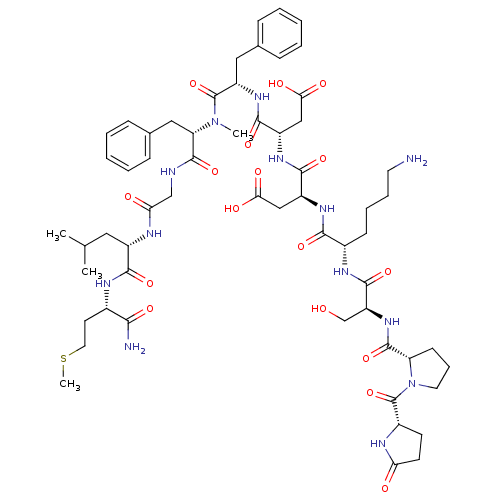
(CHEMBL2347662)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCC(=O)N1)C(N)=O |r| Show InChI InChI=1S/C59H85N13O17S/c1-33(2)26-39(52(82)65-36(50(61)80)22-25-90-4)64-47(75)31-62-56(86)45(28-35-16-9-6-10-17-35)71(3)58(88)42(27-34-14-7-5-8-15-34)69-54(84)41(30-49(78)79)68-53(83)40(29-48(76)77)67-51(81)37(18-11-12-23-60)66-55(85)43(32-73)70-57(87)44-19-13-24-72(44)59(89)38-20-21-46(74)63-38/h5-10,14-17,33,36-45,73H,11-13,18-32,60H2,1-4H3,(H2,61,80)(H,62,86)(H,63,74)(H,64,75)(H,65,82)(H,66,85)(H,67,81)(H,68,83)(H,69,84)(H,70,87)(H,76,77)(H,78,79)/t36-,37-,38-,39-,40-,41-,42-,43-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of ([125I]-His3, MePhe7)-NKB from NK3R (unknown origin) transfected in CHO cells by gamma counting analysis |
Bioorg Med Chem 21: 2413-7 (2013)
Article DOI: 10.1016/j.bmc.2013.01.036
BindingDB Entry DOI: 10.7270/Q20K29X2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


















































